Different Clinical Outcomes of COVID-19 in Two Healthcare Workers Vaccinated with BNT162b2 Vaccine, Infected with the Same Viral Variant but with Different Predisposing Conditions for the Progression of the Disease †
Abstract
:1. Introduction
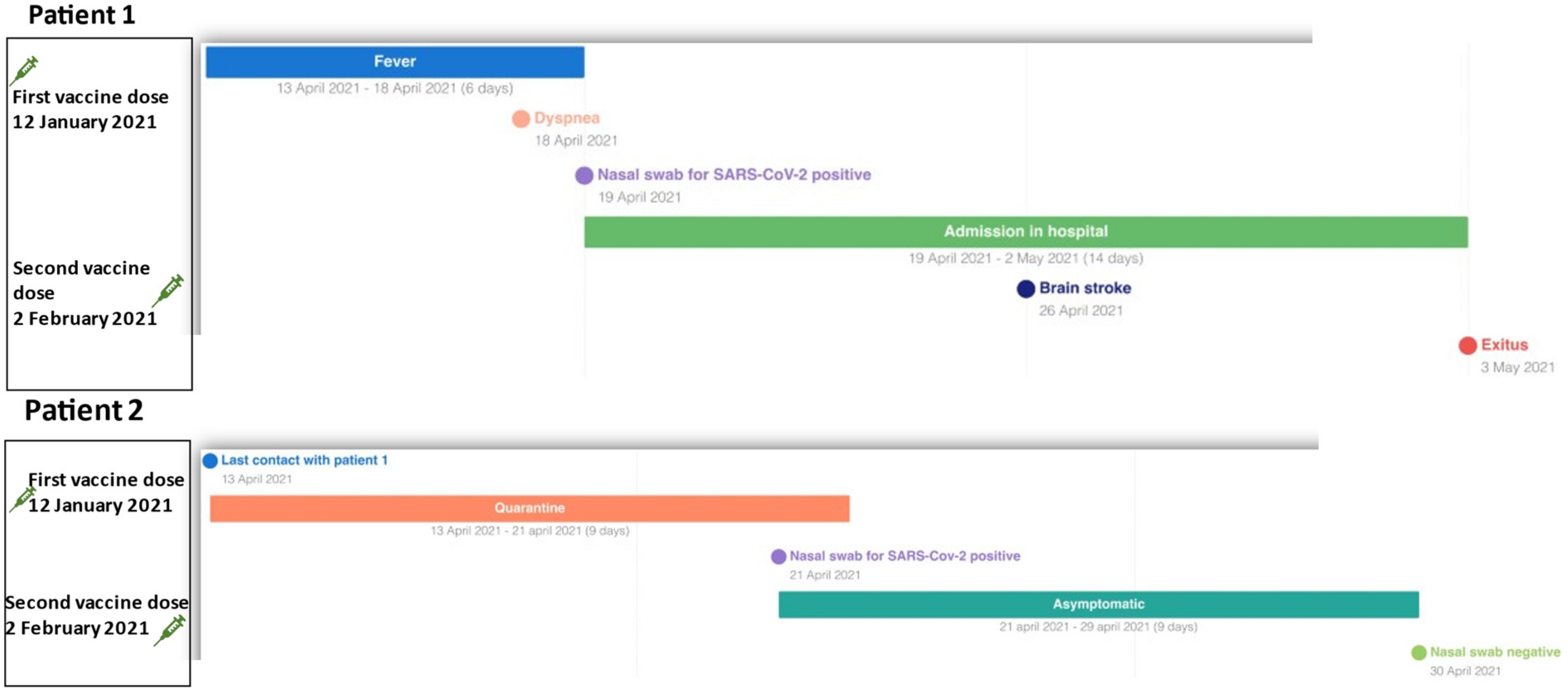
2. Clinical Cases
3. Molecular Methods
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological Update on COVID-19, 20 July 2021, Edition 49. 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---20-july-2021 (accessed on 20 July 2021).
- Rawat, K.; Kumari, P.; Saha, L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol. 2021, 892, 173751. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, N.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- John, B.V.; Deng, Y.; Scheinberg, A.; Mahmud, N.; Taddei, T.H.; Kaplan, D.; Labrada, M.; Baracco, G.; Dahman, B. Association of BNT162b2 mRNA and mRNA-1273 Vaccines with COVID-19 Infection and Hospitalization Among Patients with Cirrhosis. JAMA Intern. Med. 2021, 181, 1306–1314. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Yang, R.; Li, X.; Liu, H.; Zhen, Y.; Zhang, X.; Xiong, Q.; Luo, Y.; Gao, C.; Zeng, W. Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiol. Cardiothorac. Imaging 2020, 2, e200047. [Google Scholar] [CrossRef] [Green Version]
- Carfora, V.; Spiniello, G.; Ricciolino, R.; Di Mauro, M.; Migliaccio, M.G.; Mottola, F.F.; Verde, N.; Coppola, N. Anticoagulant treatment in COVID-19: A narrative review. J. Thromb. Thrombolysis 2021, 51, 642–648. [Google Scholar] [CrossRef]
- Sagnelli, C.; Celia, B.; Monari, C.; Cirllo, S.; De Angelis, G.; Bianco, A.; Coppla, N. Management of SARS-CoV-2 Pneumonia. J. Med. Virol. 2020, 93, 1276–1287. [Google Scholar] [CrossRef]
- Macera, M.; De Angelis, G.; Sagnelli, C.; Coppola, N. Clinical Presentation of COVID-19: Case Series and Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 5062. [Google Scholar] [CrossRef]
- Du, Y.; Tu, L.; Zhu, P.; Mu, M.; Wang, R.; Yang, P.; Wang, X.; Hu, C.; Ping, R.; Hu, P.; et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am. J. Respir. Crit. Care Med. 2020, 201, 1372–1379. [Google Scholar] [CrossRef] [Green Version]
- Sardu, C.; D′Onofrio, N.; Balestrieri, M.L.; Barbieri, M.; Rizzo, M.R.; Messina, V.; Maggi, P.; Coppola, N.; Paolisso, G.; Marfella, R. Hyperglycaemia on admission to hospital and COVID-19. Diabetologia 2020, 63, 2486–2487. [Google Scholar] [CrossRef] [PubMed]
- Monari, M.; Sagnelli, C.; Maggi, P.; Sangiovanni, V.; Numis, F.G.; Gentile, I.; Masullo, A.; Rescigno, C.; Calabria, G.; Megna, A.S.; et al. More severe COVID-19 in patients with active cancer: The results of a multicenter cohort study. Front. Oncol. 2021, 11, 662746. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; Li, P.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Collier, D.A.; De Marco, A.; Ferreira, I.A.T.M.; Meng, B.; Datir, R.P.; Walls, A.C.; Kemp, S.A.; Bassi, J.; Pinto, D.; Bianchi, S.; et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 2021, 593, 136–141. [Google Scholar] [CrossRef]
- Chodick, G.; Tene, L.; Rotem, R.S.; Patalon, T.; Gazit, S.; Ben-Tov, A.; Weil, C.; Goldshtein, I.; Twig, G.; Cohen, D.; et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: Analysis of real-world data. Clin. Infect. Dis. 2021, ciab438. [Google Scholar] [CrossRef]
- Levy, I.; Wieder-Finesod, A.; Litchevsky, V.; Biber, A.; Indenbaum, V.; Olmer, L.; Huppert, A.; Mor, O.; Goldstein, M.; Levin, E.G.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin. Microbiol. Infect. 2021, 27, 1851–1855. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Zhang, P.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with cancer appear more vulnerable to SARS-COV-2: A multi-center study during the COVID-19 outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar]
- Meng, Y.; Lu, W.; Guo, E.; Liu, J.; Yang, B.; Wu, P.; Lin, S.; Peng, T.; Fu, Y.; Li, F.; et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: A propensity score-matched analysis. J. Hematol. Oncol. 2020, 13, 75. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; Grivas, P.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Benda, M.; Mutschlechner, B.; Ulmer, H.; Grabher, C.; Severgnini, L.; Volgger, A.; Reimann, P.; Lang, T.; Atzl, M.; Huynh, M.; et al. Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients. Br. J. Haematol. 2021, 195, 523–531. [Google Scholar] [CrossRef]
- Pimpinelli, F.; Marchesi, F.; Piaggio, G.; Giannarelli, D.; Papa, E.; Falcucci, P.; Pontone, M.; Martino, S.D.; Laquintana, V.; La Malfa, A.; et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: Preliminary data from a single institution. J. Hematol. Oncol. 2021, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, F.; Pimpinelli, F.; Sperandio, E.; Papa, E.; Falcucci, P.; Pontone, M.; di Martino, S.; de Latouliere, L.; Orlandi, G.; Morrone, A.; et al. The 12-week kinetics of anti-SARS-CoV-2 antibodies in different haematological cancers after vaccination with BNT162b2. Br. J. Haematol. 2022, 196, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, F.; Pimpinelli, F.; Giannarelli, D.; Ronchetti, L.; Papa, E.; Falcucci, P.; Pontone, M.; Di Domenico, E.G.; di Martino, S.; Laquintana, V.; et al. Impact of anti-CD20 monoclonal antibodies on serologic response to BNT162b2 vaccine in B-cell Non-Hodgkin’s lymphomas. Leukemia 2022, 36, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Pimpinelli, F.; Marchesi, F.; Piaggio, G.; Giannarelli, D.; Papa, E.; Falcucci, P.; Spadea, A.; Pontone, M.; Di Martino, S.; Laquintana, V.; et al. Lower response to BNT162b2 vaccine in patients with myelofibrosis compared to polycythemia vera and essential thrombocythemia. J. Hematol. Oncol. 2021, 14, 119. [Google Scholar] [CrossRef]
- Greenberg, R.S.; Ruddy, J.A.; Boyarsky, B.J.; Werbel, W.A.; Garonzik-Wang, G.M.; Segev, D.L.; Imus, P.H. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with multiple myeloma. BMC Cancer 2021, 21, 1354. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessio, L.; Pisaturo, M.; Russo, A.; Onorato, L.; Starace, M.; Atripaldi, L.; Coppola, N. Different Clinical Outcomes of COVID-19 in Two Healthcare Workers Vaccinated with BNT162b2 Vaccine, Infected with the Same Viral Variant but with Different Predisposing Conditions for the Progression of the Disease. Vaccines 2022, 10, 298. https://doi.org/10.3390/vaccines10020298
Alessio L, Pisaturo M, Russo A, Onorato L, Starace M, Atripaldi L, Coppola N. Different Clinical Outcomes of COVID-19 in Two Healthcare Workers Vaccinated with BNT162b2 Vaccine, Infected with the Same Viral Variant but with Different Predisposing Conditions for the Progression of the Disease. Vaccines. 2022; 10(2):298. https://doi.org/10.3390/vaccines10020298
Chicago/Turabian StyleAlessio, Loredana, Mariantonietta Pisaturo, Antonio Russo, Lorenzo Onorato, Mario Starace, Luigi Atripaldi, and Nicola Coppola. 2022. "Different Clinical Outcomes of COVID-19 in Two Healthcare Workers Vaccinated with BNT162b2 Vaccine, Infected with the Same Viral Variant but with Different Predisposing Conditions for the Progression of the Disease" Vaccines 10, no. 2: 298. https://doi.org/10.3390/vaccines10020298
APA StyleAlessio, L., Pisaturo, M., Russo, A., Onorato, L., Starace, M., Atripaldi, L., & Coppola, N. (2022). Different Clinical Outcomes of COVID-19 in Two Healthcare Workers Vaccinated with BNT162b2 Vaccine, Infected with the Same Viral Variant but with Different Predisposing Conditions for the Progression of the Disease. Vaccines, 10(2), 298. https://doi.org/10.3390/vaccines10020298






