Natural Brucella melitensis Infection and Rev. 1 Vaccination Induce Specific Brucella O-Polysaccharide Antibodies Involved in Complement Mediated Brucella Cell Killing
Abstract
:1. Introduction
2. Material and Methods
2.1. Bacteria
2.2. Serum
2.3. Indirect ELISA for Brucella–OPS Antibodies
2.4. Serum Bactericidal Activity
2.5. Statistical Analysis
3. Results
3.1. Serology and Serum Bactericidal Activity
3.1.1. Serology and Serum Bactericidal Activity (SBA) of Post-Vaccination Sera of Flock 1
3.1.2. Serology and Serum Bactericidal Activity (SBA) of Post-Vaccination Sera of Flock 2
3.1.3. Serology and Serum Bactericidal Activity (SBA) of Post-Infection Sera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Institutional Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xavier, M.N.; Paixao, T.A.; den Hartigh, A.B.; Tsolis, R.M.; Santos, R.L. Pathogenesis of Brucella spp. Open Vet. Sci. J. 2010, 4, 109–118. [Google Scholar] [CrossRef]
- Seleem, M.N.; Boyle, S.M.; Sriranganathan, N. Brucellosis: A re-emerging zoonosis. Vet. Microbiol. 2010, 140, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.P.; Mulder, M.; Gilman, R.H.; Smits, H.L. Human brucellosis. Lancet Infect. Dis. 2007, 7, 775–786. [Google Scholar] [CrossRef]
- Moyle, P.M. Progress in vaccine development. Curr. Protoc. Microbiol. 2015, 36, 18.1.1–18.1.26. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q. Research progress in live attenuated Brucella vaccine development. Curr. Pharm. Biotechnol. 2013, 14, 887–896. [Google Scholar] [CrossRef]
- Herzberg, M.; Elberg, S. Immunization against brucella infection. I. Isolation and characterization of a streptomycin-dependent mutant. J. Bacteriol. 1953, 66, 585–599. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. The Development of New/Improved Brucellosis Vaccines: Report of WHO Meeting, Geneva, Switzerland, 11–12 December 1997; World Health Organization: Geneva, Switzerland, 1998; pp. 1–48. [Google Scholar]
- Ghanem-Zoubi, N.; Eljay, S.P.; Anis, E.; Paul, M. Reemergence of Human Brucellosis in Israel. Isr. Med. Assoc. J. 2019, 21, 10–12. [Google Scholar]
- Banai, M. Control of small ruminant brucellosis by use of Brucella melitensis Rev.1 vaccine: Laboratory aspects and field observations. Vet. Microbiol. 2002, 90, 497–519. [Google Scholar] [CrossRef]
- Ko, J.; Splitter, G.A. Molecular host-pathogen interaction in brucellosis: Current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 2003, 16, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Corbeil, L.B.; Blau, K.; Inzana, T.J.; Nielsen, K.H.; Jacobson, R.H.; Corbeil, R.R.; Winter, A.J. Killing of brucella abortus by bovine serum. Infect. Immun. 1988, 56, 3251–3261. [Google Scholar] [CrossRef] [Green Version]
- Paixão, T.A.; Costa, E.A.; Xavier, M.N.; Silva, T.M.A.; Santos, R.L. Natural resistance and innate immunity in brucellosis. Vet. Immunol. Immunopathol. 2009, 1, 21–37. [Google Scholar]
- Skendros, P.; Boura, P. Immunity to brucellosis. OIE Rev. Sci. Tech. 2013, 32, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forget, A.; Riendeau, C. Enhancement activity of anti-brucella sera in experimental Brucella abortus infection in mice. Rev. Can. Biol. 1977, 36, 239–243. [Google Scholar] [PubMed]
- Limet, J.; Plommet, A.M.; Dubray, G.; Plommet, M. Immunity conferred upon mice by anti-LPS monoclonal antibodies in murine Brucellosis. Ann. l’Institut Pasteur Immunol. 1987, 138, 417–424. [Google Scholar] [CrossRef]
- Phillips, M.; Deyoe, B.L.; Canning, P.C. Protection of mice against Brucella abortus infection by inoculation with monoclonal antibodies recognizing Brucella O-antigen. Am. J. Vet. Res. 1989, 50, 2158–2161. [Google Scholar]
- Adone, R.; Francia, M.; Pistoia, C.; Petrucci, P.; Pesciaroli, M.; Pasquali, P. Protective role of antibodies induced by Brucella melitensis B115 against B. melitensis and Brucella abortus infections in mice. Vaccine 2012, 30, 3992–3995. [Google Scholar] [CrossRef]
- Bundle, D.R.; McGiven, J. Brucellosis: Improved Diagnostics and Vaccine Insights from Synthetic Glycans. Acc. Chem. Res. 2017, 50, 2958–2967. [Google Scholar] [CrossRef]
- Jang, M.S.; Sahastrabuddhe, S.; Yun, C.H.; Han, S.H.; Yang, J.S. Serum bactericidal assay for the evaluation of typhoid vaccine using a semi-automated colony-counting method. Microb. Pathog. 2016, 97, 19–26. [Google Scholar] [CrossRef]
- Barquero-Calvo, E.; Chaves-Olarte, E.; Weiss, D.S.; Guzmán-Verri, C.; Chacón-Díaz, C.; Rucavado, A.; Moriyón, I.; Moreno, E. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE 2007, 2, e631. [Google Scholar] [CrossRef]
- Mandal, S.S.; Duncombe, L.; Ganesh, N.V.; Sarkar, S.; Howells, L.; Hogarth, P.J.; Bundle, D.R.; McGiven, J. Novel Solutions for Vaccines and Diagnostics to Combat Brucellosis. ACS Cent. Sci. 2017, 3, 224–231. [Google Scholar] [CrossRef] [Green Version]
- McGiven, J.; Howells, L.; Duncombe, L.; Stack, J.; Ganesh, N.V.; Guiard, J.; Bundle, D.R. Improved serodiagnosis of bovine brucellosis by novel synthetic oligosaccharide antigens representing the capping M epitope elements of Brucella O-Polysaccharide. J. Clin. Microbiol. 2015, 53, 1204–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alton, G.G.; Jones, L.M.; Pietz, D.E.; World Health Organization; Food and Agriculture Organization of the United Nations. Laboratory Techniques in Brucellosis, 2nd ed.; World Health Organization: Geneva, Switzerland, 1975. [Google Scholar]
- Baum, M.; Zamir, O.; Bergman-Rios, R.; Katz, E.; Beider, Z.; Cohen, A.; Banai, M. Comparative evaluation of microagglutination test and serum agglutination test as supplementary diagnostic methods for brucellosis. J. Clin. Microbiol. 1995, 33, 2166–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oswald, I.P.; Lantier, F.; Bourgy, G. Classical and alternative pathway haemolytic activities of ovine complement: Variations with age and sex. Vet. Immunol. Immunopathol. 1990, 24, 259–266. [Google Scholar] [CrossRef]
- Estein, S.M.; Cheves, P.C.; Fiorentino, M.A.; Cassataro, J.; Paolicchi, F.A.; Bowden, R.A. Immunogenicity of recombinant Omp31 from Brucella melitensis in rams and serum bactericidal activity against B. ovis. Vet. Microbiol. 2004, 102, 203–213. [Google Scholar] [CrossRef]
- Díaz, A.G.; Clausse, M.; Paolicchi, F.A.; Fiorentino, M.A.; Ghersi, G.; Zylberman, V.; Goldbaum, F.A.; Estein, S.M. Immune response and serum bactericidal activity against Brucella ovis elicited using a short immunization schedule with the polymeric antigen BLSOmp31 in rams. Vet. Immunol. Immunopathol. 2013, 154, 36–41. [Google Scholar] [CrossRef]
- Banai, M.; Itin, R.; Bardenstein, S. Perspectives and outcomes of the activity of a reference laboratory for brucellosis. Front. Vet. Sci. 2018, 4, 234. [Google Scholar] [CrossRef] [Green Version]
- Corbel, M.M.J. Brucellosis in Humans and Animals; WHO Library Cataloguing-in-Publication Data; WHO/CDS/EPR/2006.7; WHO: Geneva, Switzerland, 2006; pp. 1–88. [Google Scholar]
- González, D.; Grilló, M.J.; De Miguel, M.J.; Ali, T.; Arce-Gorvel, V.; Delrue, R.M.; Conde-Álvarez, R.; Muñoz, P.; López-Goñi, I.; Iriarte, M.; et al. Brucellosis vaccines: Assessment of Brucella melitensis lipopolysaccharide rough mutants defective in core and O-polysaccharide synthesis and export. PLoS ONE 2008, 3, e2760. [Google Scholar] [CrossRef]
- Robbins, J.B.; Chu, C.; Schneerson, R. Hypothesis for vaccine development: Protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin. Infect. Dis. 1992, 15, 346–361. [Google Scholar] [CrossRef]
- Taylor, D.N.; Trofa, A.C.; Sadoff, J.; Chu, C.; Bryla, D.; Shiloach, J.; Cohen, D.; Ashkenazi, S.; Lerman, Y.; Egan, W. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei bound to bacterial toxoids. Infect. Immun. 1993, 61, 3678–3687. [Google Scholar] [CrossRef] [Green Version]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]



| Serum Identity Number | Before Vaccination (Day 0) | 15 Days Post Vaccination (Day 15) | 30 Days Post Vaccination (Day 30) | |||
|---|---|---|---|---|---|---|
| MAT | CFT | MAT | CFT | MAT | CFT | |
| 9912 | Neg | Neg | Neg | Neg | 1:80 (+3) | Neg |
| 9913 | Neg | Neg | 1:40 (+3) | Neg | 1:80 (+3) | 1:5 (+3) |
| 9914 | Neg | Neg | Neg | Neg | Neg | Neg |
| 9921 | Neg | Neg | 1:40 (+3) | Neg | 1:80 (+3) | 1:20 (+3) |
| Sheep Number | Serology Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 (19 July 2021) | Day 15 (3 August 2021) | Day 30 (19 August 2021) | |||||||
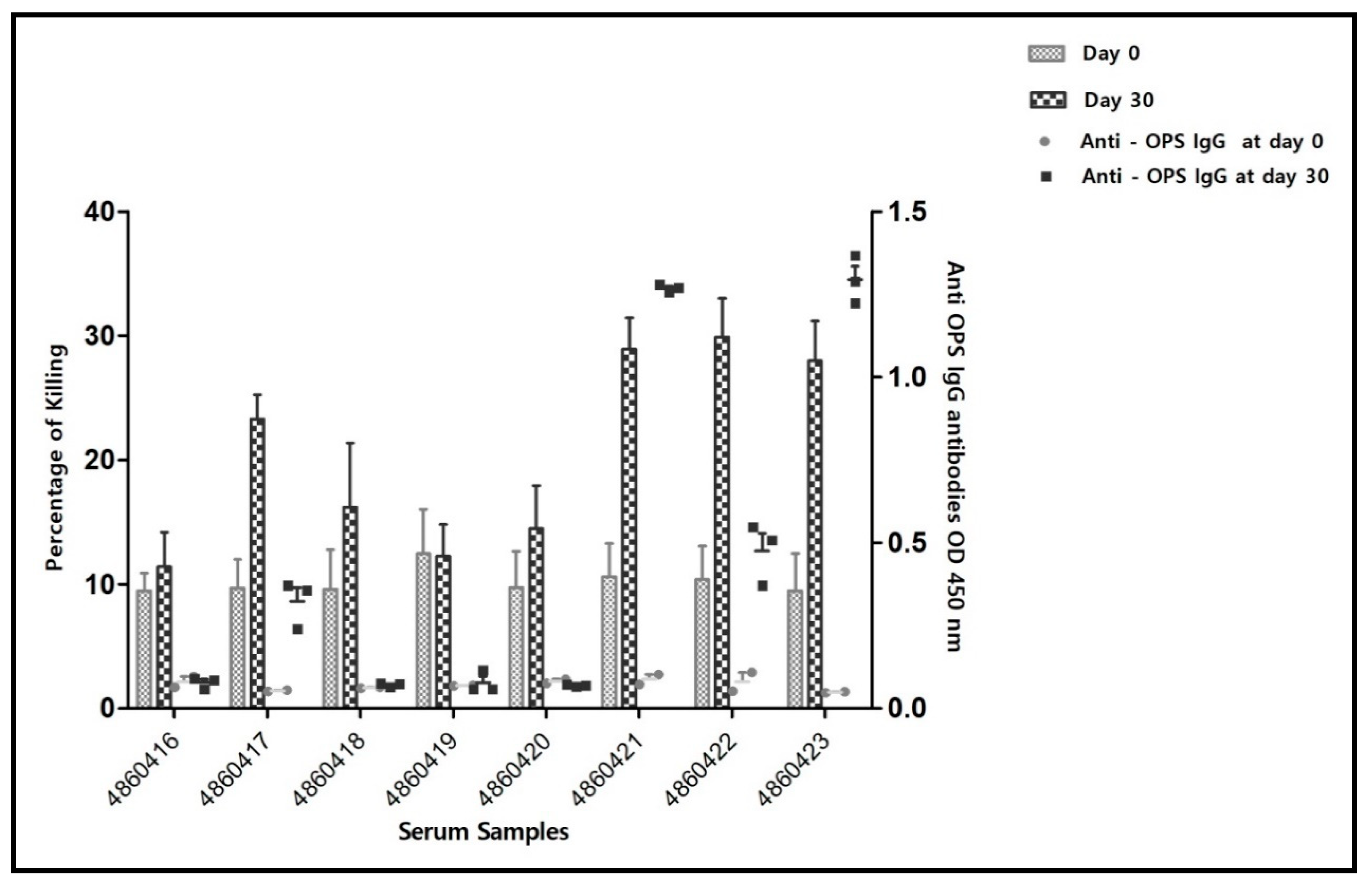
| SAT | CFT | ELISA IgG (OD) | SAT | CFT | ELISA (OD) | SAT | CFT | ELISA IgG (OD) | |
| 4860416 | Neg | Neg | 0.0805 | Neg | Neg | 0.077033333 | Neg | Neg | 0.078 |
| 4860417 | Neg | Neg | 0.053 | 1:20 (+4) | Neg | 0.056 | 1:20 (+4) | Neg | 0.323 |
| 4860418 | Neg | Neg | 0.062 | Neg | Neg | 0.054 | 1:20 (+4) | Neg | 0.071 |
| 4860419 | Neg | Neg | 0.069 | Neg | Neg | 0.058 | 1:40 (+1) | Neg | 0.077 |
| 4860420 | Neg | Neg | 0.082 | Neg | Neg | 0.056 | Neg | Neg | 0.0698 |
| 4860421 | Neg | Neg | 0.088 | Neg | Neg | 0.053 | 1:80 (+1) | 1:10+ | 1.2682 |
| 4860422 | Neg | Neg | 0.081 | Neg | Neg | 0.049 | 1:20 (+4) | Neg | 0.4757 |
| 4860423 | Neg | Neg | 0.049 | Neg | Neg | 0.054 | Neg | Neg | 1.2935 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathur, S.; Banai, M.; Cohen, D. Natural Brucella melitensis Infection and Rev. 1 Vaccination Induce Specific Brucella O-Polysaccharide Antibodies Involved in Complement Mediated Brucella Cell Killing. Vaccines 2022, 10, 317. https://doi.org/10.3390/vaccines10020317
Mathur S, Banai M, Cohen D. Natural Brucella melitensis Infection and Rev. 1 Vaccination Induce Specific Brucella O-Polysaccharide Antibodies Involved in Complement Mediated Brucella Cell Killing. Vaccines. 2022; 10(2):317. https://doi.org/10.3390/vaccines10020317
Chicago/Turabian StyleMathur, Shubham, Menachem Banai, and Dani Cohen. 2022. "Natural Brucella melitensis Infection and Rev. 1 Vaccination Induce Specific Brucella O-Polysaccharide Antibodies Involved in Complement Mediated Brucella Cell Killing" Vaccines 10, no. 2: 317. https://doi.org/10.3390/vaccines10020317
APA StyleMathur, S., Banai, M., & Cohen, D. (2022). Natural Brucella melitensis Infection and Rev. 1 Vaccination Induce Specific Brucella O-Polysaccharide Antibodies Involved in Complement Mediated Brucella Cell Killing. Vaccines, 10(2), 317. https://doi.org/10.3390/vaccines10020317






