An Attenuated HSV-1-Derived Malaria Vaccine Expressing Liver-Stage Exported Proteins Induces Sterilizing Protection against Infectious Sporozoite Challenge
Abstract
:1. Background
2. Methods
2.1. Animals and Parasites
2.2. Viral Vector Construction
2.3. Cell Culture and Viral Propagation
2.4. Immunoblotting
2.5. Vaccination
2.6. Parasite Challenge
3. Results
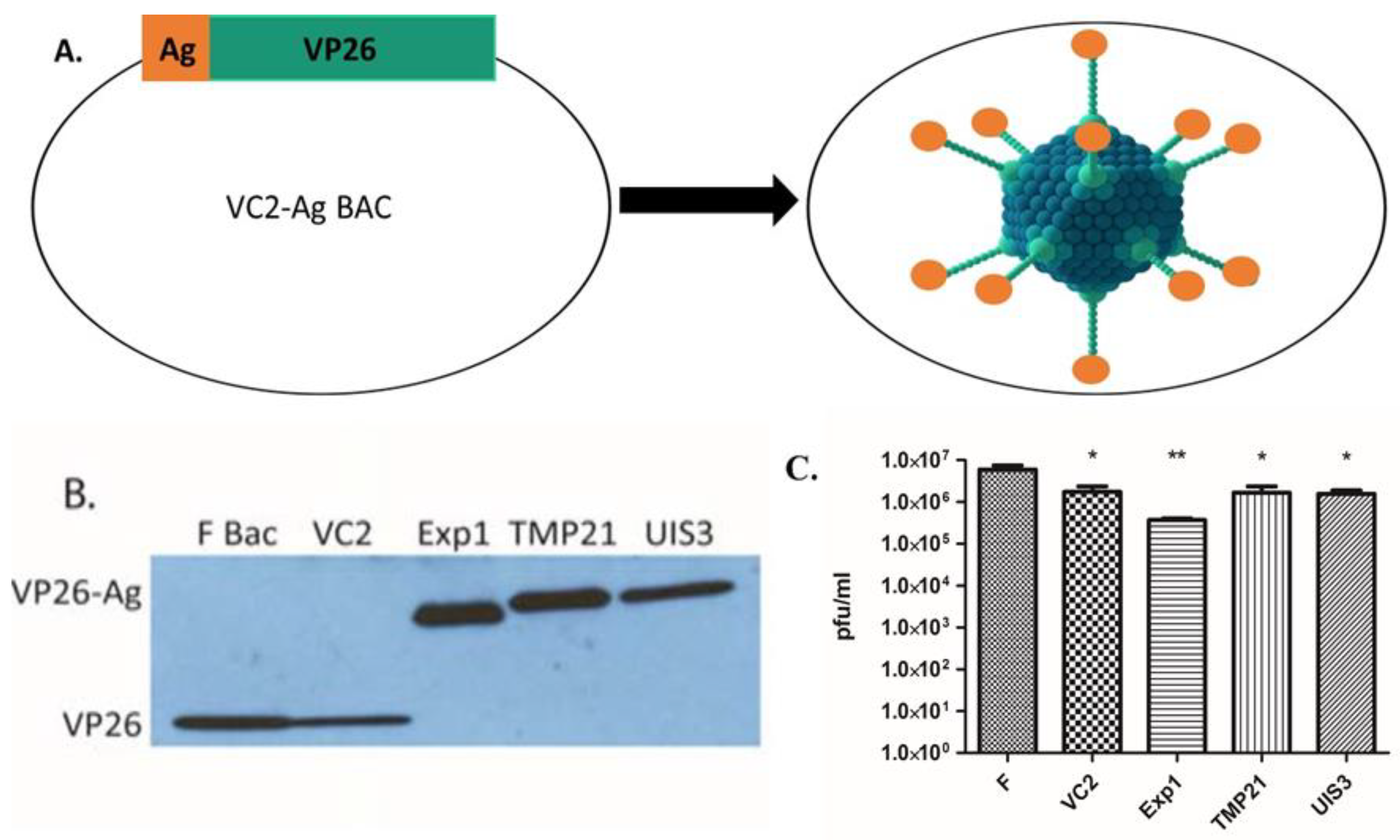
3.1. Construction of Recombinant VC2 Virus Expressing Malaria Antigens
3.2. VC2-EXP1, VC2-TMP21, and VC2-UIS3 Pooled Vaccine Demonstrates Sterile Protection in Immunized Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Duffy, P.E.; Gorres, J.P. Malaria vaccines since 2000: Progress, priorities, products. Npj Vaccines 2020, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Uche, I.K.; Fowlkes, N.; Vu, L.; Watanabe, T.; Carossino, M.; Nabi, R.; del Piero, F.; Rudd, J.S.; Kousoulas, K.G.; Rider, P.J.F. Novel Oncolytic Herpes Simplex Virus 1 VC2 Promotes Long-Lasting, Systemic Anti-melanoma Tumor Immune Responses and Increased Survival in an Immunocompetent B16F10-Derived Mouse Melanoma Model. J. Virol. 2021, 95, e01359-20. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.M.; Reuschel, E.L.; Bah, M.A.; Yun, K.; Tursi, N.; Kim, K.Y.; Chu, J.; Zaidi, F.I.; Yilmaz, I.; Hart, R.J.; et al. Synthetic DNA Vaccines Adjuvanted with pIL-33 Drive Liver-Localized T Cells and Provide Protection from Plasmodium Challenge in a Mouse Model. Vaccines 2020, 8, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, A.S.; Vaughan, A.M.; Kappe, S.H. Malaria Parasite Development in the Mosquito and Infection of the Mammalian Host. Annu. Rev. Microbiol. 2009, 63, 195–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, A.S.; Deveci, G.; Yilmaz, I.; Abraham, A.; Golshan, A.; Hart, R.J. Phenotypic Analysis of Rodent Malaria Parasite Asexual and Sexual Blood Stages and Mosquito Stages. J. Vis. Exp. 2019, 147, e55688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tischer, B.K.; Smith, G.A.; Osterrieder, N. En Passant Mutagenesis: A Two Step Markerless Red Recombination System. Methods Mol. Biol. 2010, 634, 421–430. [Google Scholar] [PubMed]
- Desai, P.; Person, S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 1998, 72, 7563–7568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, A.M.; Machado, M.; Gonçalves-Rosa, N.; Reuling, I.J.; Foquet, L.; Marques, C.; Salman, A.; Yang, A.S.P.; Moser, K.A.; Dwivedi, A.; et al. A Plasmodium berghei sporozoite-based vaccination platform against human malaria. Npj Vaccines 2018, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.F.; Gimenez, A.M.; Aliprandini, E.; Novais, J.T.; Cury, D.P.; Watanabe, I.-S.; Dominguez, M.; Silveira, E.L.V.; Amino, R.; Soares, I.S. Protective Malaria Vaccine in Mice Based on the Plasmodium vivax Circumsporozoite Protein Fused with the Mumps Nucleocapsid Protein. Vaccines 2020, 8, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, D.K.; das Mohapatra, A.; Jnawali, A.; Zuromski, J.; Jha, A.; Cham-Kpu, G.; Sherman, B.; Rudlaff, R.M.; Nixon, C.E.; Hilton, N.; et al. Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria. Nature 2020, 582, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Pan, H.; Gu, Y.; Zuo, X.; Ran, N.; Yuan, Y.; Zhang, C.; Wang, F. Prospects for Malaria Vaccines: Pre-Erythrocytic Stages, Blood Stages, and Transmission-Blocking Stages. BioMed Res. Int. 2019, 2019, 9751471. [Google Scholar] [CrossRef]
- Kobayashi, R.; Kato, A.; Sagara, H.; Watanabe, M.; Maruzuru, Y.; Koyanagi, N.; Arii, J.; Kawaguchi, Y. Herpes Simplex Virus 1 Small Capsomere-Interacting Protein VP26 Regulates Nucleocapsid Maturation. J. Virol. 2017, 91, e01068-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, S.K.; Nabi, R.; Cheemarla, N.; Stanfield, B.; Rider, P.J.; Jambunathan, N.; Chouljenko, V.N.; Carter, R.; del Piero, F.; Langohr, I.; et al. Intramuscular vaccination of mice with the human herpes simplex virus type-1(HSV-1) VC2 vaccine, but not its parental strain HSV-1(F) confers full protection against lethal ocular HSV-1 (McKrae) pathogenesis. PLoS ONE 2020, 15, e0228252. [Google Scholar] [CrossRef] [PubMed]
| Groups (N) | Immunization Route (Intervals in Days) | Protected/Challenged 1 |
|---|---|---|
| Group A: Pooled Vaccine (9) | SC (0, 21, 42) | 9/9 |
| Group B: Pooled Vaccine (8) | IM (0, 21, 42) | 8/8 |
| Group C: VC2 (5) | SC (0, 21, 42) | 0/5 |
| Group D: VC2 (5) | IM (0, 21, 42) | 0/5 |
| Group E: Naïve Control (5) | N/A | 0/5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rider, P.J.F.; Kamil, M.; Yilmaz, I.; Atmaca, H.N.; Kalkan-Yazici, M.; Ziya Doymaz, M.; Kousoulas, K.G.; Aly, A.S.I. An Attenuated HSV-1-Derived Malaria Vaccine Expressing Liver-Stage Exported Proteins Induces Sterilizing Protection against Infectious Sporozoite Challenge. Vaccines 2022, 10, 300. https://doi.org/10.3390/vaccines10020300
Rider PJF, Kamil M, Yilmaz I, Atmaca HN, Kalkan-Yazici M, Ziya Doymaz M, Kousoulas KG, Aly ASI. An Attenuated HSV-1-Derived Malaria Vaccine Expressing Liver-Stage Exported Proteins Induces Sterilizing Protection against Infectious Sporozoite Challenge. Vaccines. 2022; 10(2):300. https://doi.org/10.3390/vaccines10020300
Chicago/Turabian StyleRider, Paul J. F., Mohd Kamil, Ilknur Yilmaz, Habibe N. Atmaca, Merve Kalkan-Yazici, Mehmet Ziya Doymaz, Konstantin G. Kousoulas, and Ahmed S. I. Aly. 2022. "An Attenuated HSV-1-Derived Malaria Vaccine Expressing Liver-Stage Exported Proteins Induces Sterilizing Protection against Infectious Sporozoite Challenge" Vaccines 10, no. 2: 300. https://doi.org/10.3390/vaccines10020300
APA StyleRider, P. J. F., Kamil, M., Yilmaz, I., Atmaca, H. N., Kalkan-Yazici, M., Ziya Doymaz, M., Kousoulas, K. G., & Aly, A. S. I. (2022). An Attenuated HSV-1-Derived Malaria Vaccine Expressing Liver-Stage Exported Proteins Induces Sterilizing Protection against Infectious Sporozoite Challenge. Vaccines, 10(2), 300. https://doi.org/10.3390/vaccines10020300





