Demographic Characteristics and Status of Vaccinated Individuals with a History of COVID-19 Infection Pre- or Post-Vaccination: A Descriptive Study of a Nationally Representative Sample in Saudi Arabia
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Statistical Analysis
2.3. Ethical Approval for the Study Protocol
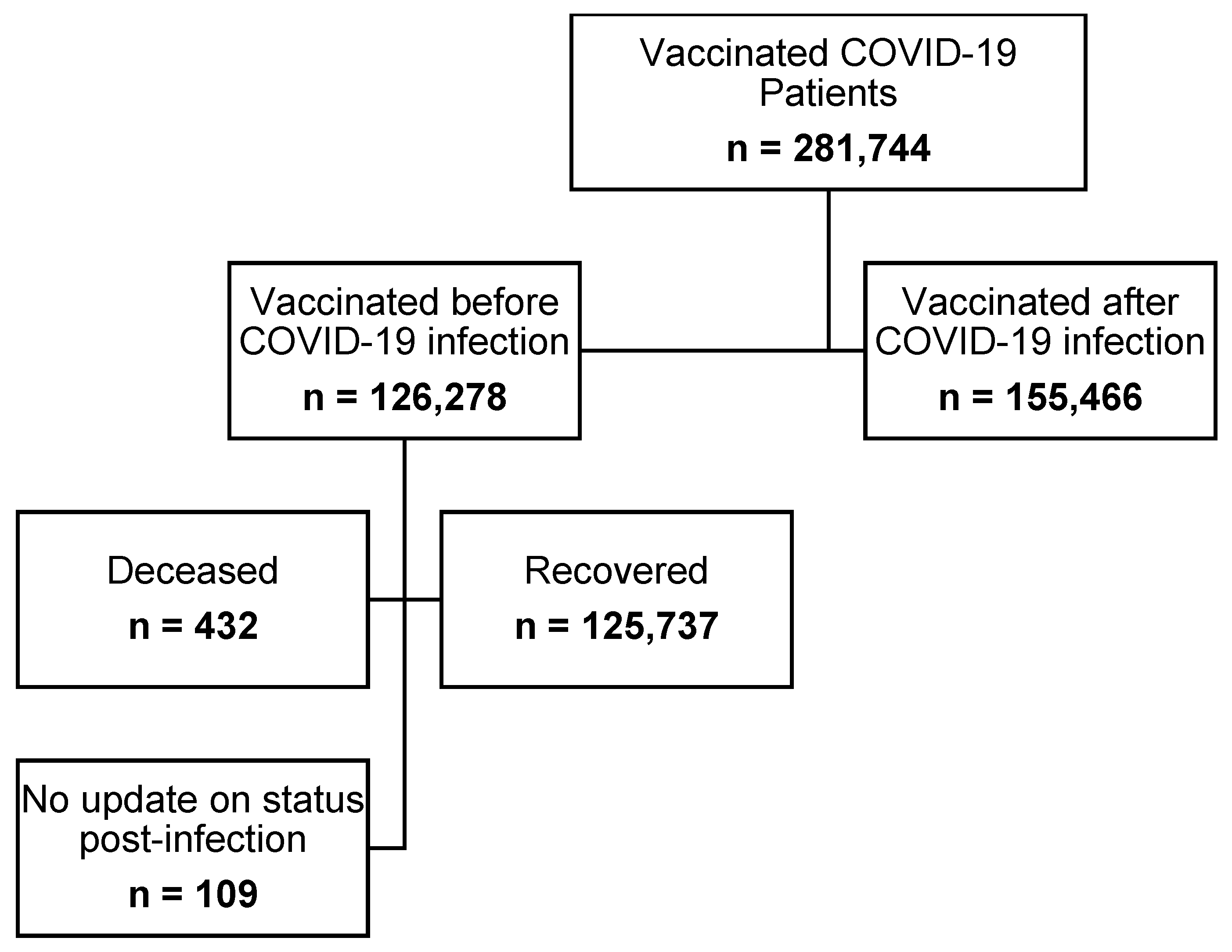
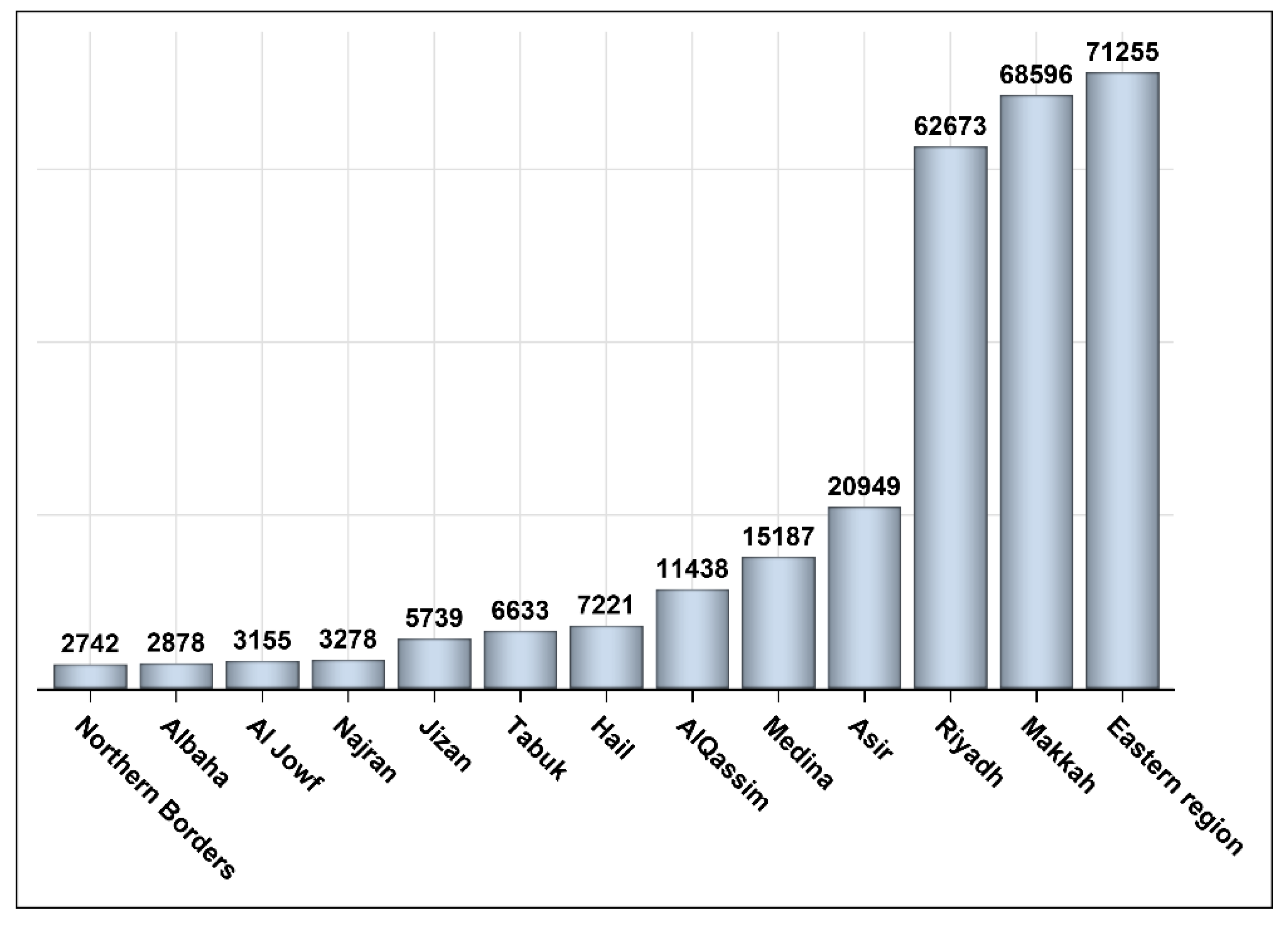
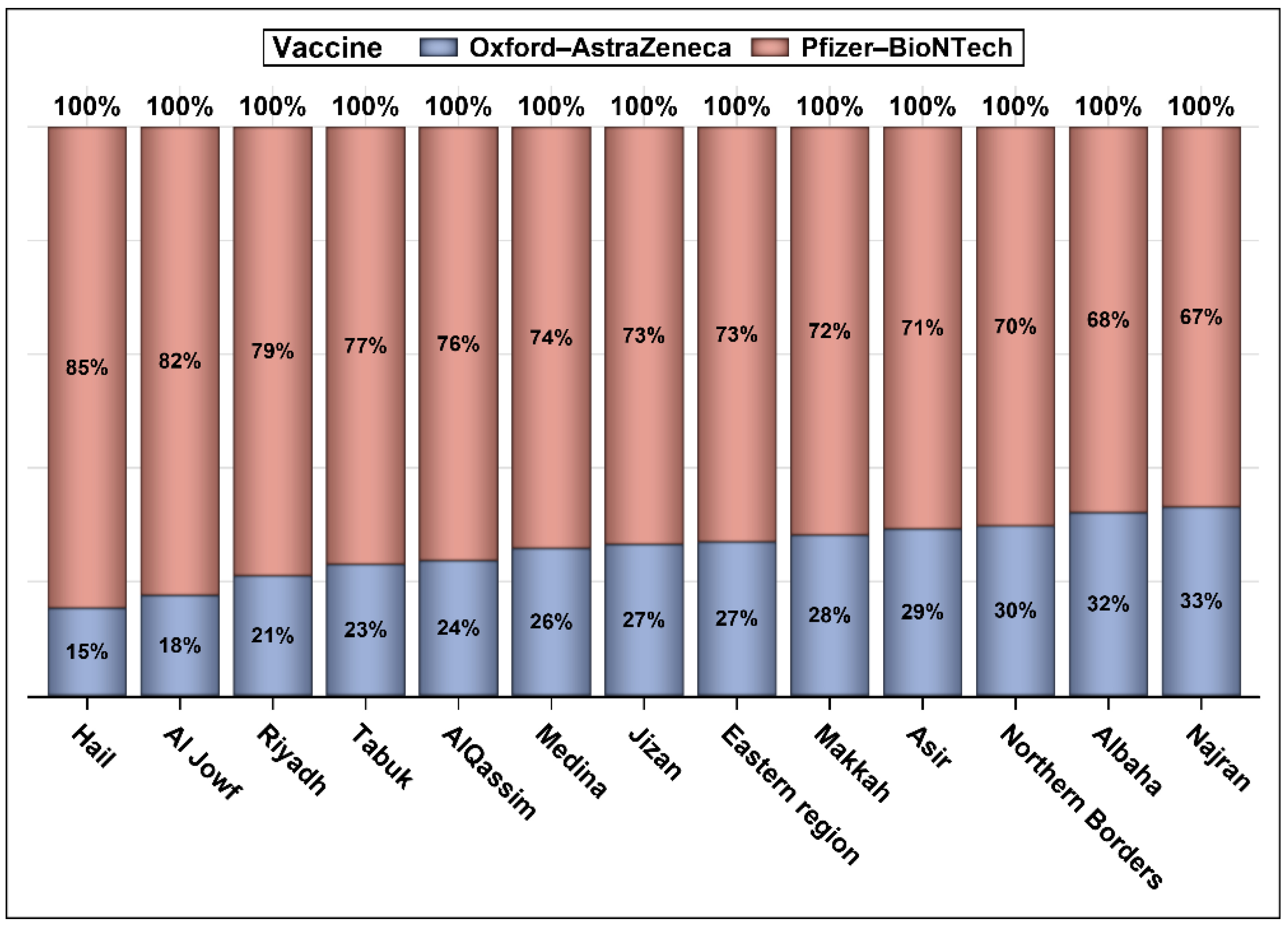
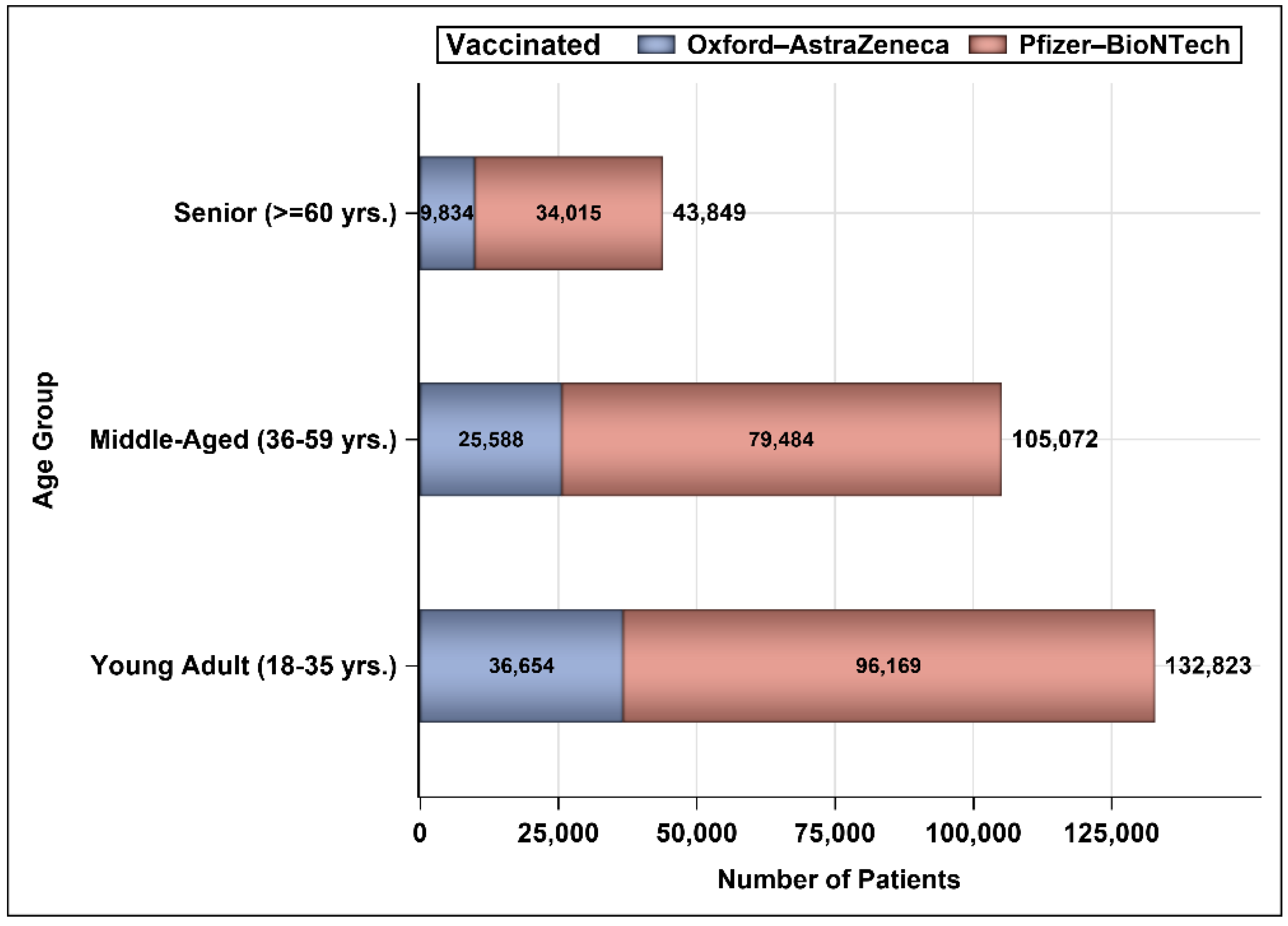
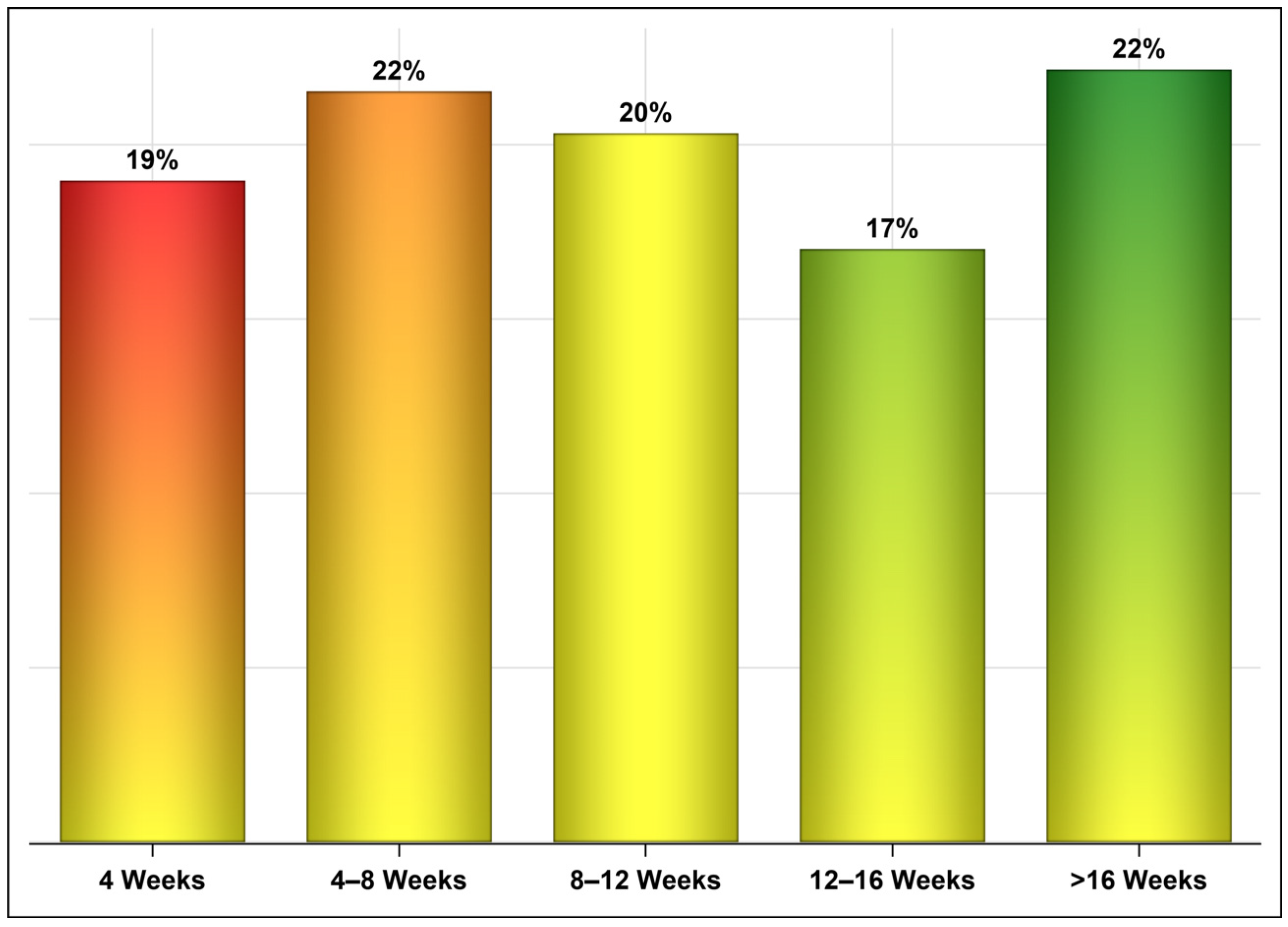
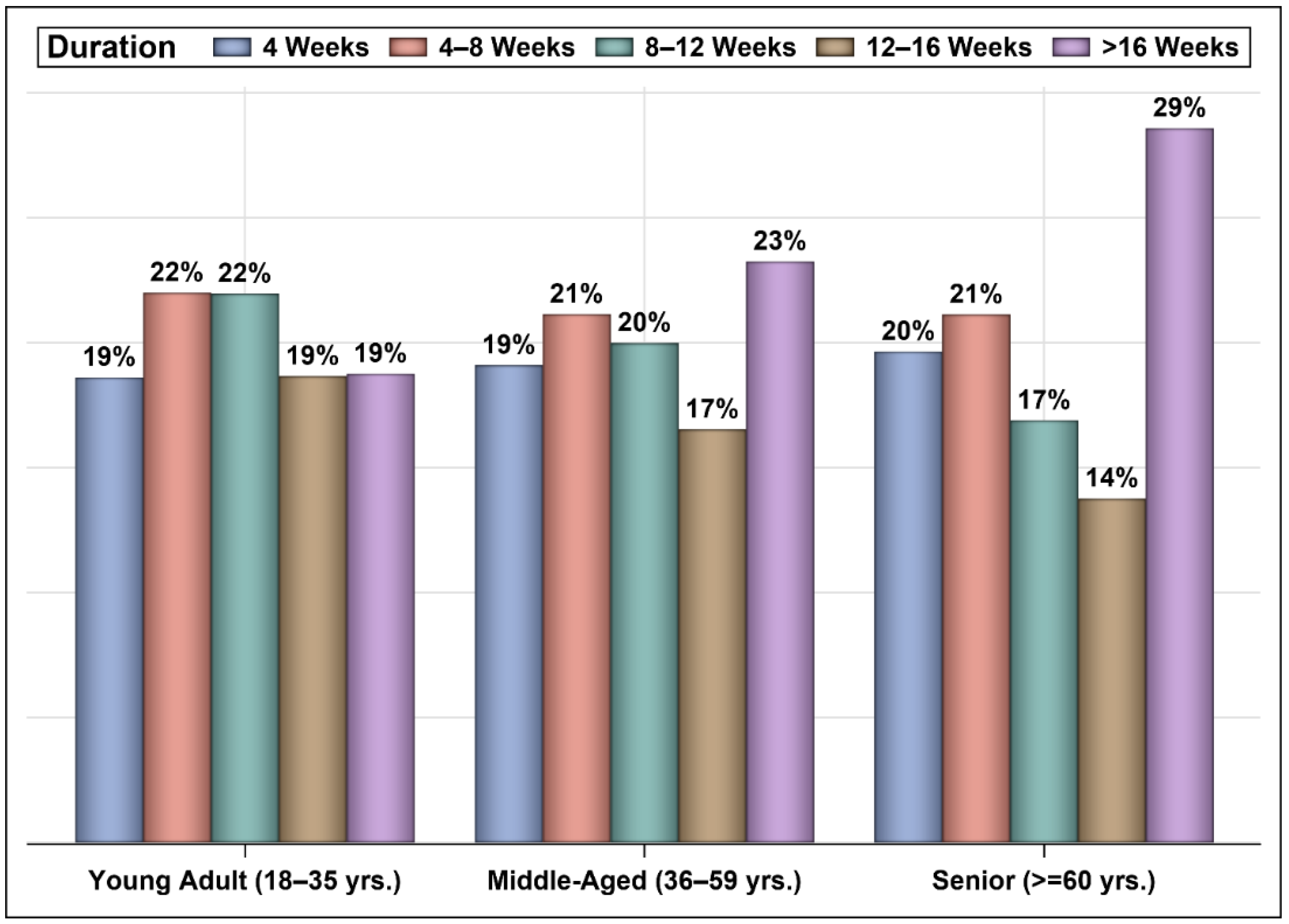
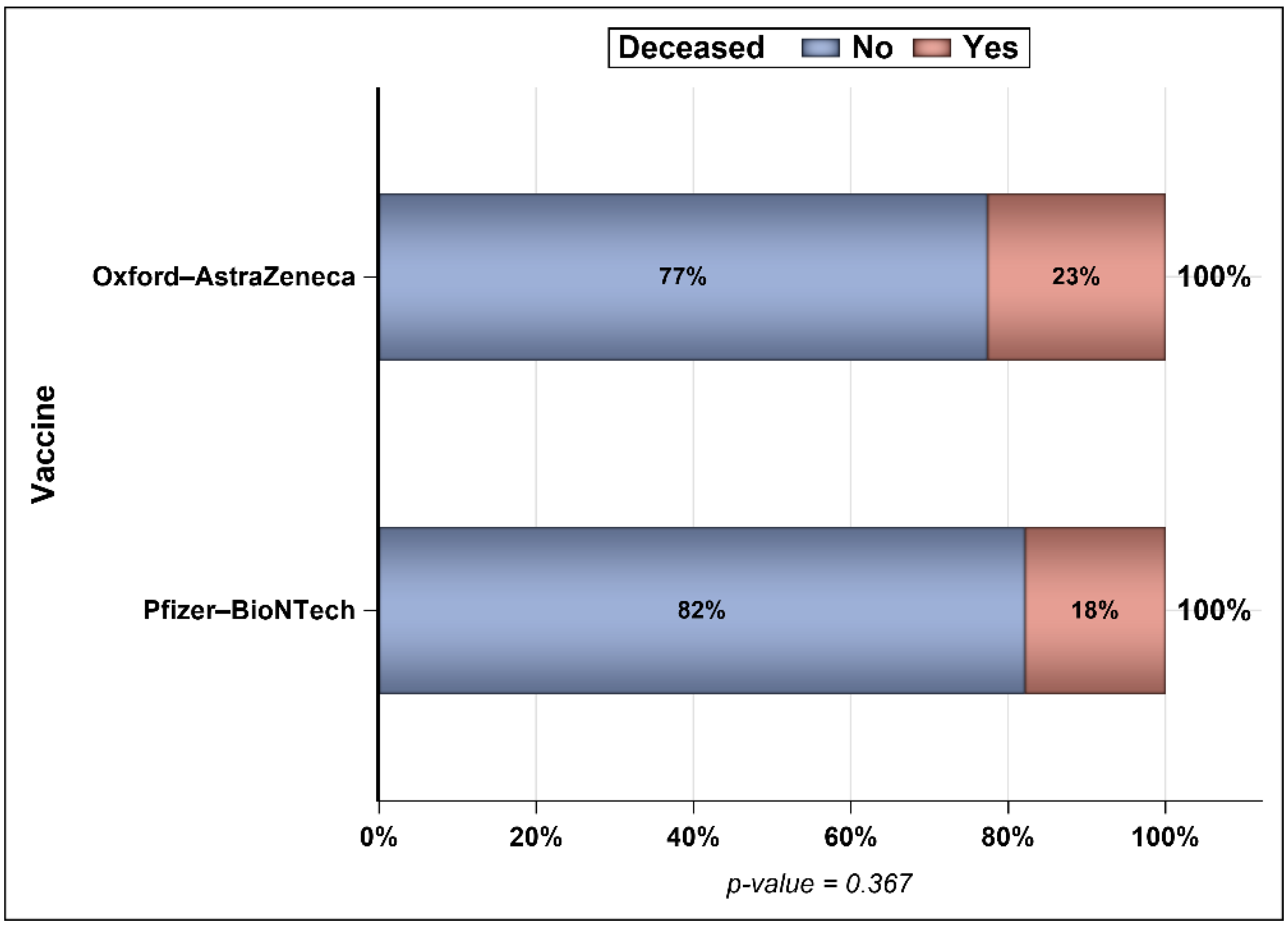
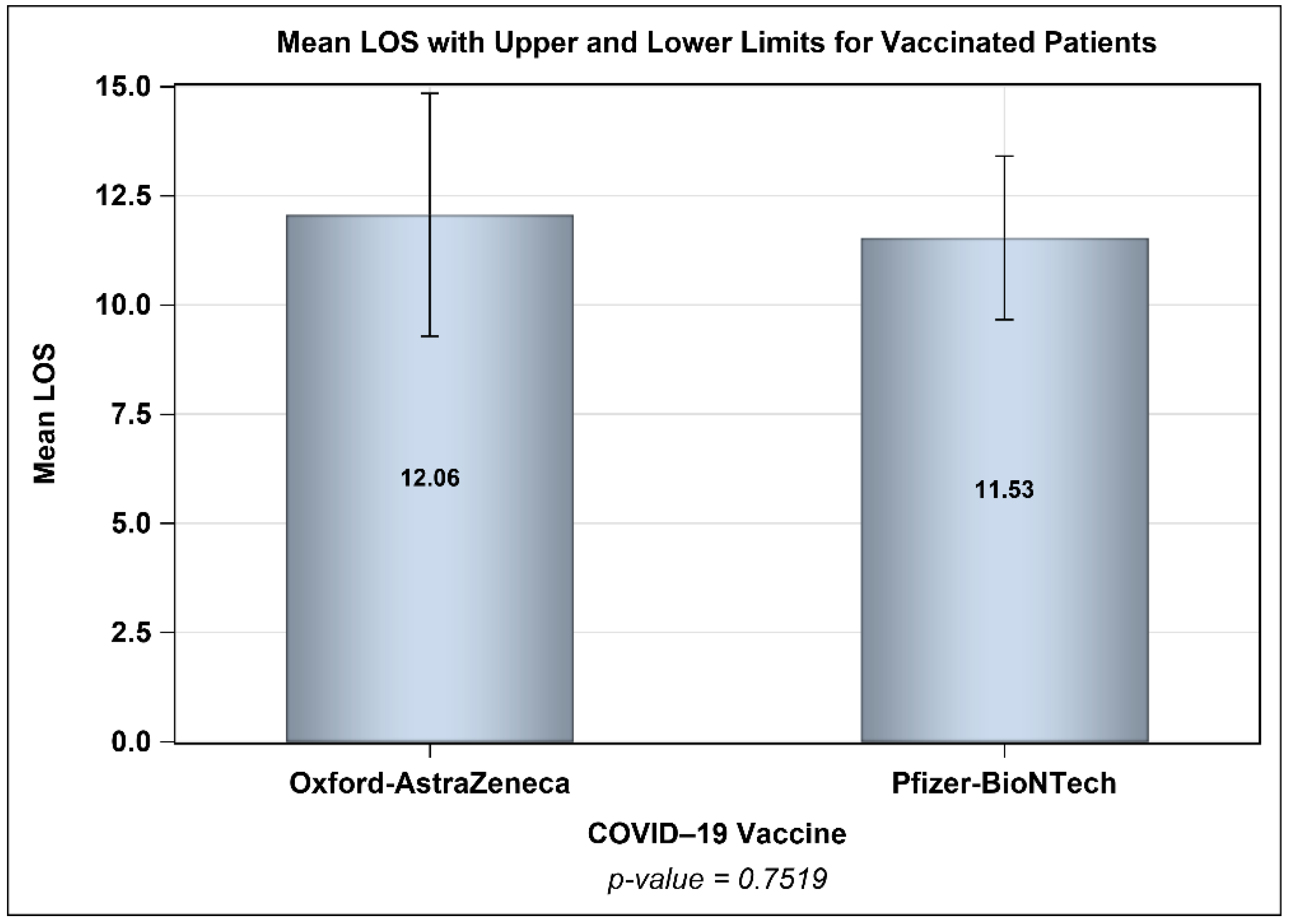
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bchetnia, M.; Girard, C.; Duchaine, C.; Laprise, C. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A review of the current global status. J. Infect. Public Health 2020, 13, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Coronavirus (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Alsofayan, Y.M.; Althunayyan, S.M.; A Khan, A.; Hakawi, A.M.; Assiri, A.M. Clinical characteristics of COVID-19 in Saudi Arabia: A national retrospective study. J. Infect. Public Health 2020, 13, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Moh, S. Saudi Ministry of Health COVID-19 Dashboard. Available online: https://covid19.moh.gov.sa/ (accessed on 13 January 2022).
- Siddiqui, S.H.; Sarfraz, A.; Rizvi, A.; Shaheen, F.; Yousafzai, M.T.; Ali, S.A. Global variation of COVID-19 mortality rates in the initial phase. Osong Public Health Res. Perspect. 2021, 12, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.K.; Navaratnam, A.V.; Day, J.; Babu, P.; Mackinnon, S.; Adelaja, I.; Bartlett-Pestell, S.; Moulton, C.; Mann, C.; Batchelor, A.; et al. Variability in COVID-19 in-hospital mortality rates between national health service trusts and regions in England: A national observational study for the Getting It Right First Time Programme. eClinicalMedicine 2021, 35, 100859. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Merone, L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int. J. Infect. Dis. 2020, 101, 138–148. [Google Scholar] [CrossRef]
- Al-Omari, A.; Alhuqbani, W.N.; Zaidi, A.R.Z.; Al-Subaie, M.F.; AlHindi, A.M.; Abogosh, A.K.; Alrasheed, A.K.; Alsharafi, A.A.; Alhuqbani, M.N.; Salih, S.; et al. Clinical characteristics of non-intensive care unit COVID-19 patients in Saudi Arabia: A descriptive cross-sectional study. J. Infect. Public Health 2020, 13, 1639–1644. [Google Scholar] [CrossRef]
- Elliott, J.; Whitaker, M.; Bodinier, B.; Eales, O.; Riley, S.; Ward, H.; Cooke, G.; Darzi, A.; Chadeau-Hyam, M.; Elliott, P. Predictive symptoms for COVID-19 in the community: REACT-1 study of over 1 million people. PLoS Med. 2021, 18, e1003777. [Google Scholar] [CrossRef]
- Chiu, N.-C.; Chi, H.; Tai, Y.-L.; Peng, C.-C.; Tseng, C.-Y.; Chen, C.-C.; Tan, B.F.; Lin, C.-Y. Impact of Wearing Masks, Hand Hygiene, and Social Distancing on Influenza, Enterovirus, and All-Cause Pneumonia During the Coronavirus Pandemic: Retrospective National Epidemiological Surveillance Study. J. Med. Internet Res. 2020, 22, e21257. [Google Scholar] [CrossRef]
- Alrashed, S.; Min-Allah, N.; Saxena, A.; Ali, I.; Mehmood, R. Impact of lockdowns on the spread of COVID-19 in Saudi Arabia. Informatics Med. Unlocked 2020, 20, 100420. [Google Scholar] [CrossRef]
- Violato, C.; Violato, E.M.; Violato, E.M. Impact of the stringency of lockdown measures on COVID-19: A theoretical model of a pandemic. PLoS ONE 2021, 16, e0258205. [Google Scholar] [CrossRef]
- Blumenthal, D.; Fowler, E.J.; Abrams, M.; Collins, S.R. COVID-19—Implications for the health care system. Mass Med. Soc. 2020, 383, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, R.; Sanders, S.; Michaleff, Z.A.; Scott, A.M.; Clark, J.; To, E.J.; Jones, M.; Kitchener, E.; Fox, M.; Johansson, M.; et al. Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open 2021, 11, e045343. [Google Scholar] [CrossRef] [PubMed]
- Paintsil, E. COVID-19 threatens health systems in sub-Saharan Africa: The eye of the crocodile. J. Clin. Investig. 2020, 130, 2741–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Alruthia, Y.; Balkhi, B.; Alghadeer, S.M.; Temsah, M.-H.; Althunayyan, S.M.; Alsofayan, Y.M. Survival and Estimation of Direct Medical Costs of Hospitalized COVID-19 Patients in the Kingdom of Saudi Arabia. Int. J. Environ. Res. Public Health 2020, 17, 7458. [Google Scholar] [CrossRef] [PubMed]
- Aljadeed, R.; AlRuthia, Y.; Balkhi, B.; Sales, I.; Alwhaibi, M.; Almohammed, O.; Alotaibi, A.; Alrumaih, A.; Asiri, Y. The Impact of COVID-19 on Essential Medicines and Personal Protective Equipment Availability and Prices in Saudi Arabia. Healthcare 2021, 9, 290. [Google Scholar] [CrossRef]
- Le Page, M. The rush to develop a vaccine. New Sci. 2020, 247, 8–10. [Google Scholar] [CrossRef]
- Graham, B.S. Rapid COVID-19 vaccine development. Science 2020, 368, 945–946. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- COVID NJI. Vaccine: Interim Analysis of Phase 3 Clinical Data Released. Natl. Inst. Health News Release 2021. Available online: https://www.nih.gov/news-events/news-releases/janssen-investigational-covid-19-vaccine-interim-analysis-phase-3-clinical-data-released (accessed on 11 December 2021).
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef]
- Haas, E.J.; McLaughlin, J.M.; Khan, F.; Angulo, F.J.; Anis, E.; Lipsitch, M.; Singer, S.R.; Mircus, G.; Brooks, N.; Smaja, M.; et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: A retrospective surveillance study. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Flacco, M.; Soldato, G.; Martellucci, C.A.; Carota, R.; Di Luzio, R.; Caponetti, A.; Manzoli, L. Interim Estimates of COVID-19 Vaccine Effectiveness in a Mass Vaccination Setting: Data from an Italian Province. Vaccines 2021, 9, 628. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Al-Tawfiq, J.A.; Alghnam, S.; Alwehaibe, A.; Alasmari, A.; Alsagaby, S.A.; Alotaibi, F.; Alsubaie, F.; Alshomrani, M.; Farahat, F.M.; et al. Effectiveness of COVID-19 Vaccines: Eight Months Post Single Dose Vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Albogami, Y.; Alkofide, H.; Alrwisan, A. COVID-19 Vaccine Surveillance in Saudi Arabia: Opportunities for Real-Time Assessment. Saudi Pharm. J. 2021, 29, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Saudi Food and Drug Authority. Saudi Food and Drug Authority Allows the Import and Use of AstraZeneca Covid19 Vaccine. Available online: https://sfda.gov.sa/en/news/79059 (accessed on 27 December 2021).
- Gazette, S. MoH: Six COVID-19 Vaccines Approved in Saudi Arabia In. Saudi Gazette. Saudi Arabia. 2021. Available online: https://saudigazette.com.sa/article/610193 (accessed on 14 December 2021).
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Co-Operation and Development (OECD). COVID-19 Crisis Response in MENA Countries. Available online: https://www.oecd.org/coronavirus/policy-responses/covid-19-crisis-response-in-mena-countries-4b366396/ (accessed on 13 January 2021).
- Skegg, D.; Pearce, N. Unwarranted optimism about vaccine efficacy. BMJ 2020, 371, m4918. [Google Scholar] [CrossRef]
- Duch, R.; Roope, L.S.J.; Violato, M.; Becerra, M.F.; Robinson, T.S.; Bonnefon, J.-F.; Friedman, J.; Loewen, P.J.; Mamidi, P.; Melegaro, A.; et al. Citizens from 13 countries share similar preferences for COVID-19 vaccine allocation priorities. Proc. Natl. Acad. Sci. USA 2021, 118, e2026382118. [Google Scholar] [CrossRef]
- Ribas, A.; Sengupta, R.; Locke, T.; Zaidi, S.K.; Campbell, K.M.; Carethers, J.M.; Jaffee, E.M.; Wherry, E.J.; Soria, J.-C.; D’Souza, G. Priority COVID-19 Vaccination for Patients with Cancer while Vaccine Supply Is Limited. Cancer Discov. 2020, 11, 233–236. [Google Scholar] [CrossRef]
- Su, Z.; McDonnell, D.; Li, X.; Bennett, B.; Šegalo, S.; Abbas, J.; Cheshmehzangi, A.; Xiang, Y.-T. COVID-19 Vaccine Donations—Vaccine Empathy or Vaccine Diplomacy? A Narrative Literature Review. Vaccines 2021, 9, 1024. [Google Scholar] [CrossRef]
- Nhamo, G.; Chikodzi, D.; Kunene, H.P.; Mashula, N. COVID-19 vaccines and treatments nationalism: Challenges for low-income countries and the attainment of the SDGs. Glob. Public Health 2020, 16, 319–339. [Google Scholar] [CrossRef]
- AlFattani, A.; AlMeharish, A.; Nasim, M.; AlQahtani, K.; AlMudraa, S. Ten public health strategies to control the Covid-19 pandemic: The Saudi Experience. IJID Reg. 2021, 1, 12–19. [Google Scholar] [CrossRef]
- Torreele, E.; Amon, J.J. Equitable COVID-19 Vaccine Access. Health Hum. Rights 2021, 23, 273–288. [Google Scholar]
- Torjesen, I. COVID-19 vaccine shortages: What is the cause and what are the implications? BMJ 2021, 372, n781. [Google Scholar] [CrossRef]
- Agrawal, U.; Katikireddi, S.V.; McCowan, C.; Mulholland, R.H.; Azcoaga-Lorenzo, A.; Amele, S.; Fagbamigbe, A.F.; Vasileiou, E.; Grange, Z.; Shi, T.; et al. COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2·57 million people in Scotland (EAVE II): A prospective cohort study. Lancet Respir. Med. 2021, 9, 1439–1449. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.-K.; Moore, Z.; Zeng, D. Effectiveness of Covid-19 Vaccines over a 9-Month Period in North Carolina. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to Covid-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Chinn, J.; De Ferrante, M.; Kirby, K.A.; Hohmann, S.F.; Amin, A. Male gender is a predictor of higher mortality in hospitalized adults with COVID-19. PLoS ONE 2021, 16, e0254066. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Rahaman, S.; Biswas, T.K.; Haque, Z.; Ibrahim, B. Association of Sex, Age, and Comorbidities with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Intervirology 2020, 64, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Kane, A.D.; Kursumovic, E.; Oglesby, F.C.; Cook, T.M. Mortality in patients admitted to intensive care with COVID-19: An updated systematic review and meta-analysis of observational studies. Anaesthesia 2021, 76, 537–548. [Google Scholar] [CrossRef]
- Loomba, S.; de Figueiredo, A.; Piatek, S.J.; de Graaf, K.; Larson, H.J. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat. Hum. Behav. 2021, 5, 337–348, Correction in 2021, 5, 960. [Google Scholar] [CrossRef]
- Shams, A.B.; Apu, E.H.; Rahman, A.; Raihan, S.; Siddika, N.; Preo, R.; Hussein, M.; Mostari, S.; Kabir, R. Web Search Engine Misinformation Notifier Extension (SEMiNExt): A Machine Learning Based Approach during COVID-19 Pandemic. Healthcare 2021, 9, 156. [Google Scholar] [CrossRef]
- Almujarri, F.; Aldayel, A.; Almoushawah, A.; Alsuliman, A.; Alshehri, M.; Alosaimi, S. The characteristics and comorbidities of chronic diseases in patients visiting in a major governmental clinic in Riyadh, Saudi Arabia. J. Health Inform. Dev. Ctries. 2020, 14, 1–9. [Google Scholar]
- Saquib, N.; Saquib, J.; Alhadlag, A.; Albakour, M.A.; Aljumah, B.; Sughayyir, M.; AlHomidan, Z.; Alminderej, O.; Aljaser, M.; Al-Mazrou, A. Chronic disease prevalence among elderly Saudi men. Int. J. Health Sci. 2017, 11, 11–16. [Google Scholar]

| Characteristic | COVID-19 Vaccine | p-Value * | Total | |
|---|---|---|---|---|
| Pfizer–BioNTech N (%) | Oxford–AstraZeneca N (%) | |||
| COVID-19 Infection | ||||
| Pre-Vaccination | 122,706 (58.52) | 32,760 (45.45) | <0.0001 | 155,466 (55.18) |
| Post-Vaccination | 86,962 (41.48) | 39,316 (54.55) | 126,278 (44.82) | |
| Gender | ||||
| Female | 112,575 (53.69) | 33,522 (46.51) | <0.0001 | 146,097 (51.85) |
| Male | 97,093 (46.31) | 38,554 (53.49) | 135,647 (48.15) | |
| COVID-19 related ICU Admissions | ||||
| Yes | 331 (0.16) | 116 (0.16) | 0.858 | 447 (0.16) |
| No | 209,337 (99.84) | 71,960 (99.84) | 281,297 (99.84) | |
| Number of injections | ||||
| 1 | 72,000 (34.34) | 38,314 (53.16) | <0.0001 | 110,314 (39.15) |
| 2 | 137,668 (65.66) | 33,762 (46.84) | 171,430 (60.85) | |
| Variable | Odds Ratio (OR) | p Value | 95% Confidence Interval |
|---|---|---|---|
| Pfizer–BioNTech vs. Oxford–AstraZeneca | 1.121 | 0.2916 | 0.907–1.386 |
| Female vs. Male | 0.712 | 0.0009 | 0.583–0.871 |
| Age | 1.099 | <0.0001 | 1.091–1.106 |
| ICU admission | 15.746 | <0.0001 | 10.757–23.048 |
| Number of injections (2 injections vs. 1 injection) | 0.149 | <0.0001 | 0.118–0.189 |
| Variable | Odds Ratio (OR) | p Value | 95% Confidence Interval |
|---|---|---|---|
| Number of injections (2 injections vs. 1 injection) | 0.215 | <0.0001 | 0.141–0.328 |
| Female vs. Male | 0.880 | 0.4755 | 0.620–1.250 |
| Age | 1.105 | <0.0001 | 1.092–1.118 |
| ICU admission | 19.001 | <0.0001 | 10.125–35.660 |
| Variable | Odds Ratio (OR) | p Value | 95% Confidence Interval |
|---|---|---|---|
| Number of injections (2 injections vs. 1 injection) | 0.127 | <0.0001 | 0.096–0.168 |
| Female vs. Male | 0.634 | 0.0010 | 0.496–0.810 |
| Age | 1.095 | <0.0001 | 1.086–1.104 |
| ICU admission | 13.730 | <0.0001 | 8.505–22.164 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlRuthia, Y.; Al-Salloum, H.F.; Almohammed, O.A.; Alqahtani, A.S.; Al-Abdulkarim, H.A.; Alsofayan, Y.M.; Almudarra, S.S.; AlQahtani, S.H.; Almutlaq, A.; Alabdulkareem, K.; et al. Demographic Characteristics and Status of Vaccinated Individuals with a History of COVID-19 Infection Pre- or Post-Vaccination: A Descriptive Study of a Nationally Representative Sample in Saudi Arabia. Vaccines 2022, 10, 323. https://doi.org/10.3390/vaccines10020323
AlRuthia Y, Al-Salloum HF, Almohammed OA, Alqahtani AS, Al-Abdulkarim HA, Alsofayan YM, Almudarra SS, AlQahtani SH, Almutlaq A, Alabdulkareem K, et al. Demographic Characteristics and Status of Vaccinated Individuals with a History of COVID-19 Infection Pre- or Post-Vaccination: A Descriptive Study of a Nationally Representative Sample in Saudi Arabia. Vaccines. 2022; 10(2):323. https://doi.org/10.3390/vaccines10020323
Chicago/Turabian StyleAlRuthia, Yazed, Haya F. Al-Salloum, Omar A. Almohammed, Amani S. Alqahtani, Hana A. Al-Abdulkarim, Yousef M. Alsofayan, Sami S. Almudarra, Sara H. AlQahtani, Abdullah Almutlaq, Khaled Alabdulkareem, and et al. 2022. "Demographic Characteristics and Status of Vaccinated Individuals with a History of COVID-19 Infection Pre- or Post-Vaccination: A Descriptive Study of a Nationally Representative Sample in Saudi Arabia" Vaccines 10, no. 2: 323. https://doi.org/10.3390/vaccines10020323
APA StyleAlRuthia, Y., Al-Salloum, H. F., Almohammed, O. A., Alqahtani, A. S., Al-Abdulkarim, H. A., Alsofayan, Y. M., Almudarra, S. S., AlQahtani, S. H., Almutlaq, A., Alabdulkareem, K., Balkhi, B., Almutairi, H. T., Alanazi, A. S., & Asiri, Y. A. (2022). Demographic Characteristics and Status of Vaccinated Individuals with a History of COVID-19 Infection Pre- or Post-Vaccination: A Descriptive Study of a Nationally Representative Sample in Saudi Arabia. Vaccines, 10(2), 323. https://doi.org/10.3390/vaccines10020323






