Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Laboratory Analyses
2.3. Statistical Analysis
3. Results
3.1. Safety and Adverse Reactions to BNT162b2 Vaccination
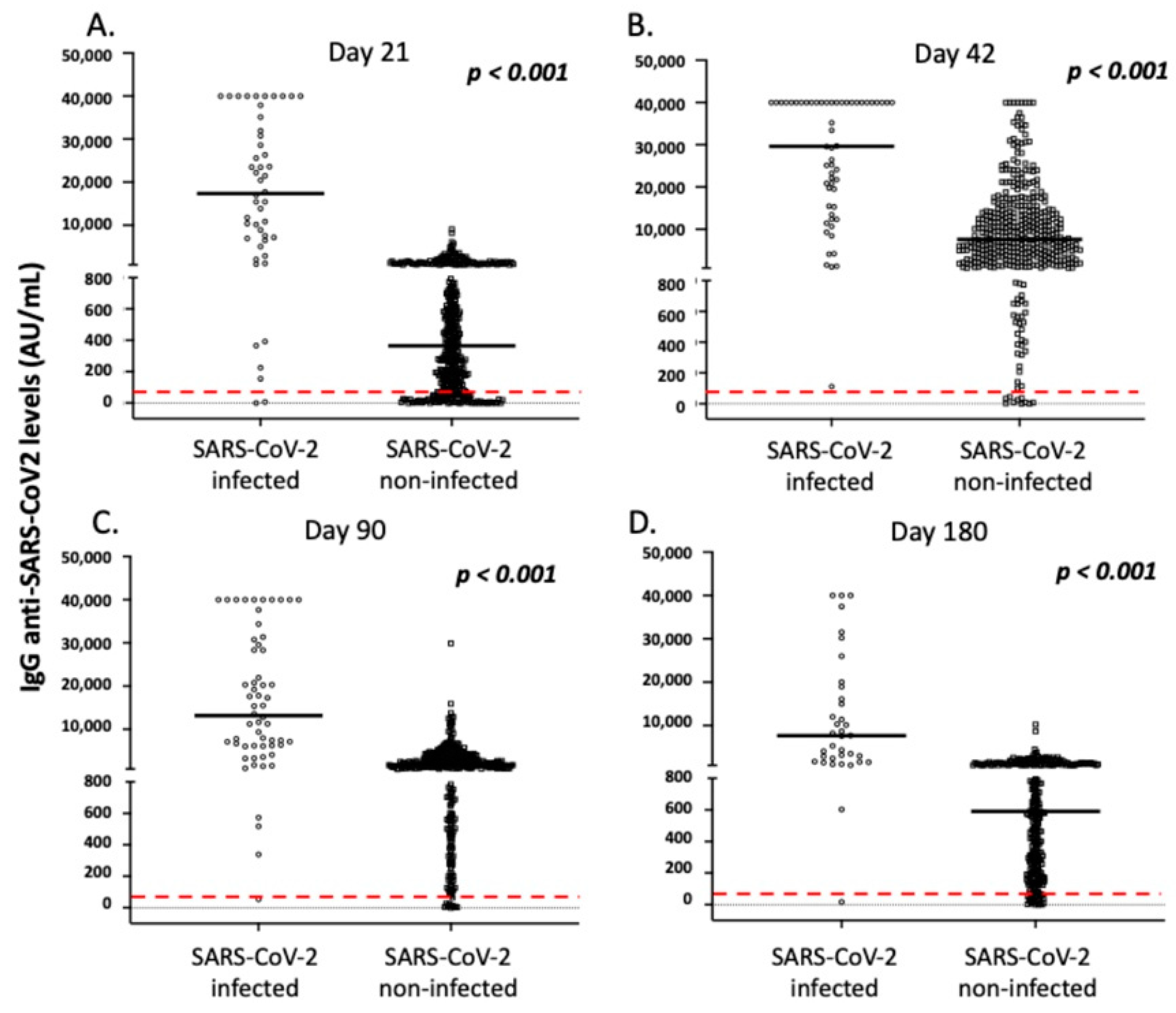
3.2. COVID-19 Disease before and after Vaccination in the Study Individuals
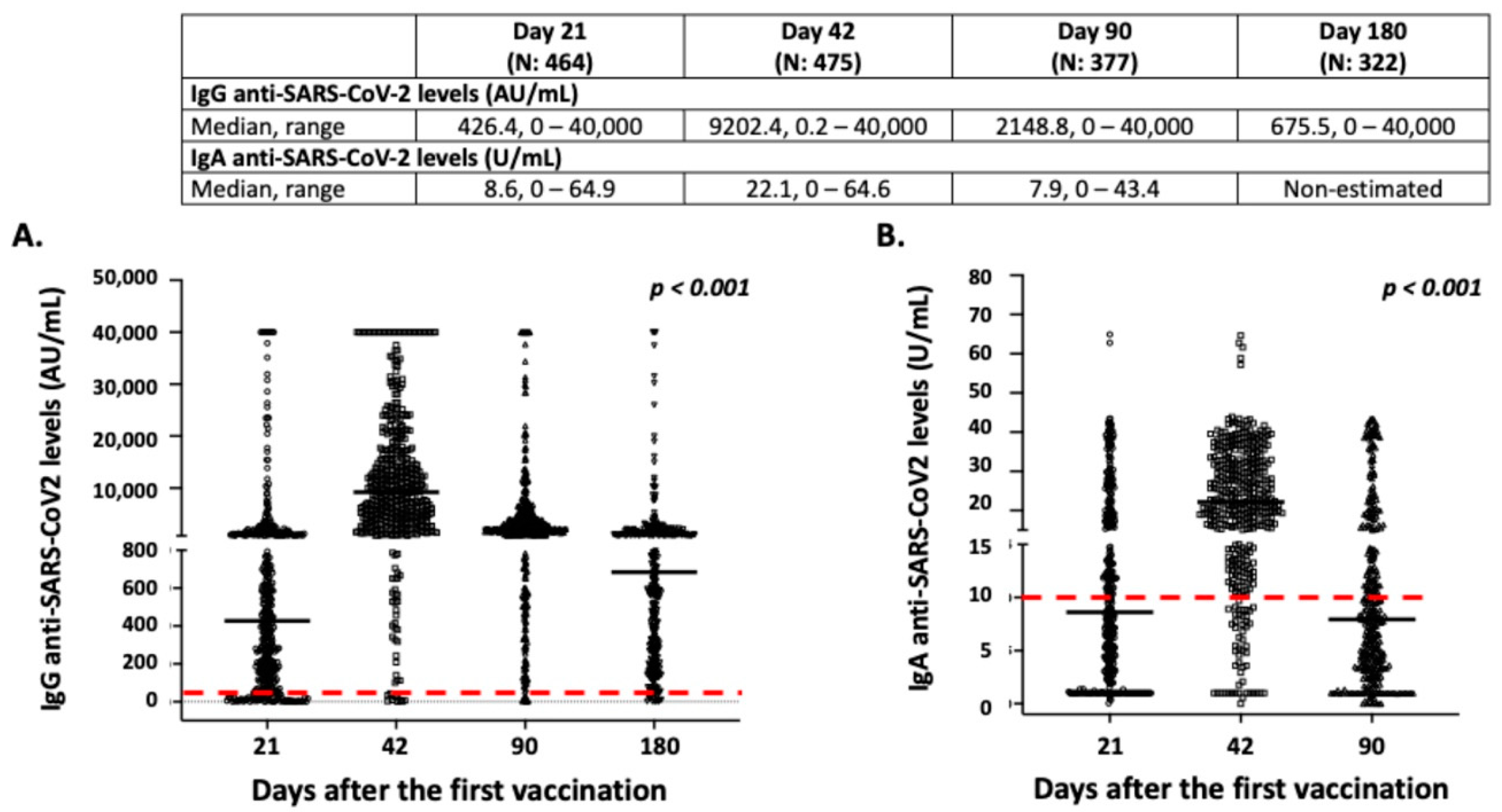
3.3. Intensity and Dynamics of IgG and IgA Responses after BNT162b2 Vaccination
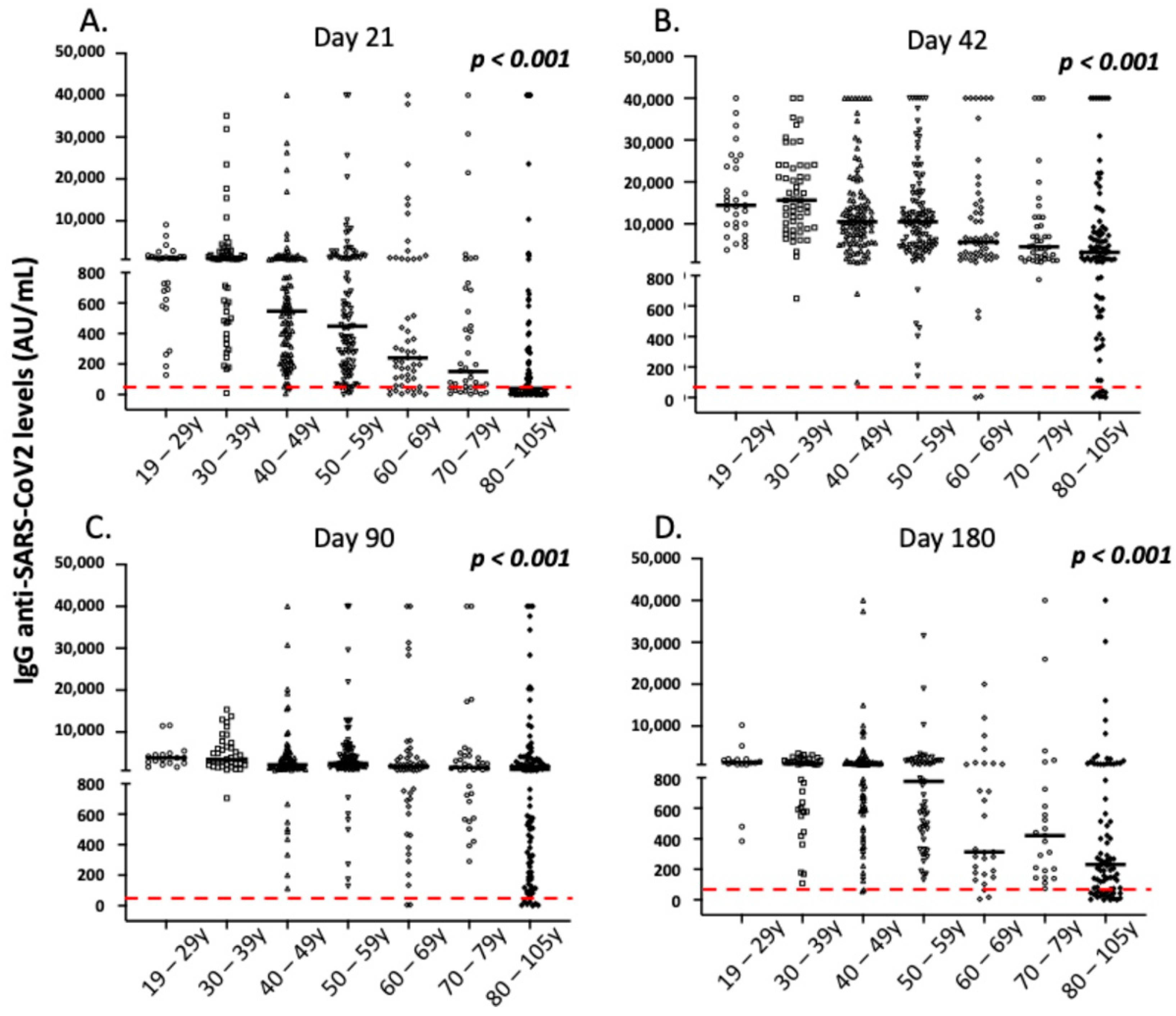
3.4. Correlation of IgG Antibody Responses with Demographic and Clinical Parameters of the Vaccinated Individuals
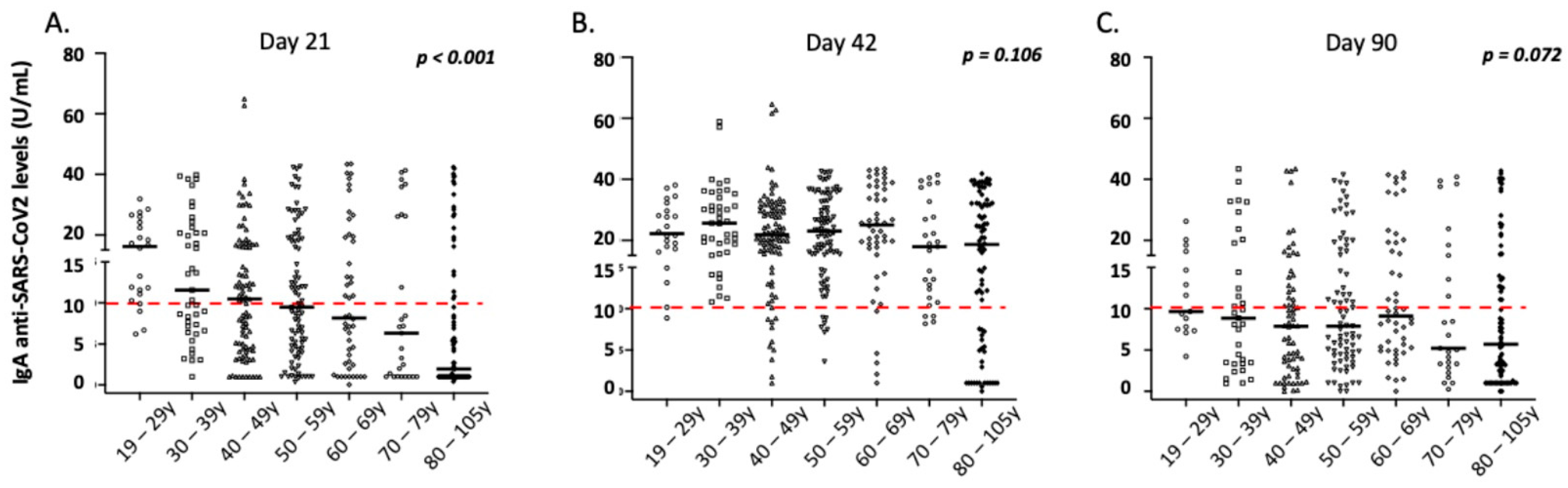
3.5. Correlation of IgA Antibody Responses with Demographic and Clinical Parameters of the Vaccinated Individuals
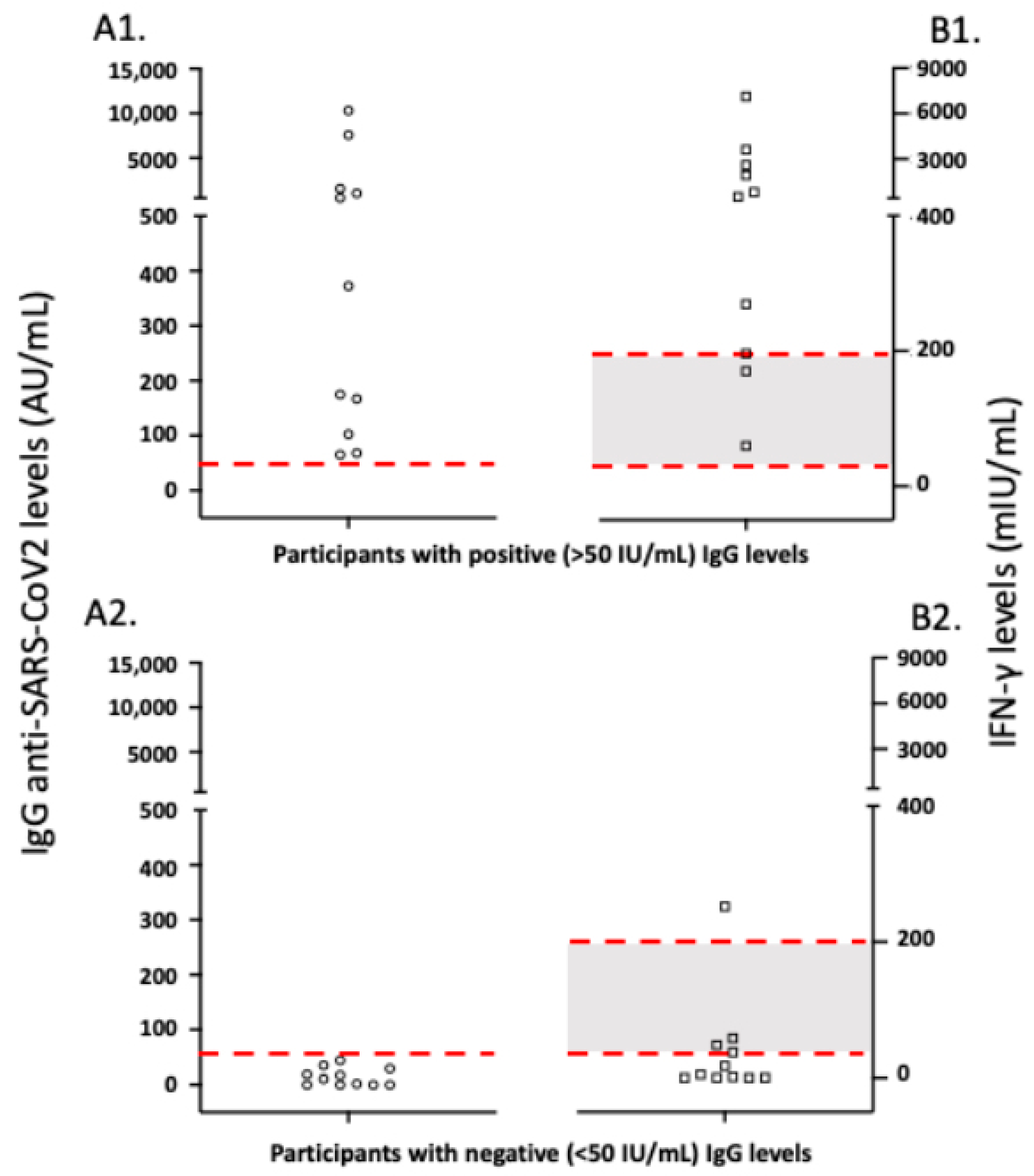
3.6. Correlation of Antibody to Cellular Anti-SARS-CoV-2 Immune Responses in Vaccinated Study Participants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolf, S.H.; Chapman, D.A.; Lee, J.H. COVID-19 as the Leading Cause of Death in the United States. JAMA 2021, 325, 123–124. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 1, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agenzia Italiana del Farmaco. Release n. 620 22nd December 2020 AIFA Approbation of BioNTech/Pfizer Vaccine. Available online: https://www.aifa.gov.it/en/-/autorizzato-il-vaccino-biontech-pfizer (accessed on 27 December 2020).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 MRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Coppeta, L.; Somma, G.; Ferrari, C.; Mazza, A.; Rizza, S.; Trabucco Aurilio, M.; Perrone, S.; Magrini, A.; Pietroiusti, A. Persistence of Anti-S Titre among Healthcare Workers Vaccinated with BNT162b2 MRNA COVID-19. Vaccines 2021, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and MRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Speletas, M.; Kyritsi, M.A.; Vontas, A.; Theodoridou, A.; Chrysanthidis, T.; Hatzianastasiou, S.; Petinaki, E.; Hadjichristodoulou, C. Evaluation of Two Chemiluminescent and Three ELISA Immunoassays for the Detection of SARS-CoV-2 IgG Antibodies: Implications for Disease Diagnosis and Patients’ Management. Front. Immunol. 2020, 11, 609242. [Google Scholar] [CrossRef]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The Neonatal Fc Receptor (FcRn): A Misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef]
- Zeng, F.; Wu, M.; Wang, J.; Li, J.; Hu, G.; Wang, L. Over 1-Year Duration and Age Difference of SARS-CoV-2 Antibodies in Convalescent COVID-19 Patients. J. Med. Virol. 2021, 93, 6506–6511. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Hu, M.; Wen, L.; Wen, C.; Wang, Y.; Zhu, W.; Tai, S.; Jiang, Z.; Xiao, K.; et al. Antibody Seroconversion in Asymptomatic and Symptomatic Patients Infected with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Transl. Immunol. 2020, 9, e1182. [Google Scholar] [CrossRef]
- Ponticelli, D.; Antonazzo, I.C.; Caci, G.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; Ferrara, P. Dynamics of Antibody Response to BNT162b2 MRNA COVID-19 Vaccine after 6 Months. J. Travel. Med. 2021, 28, taab173. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta Variant of SARS-CoV-2: A Comparative Computational Study of Spike Protein. J. Med. Virol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M.; Salvagno, G.L.; Peserico, D.; Pighi, L.; De Nitto, S.; Henry, B.M.; Porru, S.; Lippi, G. Comprehensive Assessment of Humoral Response after Pfizer BNT162b2 MRNA Covid-19 Vaccination: A Three-Case Series. Clin. Chem. Lab. Med. 2021, 59, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Wisnewski, A.V.; Campillo Luna, J.; Redlich, C.A. Human IgG and IgA Responses to COVID-19 MRNA Vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e13. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Acero, R.; Castelletti, N.; Fingerle, V.; Olbrich, L.; Bakuli, A.; Wölfel, R.; Girl, P.; Müller, K.; Jochum, S.; Strobl, M.; et al. In Search of the SARS-CoV-2 Protection Correlate: Head-to-Head Comparison of Two Quantitative S1 Assays in Pre-Characterized Oligo-/Asymptomatic Patients. Infect. Dis. Ther. 2021, 10, 1505–1518. [Google Scholar] [CrossRef]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Rydyznski Moderbacher, C.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-Dose MRNA-1273 COVID-19 Vaccine Generates Durable Memory Enhanced by Cross-Reactive T Cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Vita, R.; Peters, B.; Crotty, S.; Weiskopf, D.; Sette, A. SARS-CoV-2 Human T Cell Epitopes: Adaptive Immune Response against COVID-19. Cell Host Microbe 2021, 29, 1076–1092. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 Variants on the Total CD4+ and CD8+ T Cell Reactivity in Infected or Vaccinated Individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-Specific T Cell Immunity Is Maintained at 6 Months Following Primary Infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef] [PubMed]

| No. | 511 |
|---|---|
| Sex (male/female) | 190/321 |
| Age (median, range) | 54 years, 19–105 |
| Hypertension (n, %) | 140, 27.4% |
| Diabetes mellitus (n, %) | 68, 13.3% |
| Dyslipidemia (n, %) | 89, 17.4% |
| Chronic heart disease (n, %) ^ | 74, 14.5% |
| Chronic respiratory disease (n, %) * | 29, 5.7% |
| Stroke and transient ischemic attacks (n, %) | 27, 5.3% |
| Thrombotic attacks (venous and/or arterial) (n, %) | 11, 2.2% |
| Chronic liver disease (n, %) # | 5, 1.0% |
| Chronic kidney disease (n, %) ^^ | 7, 1.4% |
| Thyroid disease (n, %) ** | 60, 11.7% |
| Autoimmune or autoinflammatory diseases (n, %) ## | 41, 8.0% |
| Cancer (n, %) ^^^ | 27, 5.3% |
| Insomnia or psychiatric diseases (n, %) *** | 126, 24.7% |
| Others ### | 91, 17.8% |
| Comorbidities > 2 (n, %) | 128, 25.0% |
| History of previous COVID-19 disease (n, %) | 60, 11.7% |
| 1st Dosage (n, %) | 2nd Dosage (n, %) | |
|---|---|---|
| Local pain | 134, 36.9% | 103, 28.5% |
| Fever | 11, 2.4% | 45, 9.7% |
| Myalgias | 11, 3.0% | 39, 10.8% |
| Fatigue | 19, 5.2% | 65, 18.0% |
| Headache | 30, 8.3% | 34, 9.4% |
| Flu-like symptoms | 1, 0.3% | 5, 1.4% |
| Others * | 25, 6.9% | 43, 11.9% |
| Age 19–29 (N 29) | Age 30–39 (N 57) | Age 40–49 (N 109) | Age 50–59 (N 108) | Age 60–69 (N 53) | Age 70–79 (N 43) | Age 80–105 (N 112) | p * | |
|---|---|---|---|---|---|---|---|---|
| A. Day 21 after the first vaccination | ||||||||
| IgG levels (median, range) | 1093.1, 127.7–9067.3 | 1008.2, 9.3–35,115.6 | 546.5, 7.0–40,000.0 | 448.4, 0.0–40,000.0 | 240.5, 0.0–40,000.0 | 151.2, 2.9–40,000.0 | 39.8, 0.0–40,000.0 | <0.001 |
| IgA levels (median, range) | 16.2, 6.2–31.8 | 11.6, 1.0–39.9 | 10.5, 1.0–64.9 | 9.5, 0.4–42.6 | 8.2, 0.1–43.5 | 6.3, 1.0–41.3 | 1.9, 0.4–42.4 | <0.001 |
| B. Day 42 after the first vaccination | ||||||||
| IgG levels (median, range) | 14,434.8, 3757.5–40,000.0 | 15,631.0, 649.3–40,000.0 | 10,485.9, 100.4–40,000.0 | 10,484.7, 140.5–40,000.0 | 5650.5, 0.2–40,000.0 | 4509.4, 773.4–40,000.0 | 3206.2, 1.6–40,000.0 | <0.001 |
| IgA levels (median, range) | 22.2, 8.9–38.1 | 25.6, 10.8–58.9 | 21.8, 1.0–64.3 | 23.0, 3.6–42.6 | 25.0, 1.0–43.4 | 17.9, 8.2–41.4 | 18.6, 0.0–41.9 | 0.106 |
| C. Day 90 after the first vaccination | ||||||||
| IgG levels (median, range) | 3836.2, 1621.5–11,572.8 | 3376.9, 704.9–15,365.8 | 2180.1, 113.2–40,000.0 | 2491.6, 127.5–40,000.0 | 1713.7, 5.4–40,000.0 | 1550.3, 290.8–40,000.0 | 1092.7, 0.0–40,000.0 | <0.001 |
| IgA levels (median, range) | 9.7, 4.2–26.2 | 8.9, 0.9–43.4 | 7.9, 0.0–43.9 | 7.9, 0.0–41.5 | 9.1, 0.1–42.1 | 5.2, 0.3–40.8 | 5.7, 0.0–42.7 | 0.072 |
| D. Day 180 after the first vaccination | ||||||||
| IgG levels (median, range) | 1303.1, 384.6–10,218.0 | 937.5, 108.2–3494.9 | 885.1, 55.2–40,000.0 | 776.1, 130.9–31,532.6 | 312.9, 5.0–20,003.0 | 420.6, 72.9–40,000.0 | 227.6, 0.0–40,000.0 | <0.001 |
| IgA levels | NE | NE | NE | NE | NE | NE | NE | |
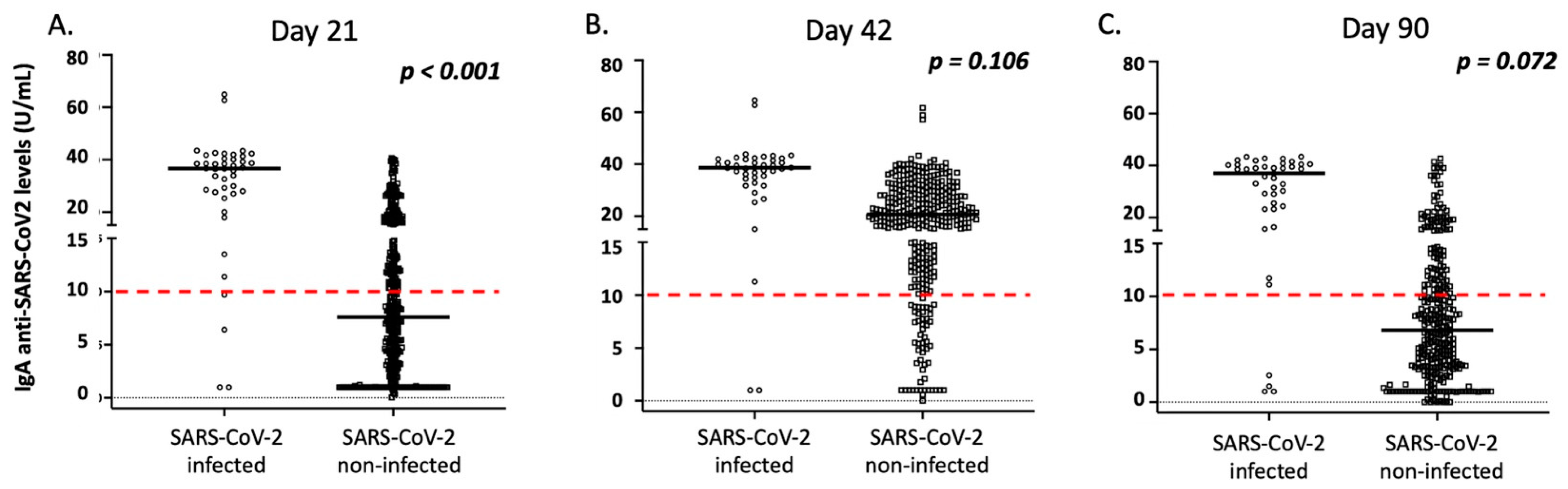
| SARS-CoV-2 Infected | SARS-CoV-2 Non-Infected | p | |
|---|---|---|---|
| A. Day 21 after the first vaccination | |||
| N | 48 | 416 | |
| IgG levels (median, range) | 17,353.0, 0.0–40.000 | 364.8, 0.0–9067.3 | <0.001 |
| IgA levels (median, range) | 36.0, 1.0–64.9 | 7.7, 0.1–40.7 | <0.001 |
| B. Day 42 after the first vaccination | |||
| N | 56 | 419 | |
| IgG levels (median, range) | 29,646.0, 111.8–40,000.0 | 7593.0, 0.2–40,000.0 | <0.001 |
| IgA levels (median, range) | 38.7, 1.0–64.3 | 20.8, 0.0–61.7 | <0.001 |
| C. Day 90 after the first vaccination | |||
| N | 58 | 349 | |
| IgG levels (median, range) | 13,163.7, 54.9–40,000.0 | 6788.0, 0.0–29,894.9 | <0.001 |
| IgA levels (median, range) | 37.0, 1.0–41.4 | 6.9, 0.0–42.7 | <0.001 |
| D. Day 180 after the first vaccination | |||
| N | 36 | 286 | |
| IgG levels (median, range) | 7646.2, 17.3–40,000.0 | 589.8, 0.0–10,218.0 | <0.001 |
| IgA levels (U/mL) | NE | NE | |
| Parameter | N, Median IgG Levels (mg/dL) (IQR) | p, Univariate Analysis | p, Multivariate Analysis | Coefficient, 95% CI |
|---|---|---|---|---|
| Sex (male/female) | Male: 181, 8902 (16,190) Female: 294, 9462 (11,759) | 0.789 | - | |
| Age | ρ (rho) = −0.392 | <0.001 | 0.026 | −126.96, −238.69–−15.24 |
| Comorbidities (≤2 vs. ≥3) | ≥3: 111, 4828.8 (11,196.1) No: 364, 10,295.8 (12,503.9) | <0.001 | 0.518 | −554.95, −2243.57–1133.66 |
| Local pain | Yes: 102, 11,441.6 (12,279.0) No: 255, 9745.2 (12,705.1) | 0.358 | 0.667 | −563.54, −3146.07–2018.99 |
| Fever | Yes: 45, 17,225.8 (17,149.2) No: 414, 7772.5 (11,209.8) | <0.001 | 0.004 | 5865.08, 1940.54–9789.61 |
| Myalgias | Yes: 39, 17,501.4 (12,504.7) No: 318, 9308.1 (11,434.3) | <0.001 | 0.038 | 3911.15, 215.17–7607.14 |
| Fatigue | Yes: 64, 14,209.7 (14,919.9) No: 293, 9050.9 (11,839.9) | <0.001 | 0.758 | 517.39, −2794.27–3829.05 |
| Headache | Yes: 34, 10,152.8 (11,980.4) No: 323, 10,094.5 (12,170.3) | 0.756 | - | |
| Flu-like symptoms | Yes: 5, 15,764.4 (29,612.3) No: 352, 10,034.6 (12,538.6) | 0.109 | 0.135 | 7443.16, −2325.49–17,211.82 |
| Others * | Yes: 42, 11,899.3 (15,455.4) No: 315, 9640.2 (11,653.1) | 0.119 | 0.798 | 446.68, −2993.8–3887.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speletas, M.; Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Mouchtouri, V.A.; Nikoulis, D.J.; Tsakona, M.; Kyritsi, M.A.; Peristeri, A.-M.; Avakian, I.; et al. Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies. Vaccines 2022, 10, 316. https://doi.org/10.3390/vaccines10020316
Speletas M, Voulgaridi I, Sarrou S, Dadouli A, Mouchtouri VA, Nikoulis DJ, Tsakona M, Kyritsi MA, Peristeri A-M, Avakian I, et al. Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies. Vaccines. 2022; 10(2):316. https://doi.org/10.3390/vaccines10020316
Chicago/Turabian StyleSpeletas, Matthaios, Ioanna Voulgaridi, Styliani Sarrou, Aikaterini Dadouli, Varvara A. Mouchtouri, Dimitrios J. Nikoulis, Maria Tsakona, Maria A. Kyritsi, Athanasia-Marina Peristeri, Ioanna Avakian, and et al. 2022. "Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies" Vaccines 10, no. 2: 316. https://doi.org/10.3390/vaccines10020316
APA StyleSpeletas, M., Voulgaridi, I., Sarrou, S., Dadouli, A., Mouchtouri, V. A., Nikoulis, D. J., Tsakona, M., Kyritsi, M. A., Peristeri, A.-M., Avakian, I., Nasika, A., Fragkou, P. C., Moschopoulos, C. D., Zoubouneli, S., Onoufriadis, I., Anagnostopoulos, L., Matziri, A., Papadamou, G., Theodoridou, A., ... Hadjichristodoulou, C. (2022). Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies. Vaccines, 10(2), 316. https://doi.org/10.3390/vaccines10020316













