Vaccination Accelerates Liver-Intrinsic Expression of Megakaryocyte-Related Genes in Response to Blood-Stage Malaria
Abstract
1. Introduction
2. Material and Methods
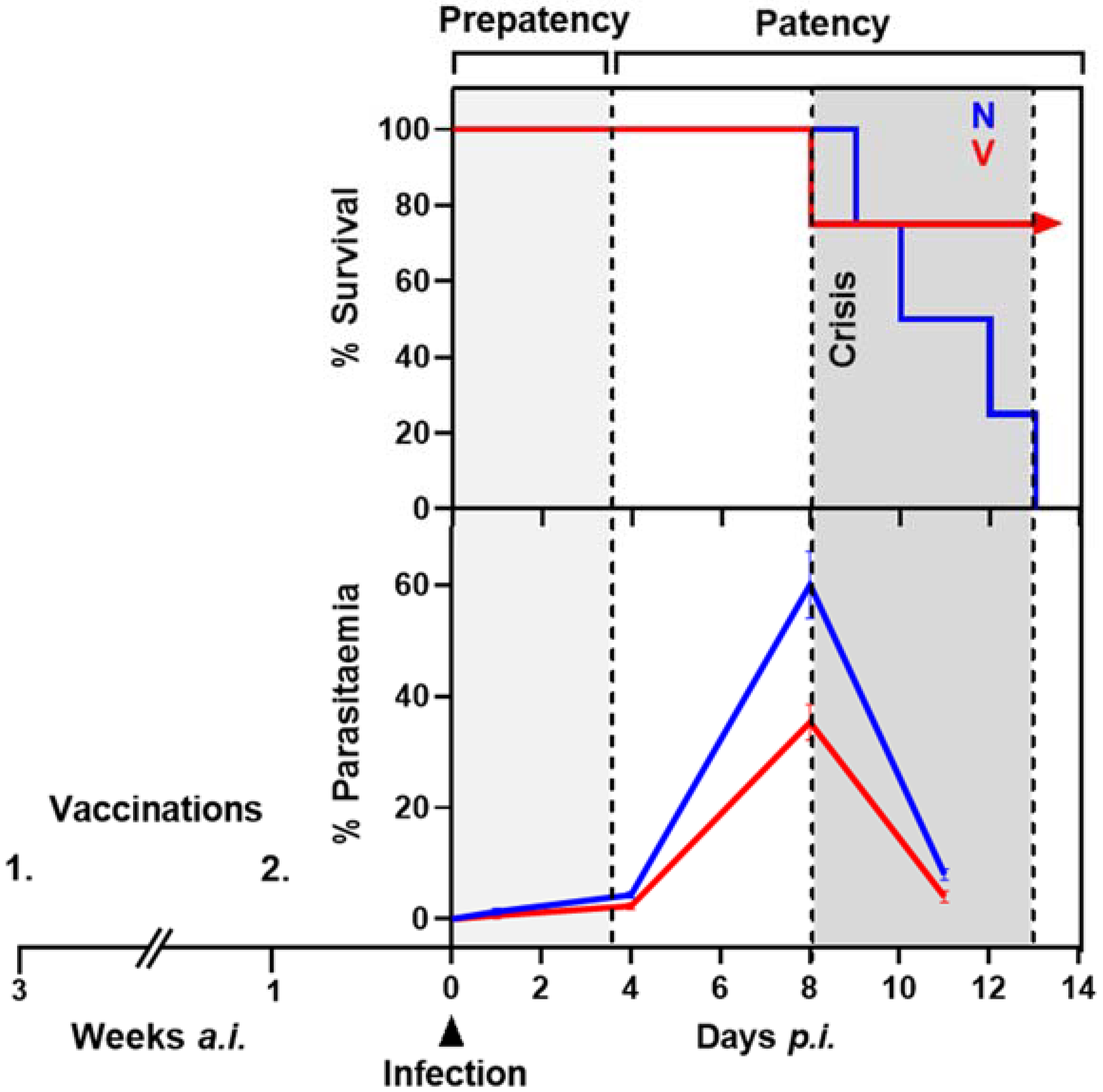
2.1. Protective Vaccination of Mice against Blood-Stage Malaria
2.2. P. chabaudi Malaria and Liver Sampling
2.3. Hybridization of Mouse Whole Genome Oligo Microarrays
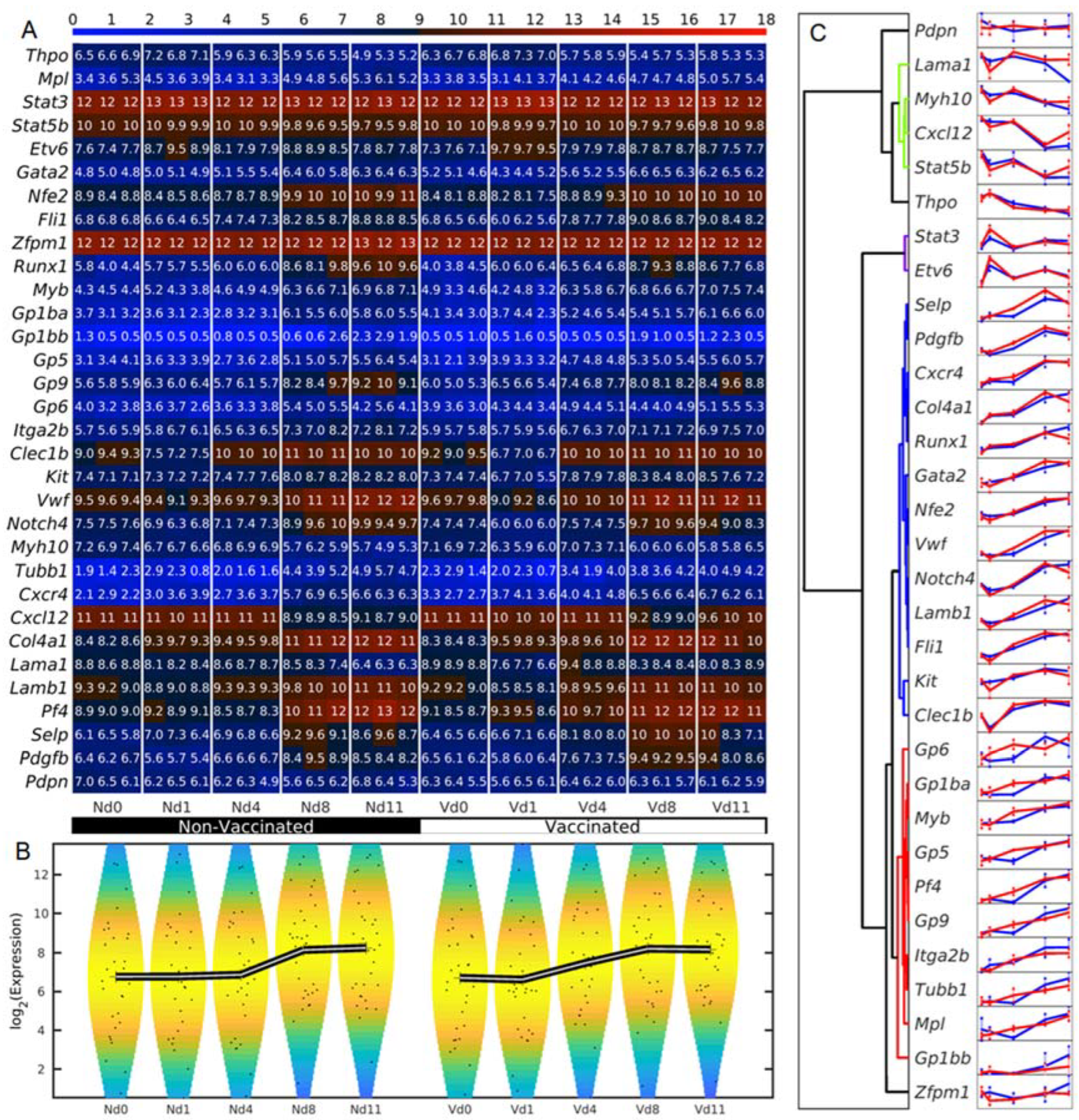
2.4. Analyses of Microarrays
2.5. Real-Time Quantitative PCR
3. Results
3.1. Megakaryocyte-Related Genes of Vaccinated Mice Increase Their Average Expression Three Days Earlier Than Non-Vaccinated Mice
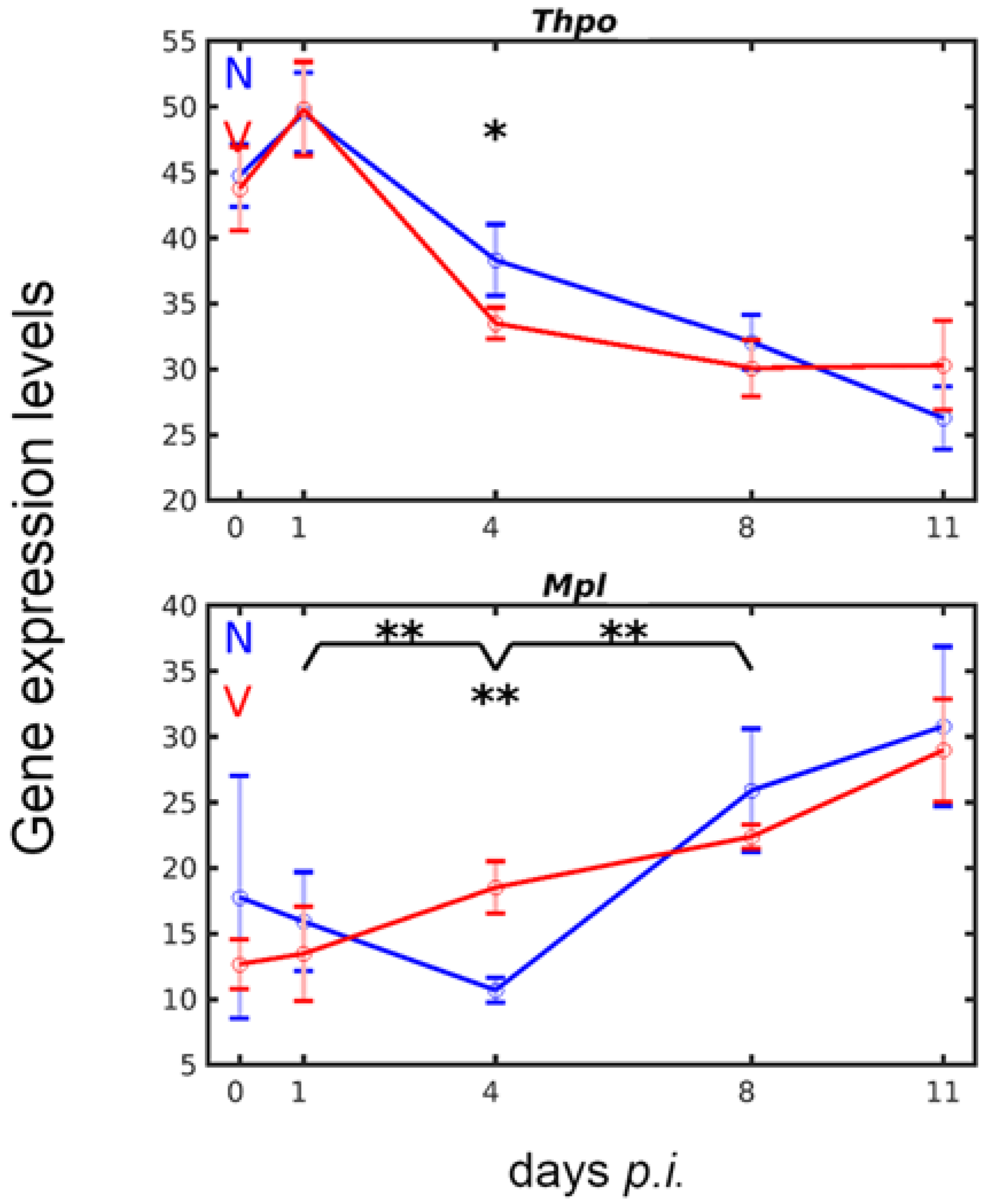
3.2. Vaccination Accelerates Inverse Down- and Upregulation of Thpo and Mpl Expression in the Liver in Response to Blood-Stage Malaria
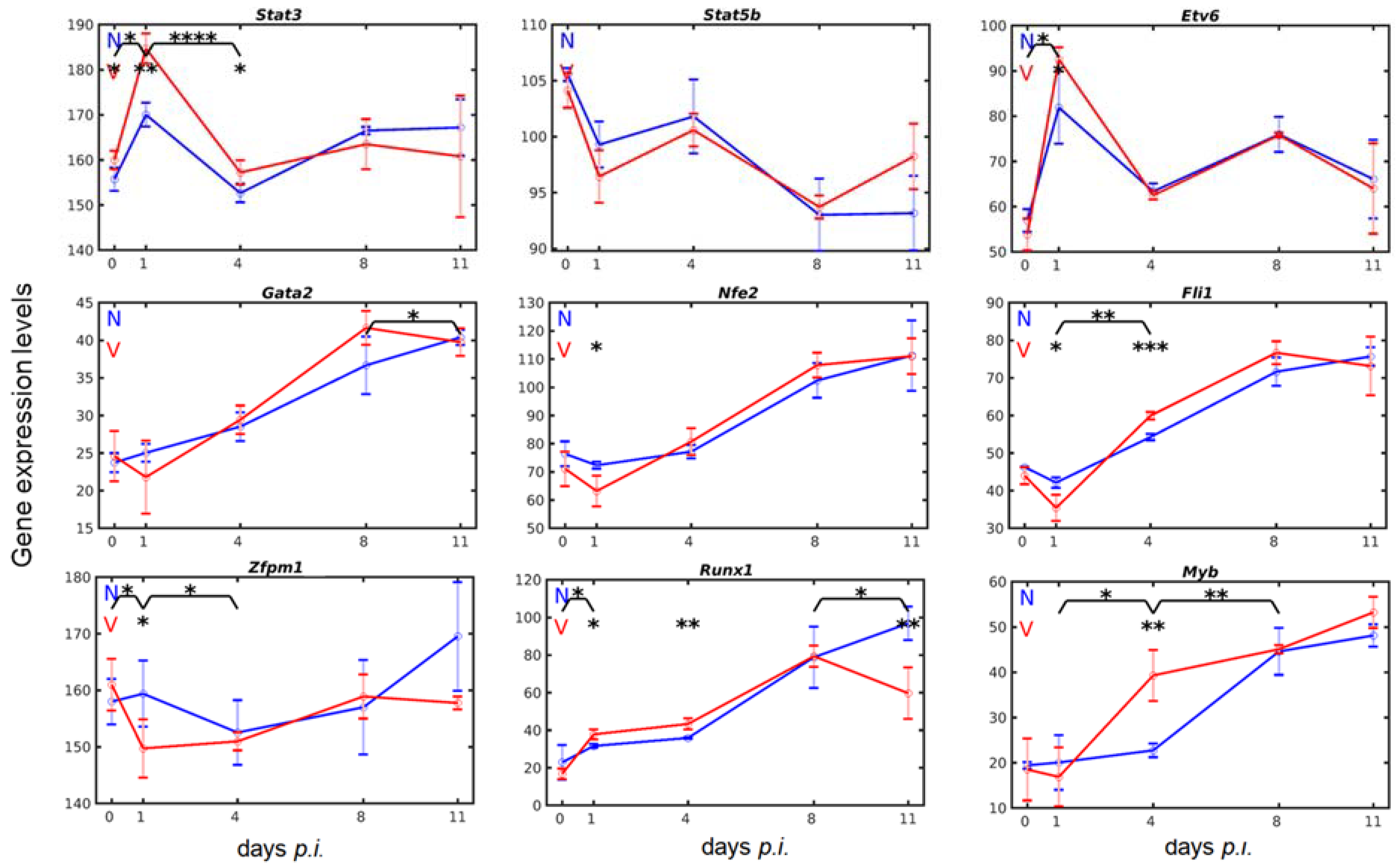
3.3. Vaccination Reshapes Malaria-Induced Hepatic Expression of Genes Encoding Transcription Regulators Involved in Megakaryopoiesis
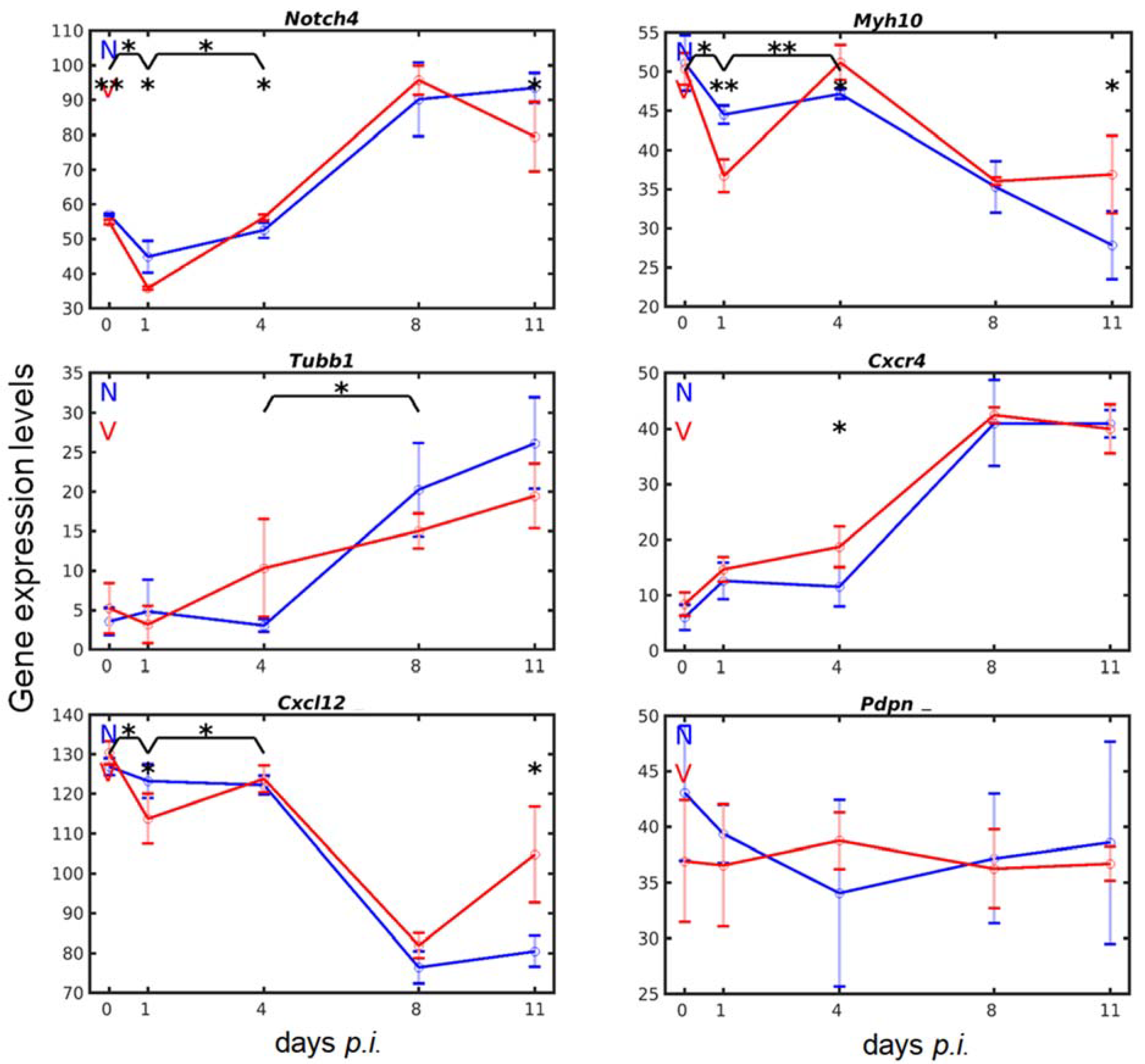
3.4. Vaccination Accelerates Malaria-Induced Expression of Genes Encoding Surface Proteins on Cells of the Megakaryocytic Lineage in the Liver
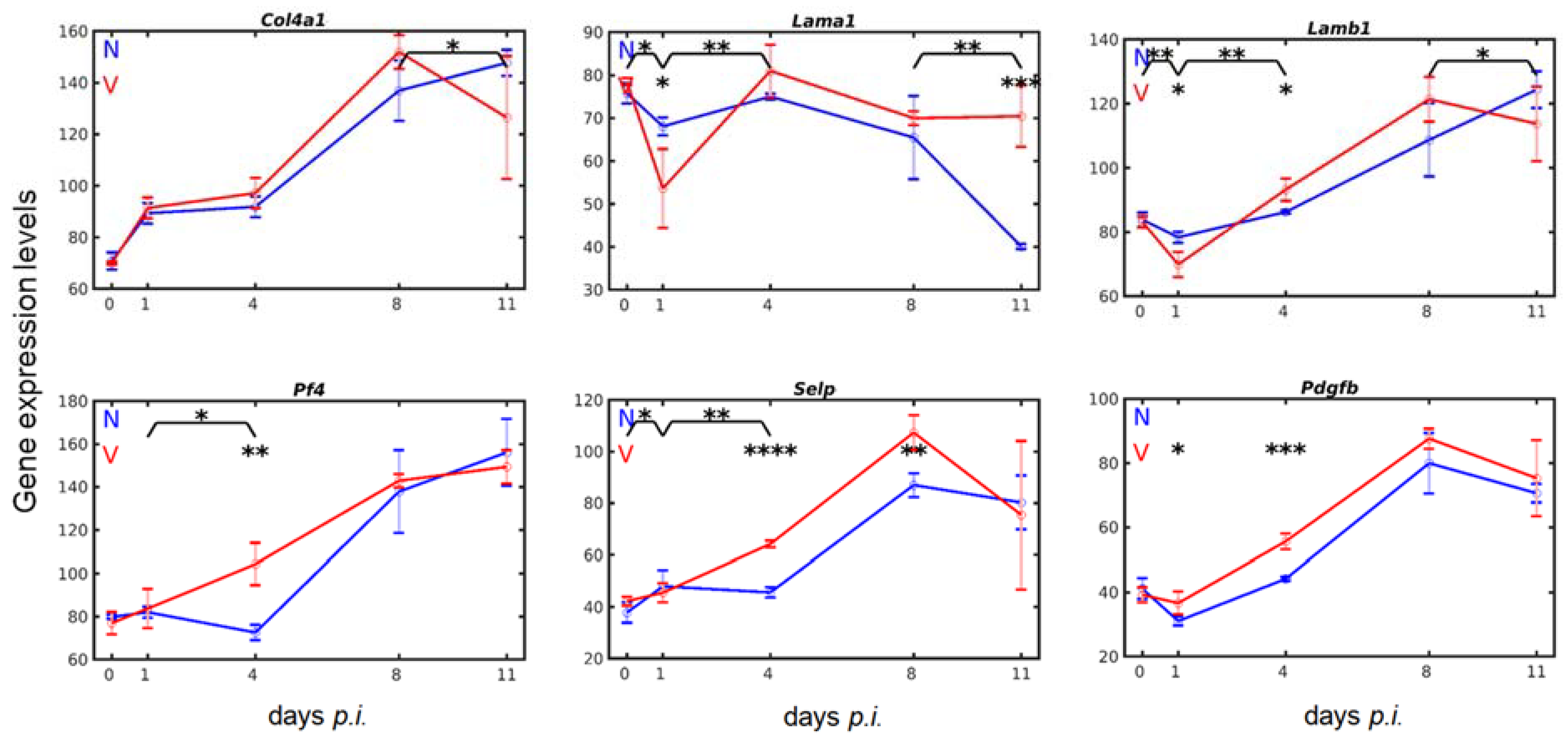
3.5. Vaccination Alters the Malaria-Responsive Expression of Genes also Involved in Megakaryocyte Development
3.6. Vaccination Enhances Transcripts of Exportable Proteins in Liver-Localized Cells of the Megakaryocyte Lineage
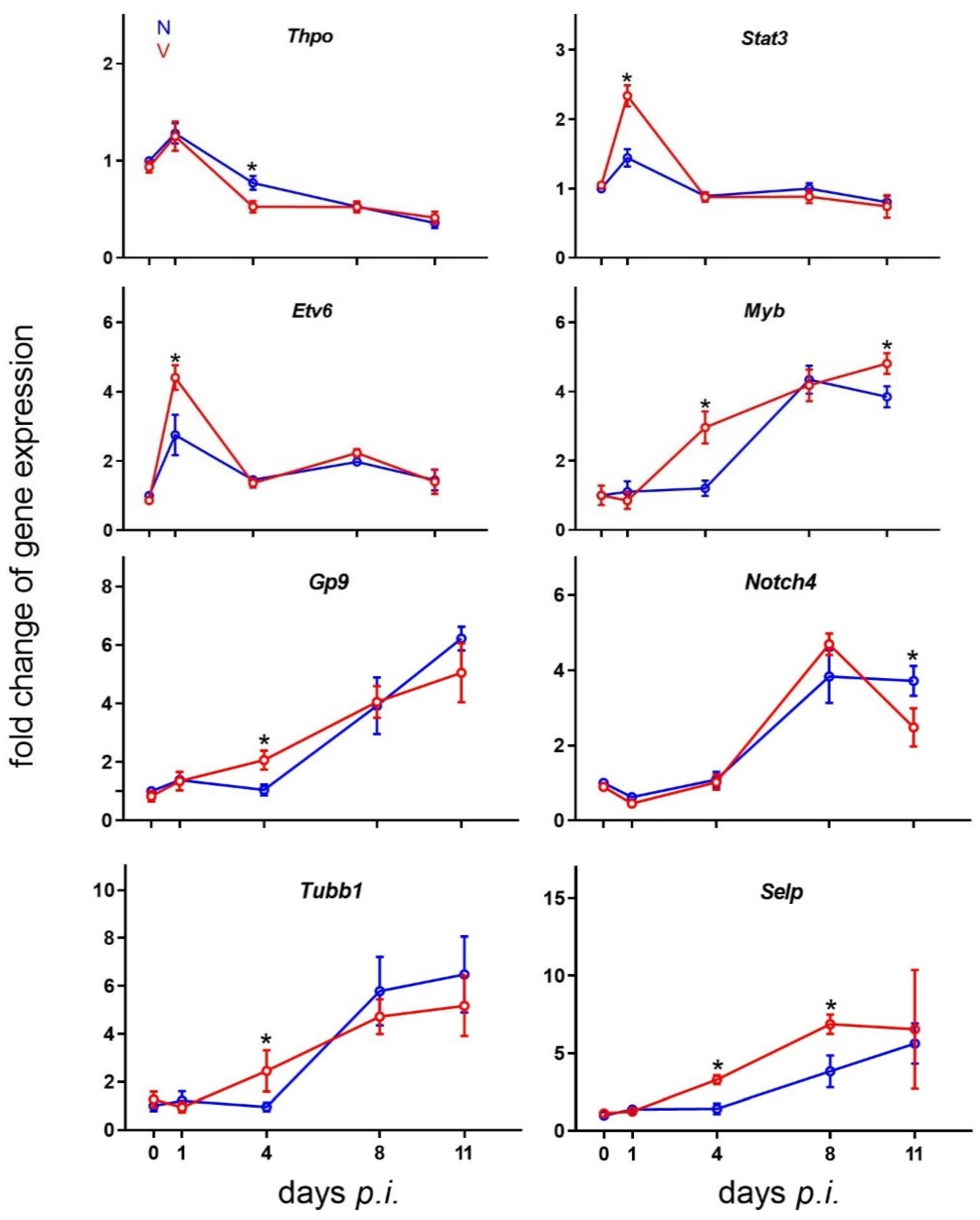
3.7. Validation of Microarray Results by Quantitative RT-PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Statement of Ethics
References
- WHO. World Malaria Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Draper, S.J.; Sack, B.K.; Richter King, C.; Nielsen, C.N.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria vaccines: Recent advances and new horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Chikondi, T. A shot of hope. Science 2019, 366, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.; Smith, C.; Fortin, A.; Berghout, J.; McMorran, B.; Burgio, G.; Foote, S.; Gros, P. Host resistance to malaria: Using mouse models to explore the host response. Mamm. Genome 2011, 22, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.; Culleton, R.L.; Lamb, T.J. The contribution of Plasmodium chabaudi to our understanding of malaria. Trends Parasitol. 2012, 28, 73–82. [Google Scholar] [CrossRef]
- Wunderlich, F.; Brenner, H.; Helwig, M. Plasmodium chabaudi malaria: Protective immunization with surface membranes of erythrocytes infected with Plasmodium chabaudi. Infect. Immun. 1988, 56, 3326–3328. [Google Scholar] [CrossRef]
- Krücken, J.; Delic, D.; Pauen, H.; Wojtalla, A.; El-Khadragy, M.; Dkhil, M.A.; Mossmann, H.; Wunderlich, F. Augmented particle trapping and attenuated inflammation in the liver by protective vaccination against Plasmodium chabaudi malaria. Malar. J. 2009, 8, 54–64. [Google Scholar] [CrossRef]
- Del Portillo, H.A.; Ferrer, M.; Brugat, T.; Martin-Jaular, L.; Langhorne, J.; Lacerda, M.V.G. The role of the spleen in malaria. Cell. Microbiol. 2012, 14, 343–355. [Google Scholar] [CrossRef]
- White, N.J. Malaria parasite clearance. Malar. J. 2017, 16, 88. [Google Scholar] [CrossRef]
- Wunderlich, F.; Al-Quraishy, S.; Dkhil, M. Liver-inherent immune system: Its role in blood-stage malaria. Front. Microbiol. 2014, 5, 559. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Dkhil, M.A.; Abdel-Baki, A.S.; Ghanjati, F.; Erichsen, L.; Santourlidis, S.; Wunderlich, F.; Araúzo-Bravo, M.J. Protective vaccination and blood-stage malaria modify DNA methylation of gene promoters in the liver of Blab/c mice. Parasitol. Res. 2017, 116, 1463–1477. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Al-Quraishy, S.A.; Abdel-Baki, A.S.; Delic, D.; Wunderlich, F. Differential miRNA expression in the liver of Balb/c protected by vaccination during crisis of Plasmodium chabaudi blood-stage malaria. Front. Microbiol. 2017, 7, 2155. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraishy, S.; Dkhil, M.A.; Al-Shaebi, E.M.; Abdel-Baki, A.S.; Araúzo-Bravo, M.J.; Delic, D.; Wunderlich, F. Gene expression of the liver of vaccination-protected mice in response to early patent infections of Plasmodium chabaudi blood-stage malaria. Malar. J. 2018, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraishy, S.; Dkhil, M.A.; Abdel-Baki, A.A.S.; Delic, D.; Wunderlich, F. Protective vaccination alters gene expression of the liver of Balb/c mice in response to early prepatent blood-stage malaria of Plasmodium chabaudi. Parasitol. Res. 2018, 117, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Araúzo-Bravo, M.J.; Delic, D.; Gerovska, D.; Wunderlich, F. Protective Vaccination Reshapes Hepatic Response to Blood-Stage Malaria of Genes Preferentially Expressed by NK Cells. Vaccines 2020, 8, 677. [Google Scholar] [CrossRef]
- Delic, D.; Wunderlich, F.; Al-Quraishy, A.; Abdel-Baki, A.; Dkhil, M.; Araúzo-Bravo, M.J. Vaccination accelerates hepatic erythroblastosis induced by blood-stage malaria. Malar. J. 2020, 19, 49. [Google Scholar] [CrossRef]
- Geddis, A.E. Megakaryopoiesis. Semin. Hematol. 2010, 47, 212–219. [Google Scholar] [CrossRef]
- Xavier-Ferrucio, J.; Krause, D.S. Concise review: Bipotent megakaryocytic-erythroid progenitors: Concepts and controversies. Stem Cells 2018, 36, 1138–1145. [Google Scholar] [CrossRef]
- Noetzli, L.J.; French, S.L.; Machlus, K.R. New insights into the differentiation of megakaryocytes from haematopoietic progenitors. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1288–1300. [Google Scholar] [CrossRef]
- Kwon, N.; Thompson, E.N.; Mayday, M.Y.; Scanlon, V.; Lu, Y.C.; Krause, D.S. Current understanding of human megakaryocytic-erythroid progenitors and their fate determinants. Curr. Opin. Hematol. 2021, 28, 28–35. [Google Scholar] [CrossRef]
- Yamamoto, R.; Morita, Y.; Oohara, J.; Hamanaka, S.; Onodera, M.; Rudolph, K.L.; Ema, H.; Nakauchi, H. Clonal anlysis unveils self-renewing lineage-restricted progenitors generated directly from haematopoietic stem cells. Cell 2013, 154, 1112–1126. [Google Scholar] [CrossRef]
- Haas, S.; Hansson, J.; Klimmeck, D.; Loeffler, D.; Velten, L.; Uckelmann, H.; Wurzer, S.; Prendergast, A.M.; Schnell, A.; Hexel, K.; et al. Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors. Cell Stem Cell 2015, 17, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Woolthuis, C.M.; Park, C.Y. Hematopoietic stem/progenitor cell commitment to the megakaryocyte lineage. Blood 2016, 127, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fraticelli, A.E.; Wolock, S.L.; Weinreb, C.S.; Panero, R.; Patel, S.H.; Jankovic, M.; Sun, J.; Calogero, R.A.; Klein, A.M.; Camargo, F.D. Clonal analysis of lineage fate in native haematopoiesis. Nature 2018, 553, 212–216. [Google Scholar] [CrossRef]
- Lefrancais, E.; Ortiz-Munoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.K.; Villacorta-Martin, C.; Hon, S.; Rock, J.R.; Murphy, G.J. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 2020, 4, 6204–6217. [Google Scholar] [CrossRef]
- Cunin, P.; Nigrovic, P.A. Megakaryocytes as immune cells. J. Leukoc. Biol. 2019, 105, 1111–1121. [Google Scholar] [CrossRef]
- Finkielsztein, A.; Schlinker, A.C.; Zhang, L.; Miller, W.M.; Datta, S.K. Human megakaryocyte progenitors derived from haematopoietic stem cells of normal individuals are MHC class II-expressing professional APC that enhance Th17 Th1/Th17 responses. Immunol. Lett. 2015, 163, 84–95. [Google Scholar] [CrossRef]
- Zufferey, A.; Speck, E.R.; Machlus, K.R.; Aslam, R.; Guo, L.; McVey, M.J.; Kim, M.; Kapur, R.; Boilard, E.; Italiano Jr, J.E.; et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 2017, 1, 1773–1785. [Google Scholar] [CrossRef]
- Christ, S.A.; Elzey, B.D.; Ahmann, M.T.; Ratliff, T.L. Early growth response -1 (EGR-1) and nuclear factor of activated T cells (NFAT) cooperate to mediate CD40L expression in megakaryocytes and platelets. J. Biol. Chem. 2013, 288, 33985–33996. [Google Scholar] [CrossRef]
- Campbell, R.A.; Schwertz, H.; Hottz, E.D.; Rowley, J.W.; Manne, P.K. Human megakaryocytes possess intrinsic antiviral immunity through regulated induction of IFITM3. Blood 2019, 133, 2013–2026. [Google Scholar] [CrossRef]
- Vogt, M.B.; Lahon, A.; Arya, R.P.; Spencer Clinton, J.L.; Rico Hesse, R. Dengue viruses infect human megakaryocytes, with probable clinical consequences. PLoS Negl. Trop. Dis. 2019, 13, e0007837. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, J.; Fang, Y.; Lu, S.; Wu, J.; Theng, X.; Deng, F. SARS-CoV-2 interacts with platelets and megakaryocytes via ACE2-independent mechanism. J. Haematol. Oncol. 2021, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.W.; Oler, A.J.; Tolley, N.D.; Hunter, B.N.; Low, E.N.; Nix, D.A.; Yost, C.C.; Zimmerman, G.A.; Weyrich, A.S. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 2011, 118, e101–e111. [Google Scholar] [CrossRef] [PubMed]
- Neu, C.; Gutschner, T.; Haemmerle, M. Post-transcriptional expression control in platelet biogenesis and function. Int. J. Mol. Sci. 2020, 21, 7614. [Google Scholar] [CrossRef] [PubMed]
- Davizon-Castillo, P.; Rowley, J.W.; Rondina, M.T. Megakaryocyte and platelet transcriptomics for discoveries in human health and disease. Arterioscler. Thromp. Vasc. Biol. 2020, 40, 1432–1440. [Google Scholar] [CrossRef]
- McMorran, B.J.; Marshall, V.M.; de Graaf, C.; Drysdale, K.E.; Shabbar, M.; Smyth, G.K.; Corbin, J.E.; Alexander, W.S.; Foote, S.J. Platlets kill intraerythrocytic parasites and mediate survival of infection. Science 2009, 323, 797–800. [Google Scholar] [CrossRef]
- Kho, S.; Barber, B.E.; Johar, E.; Andries, B.; Poespoprodjo, J.R.; Kenangalem, E.; Piera, K.A.; Ehmann, A.; Price, R.N.; William, T.; et al. Platelets kill circulating parasites of all major Plasmodium species in human malaria. Blood 2018, 132, 1332–1344. [Google Scholar] [CrossRef]
- Horstmann, R.D.; Dietrich, M.; Bienzle, U.; Rasche, H. Malaria-induced thrombocytopenia. Blut 1981, 42, 157–164. [Google Scholar] [CrossRef]
- Lacerda, M.V.G.; Mourao, M.P.G.; Coelho, H.C.C.; Santos, J.B. Thrombocytopenia in malaria: Who cares? Mem. Inst. Oswaldo Cruz 2011, 106 (Suppl. 1), 52–63. [Google Scholar] [CrossRef]
- Gupta, N.K.; Bansal, S.B.; Jain, U.C.; Sahare, K. Study of thrombocytopenia in patients of malaria. Trop. Parasitol. 2013, 3, 58–61. [Google Scholar] [CrossRef]
- Antinori, S.; Corona, A.; Ridolfo, A.L.; Galimberti, L.; Ricaboni, D.; Milazzo, L.; Corbellino, M. Imported Plasmodium vivax malaria with severe thrombocytopaenia: Can it be severe malaria or not? Malar. J. 2016, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Gramaglia, I.; Velez, J.; Combes, V.; Grau, G.E.R.; Wree, M.; van der Heyde, H.C. Platelets activate a pathogenic response to blood-stage Plasmodium infection but not a protective immune response. Blood 2017, 129, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Sharma, M.; Mitchell, W.B. Malaria and thrombopoiesis: A possible mechanism for the malarial thrombocytopenia. J. Immunol. Infect. Inflam. Dis. 2017, 2, 14. [Google Scholar]
- Naing, C.; Whittaker, M.A. Severe thrombocytopaenia in patients with vivax malaria compared to falciparum malaria: A systematic review and meta-analysis. Inf. Dis. Poverty 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Asare, R.; Opoku-Okrah, C.; Danquah, K.O.; Opare-Sem, O.; Addai-Mensah, O.; Gyamfi, D.; Amponsah, F.A.; Afriyie, E.Y.; Duneeh, R.V.; Ofosu, D.N.; et al. Assessment of platelet indices and platelet activation markers in children with Plasmodium falciparum malaria. Malar. J. 2020, 19, 143. [Google Scholar] [CrossRef]
- Gebreweld, A.; Erkihun, Y.; Feleke, D.G.; Hailu, G.; Fiseha, T. Thrombocytopenia as a diagnostic marker for malaria in patients with acute febrile illness. J. Trop. Med. 2021, 10, 5585272. [Google Scholar] [CrossRef]
- Chapnik, E.; Rivkin, N.; Mildner, A.; Beck, G.; Pasvolsky, R.; Metzl-Raz, E.; Birger, Y.; Amir, G.; Tirosh, I.; Porat, Z.; et al. Mir-142 orchestrates a network of actin cytoskeleton regulators during megakaryopoiesis. eLife 2014, 3, e01964. [Google Scholar] [CrossRef]
- Wunderlich, F.; Helwig, M.; Schillinger, G.; Vial, H.; Philippot, J.; Speth, V. Isolation and characterization of parasites and host cell ghosts from erythrocytes infected with Plasmodium chabaudi. Mol. Biochem. Parasitol. 1987, 23, 103–105. [Google Scholar] [CrossRef]
- Wunderlich, F.; Helwig, M.; Schillinger, G.; Speth, V. Cryptic disposition of antigenic parasite proteins in plasma membranes of erythrocytes infected with Plasmodium chabaudi. Mol. Biochem. Parasitol. 1988, 30, 55–65. [Google Scholar] [CrossRef]
- Wunderlich, F.; Helwig, M.; Schillinger, G.; Speth, V.; Wiser, M.F. Expression of the parasite protein Pc90 in plasma membranes of erythrocytes infected with Plasmodium chabaudi. Eur. J. Cell. Biol. 1988, 47, 157–164. [Google Scholar]
- Wunderlich, F.; Mossmann, H.; Helwig, M.; Schillinger, G. Resistance to Plasmodium chabaudi in B10 mice: Influence of the H-2 complex and testosterone. Infect. Immun. 1988, 56, 2400–2406. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bianchi, E.; Norfo, R.; Pennucci, V.; Zini, R.; Manfredini, R. Genomic landscape of megakryopoiesis and platelet function defects. Blood 2015, 127, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K. Thrombopoiesis. Semin. Hematol. 2015, 52, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y. Megakaryopoiesis and platelet biology: Roles of transcription factors and emerging clinical implications. Int. J. Mol. Sci. 2021, 22, 9615. [Google Scholar] [CrossRef] [PubMed]
- Dore, L.C.; Crispino, J.D. Transcription factor networks in erythroid cell and megakaryocyte development. Blood 2011, 118, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ikonomi, P.; Rivera, C.E.; Riordan, M.; Washington, G.; Shechter, A.N.; Noguchi, C.T. Overexpression of GATA-2 inhibits erythroid and promotes megakaryocyte differentiation. Exp. Hematol. 2000, 28, 1423–1432. [Google Scholar] [CrossRef]
- Lecine, P.; Villeval, J.L.; Vyas, P.; Swencki, B.; Xu, Y.; Shivdasani, R.A. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and how a platelet production defect that is intrinsic to megakaryocytes. Blood 1998, 92, 1608–1616. [Google Scholar] [CrossRef]
- Athanasiou, M.; Mavrothalassitis, G.; Sun-Hofmann, L.; Blair, D.G. FLI-1 is a suppressor of erythroid differentiation in human hematopoietic cells. Leukemia 2000, 14, 439–445. [Google Scholar] [CrossRef]
- Guo, T.; Wang, X.; Qu, Y.; Yin, Y.; Jing, T.; Zhang, Q. Megakaryopoiesis and platelet production: Insight into hematopoietic stem cell proliferation and differentiation. Stem Cell Investig. 2015, 2, 3. [Google Scholar]
- Kuvardina, O.N.; Herglotz, J.; Kolodziej, S.; Kohrs, N.; Herkt, S.; Wojcik, B.; Oellerich, T.; Corso, J.; Behrens, K.; Kumar, A.; et al. RUNX1 represses the erythroid gene expression program during megakaryocyte differentiation. Blood 2015, 125, 3570–3579. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.; Carpinelli, M.R.; Hyland, C.; Mifsud, S.; Dirago, L.; Nicola, N.A.; Hilton, D.J.; Alexander, W.S. Anomalous megakaryocytopoiesis in mice with mutations in the c-Myb gene. Blood 2005, 105, 3480–3487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, M.; Shivdasani, R.A. Expression analysis of primary mouse megakaryocyte differentiation and its application in identifying stage-specific molecular markers and a novel transcriptional target of NF-E2. Blood 2007, 109, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Rayes, J.; Watson, S.P.; Nieswandt, B. Functional significance of the platelet immune immune receptors GPVI and CLEC-2. J. Clin. Investig. 2019, 129, 12–23. [Google Scholar] [CrossRef]
- Oishi, S.; Tsukiji, N.; Otake, S.; Oishi, N.; Sasaki, T.; Shirai, T.; Yoshikawa, Y.; Takano, K.; Shinmori, H.; Inukai, T.; et al. Heme activates platelets and exarcabates rhabdomyolysis-induced acute kidney injury via CLEC-2 and GPVI/FcRgamma. Blood Adv. 2021, 5, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Luo, M.; Liu, B. The role of CLEC-2 and its ligands in thromboinflammation. Front. Immunol. 2021, 12, 688643. [Google Scholar] [CrossRef]
- Haining, E.J.; Cherpokova, D.; Wolf, K.; Becker, I.C.; Beck, S.; Eble, J.A.; Stegner, D.; Watson, S.P.; Nieswandt, B. CLEC-2 contributes to hemostasis independently of classical hemITAM signaling in mice. Blood 2017, 130, 2224–2228. [Google Scholar] [CrossRef]
- Nakamura-Ishizu, A.; Takubo, K.; Kobayashi, H.; Suzuki-Inoue, K.; Suda, T. CLEC-2 in megakaryocytes is critical for maintenance of haematopoietic stem cells in the bone marrow. J. Exp. Med. 2015, 212, S2133–S2146. [Google Scholar] [CrossRef]
- Shin, J.Y.; Hu, W.; Naramura, M.; Park, C.Y. High c-Kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J. Exp. Med. 2014, 211, 217–231. [Google Scholar] [CrossRef]
- Grinenko, T.; Arndt, K.; Portz, M.; Mende, N.; Günther, M.; Cosgun, K.N.; Alexopoulou, D.; Lakshmanaperumal, N.; Henry, I.; Dahl, A.; et al. Clonal expansion capacity defines two consecutive developmental stages of long-term hematopoietic stem cells. J. Exp. Med. 2014, 214, 209–215. [Google Scholar] [CrossRef]
- Li, Y.; Jin, C.; Bai, H.; Gao, Y.; Sun, S.; Chen, L.; Qin, L.; Liu, P.P.; Cheng, L.; Wang, Q.F. Human Notch4 is a key target of RUNX1 in megakaryocytic differentiation. Blood 2018, 131, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Suzuki-Inoue, K.; Tsukiji, N.; Shirai, T.; Sasaki, T.; Osada, M.; Satoh, K.; Ozaki, Y. Podoplanin-positive periarteriolar stromal cells promote megakaryocyte growth and proplatelet formation in mice by CLEC-2. Blood 2016, 127, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Currao, A.; Gruppi, C.; Celesti, G.; Viarengo, G.; Buracchi, C.; Laghi, L.; Kaplan, D.L.; Balduini, A. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen and laminin. Stem Cells 2014, 32, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Perrin, A.; Bartholdson, S.J.; Wright, G.J. P-selectin is a host receptor for Plasmodium MSP7 ligands. Malar. J. 2015, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Kadekoppala, M.; O’Donnell, R.A.; Grainger, M.; Crabb, E.S.; Holder, A.A. Deletion of the Plasmodium falciparum merozoite surface protein 7 gene impairs parasite invasion of erythrocytes. Eukaryot. Cell 2008, 7, 2123–2132. [Google Scholar] [CrossRef][Green Version]
- Lesyk, G.; Jurasz, P. Advances in platelet subpopulation research. Front. Cardivasc. Med. 2019, 6, 138. [Google Scholar] [CrossRef]
- Love, M.S.; Millholland, M.G.; Drysdale, K.E.; Chan, J.A.; Huang, H.M.; Smith, C.; Mitiku, C.; Beeson, J.G.; Burgio, G.; Foote, S.J. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science 2012, 338, 1348–1351. [Google Scholar]
- Drescher, H.K.; Brandt, E.F.; Fischer, P.; Dreschers, S.; Schwendener, R.A.; Kowalska, M.A.; Canbay, A.; Wasmuth, H.E.; Weiskirchen, R.; Trautwein, C.; et al. Platelet factor 4 attenuates experimental acute liver injury in mice. Front. Physiol. 2019, 10, 326. [Google Scholar] [CrossRef]
- Kaushansky, K. The molecular mechanisms that control thrombopoiesis. J. Clin. Investig. 2005, 115, 3339–3347. [Google Scholar] [CrossRef]
- Jung, S.M.; Moroi, M. Platelet glycoprotein VI. Adv. Exp. Med. Biol. 2008, 640, 53–63. [Google Scholar]
- Okubo, K.; Kurosawa, M.; Kamiya, M.; Urano, Y.; Suzuki, A.; Yamamoto, K.; Hase, K.; Homma, K.; Sasaki, J.; Miyauchi, H.; et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat. Med. 2018, 24, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Lordier, L.; Bluteau, D.; Jalil, A.; Legrand, C.; Pan, J.; Rameau, P.; Jouni, D.; Bluteau, O.; Mercher, T.; Leon, C.; et al. RUNX1-induced silencing of non-muscle myosin heavy chain IIB contributes to megakaryocyte polyploidization. Nat. Commun. 2012, 3, 717. [Google Scholar] [CrossRef] [PubMed]
- Borges, I.; Sena, I.; Azevedo, P.; Andreotti, J.; Almeida, V.; Paiva, A.; Santos, G.; Guerra, D.; Prazeres, P.; Mesquita, L.L.; et al. Lung as a niche for haematopoietic progenitors. Stem Cell Rev. Rep. 2017, 13, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lefrancais, E.; Looney, M.R. Platelet biogenesis in the lung circulation. Physiology 2019, 34, 392–401. [Google Scholar] [CrossRef]
- Bai, L.; Vienne, M.; Tang, L.; Kerdiles, Y.; Etiennot, M.; Escalière, B.; Galluso, J.; Wie, H.; Sun, R.; Vivier, E.; et al. Liver type 1 innate lymphoid cells develop locally via an interferon-γ-dependent loop. Science 2021, 371, eaba4177. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Neves, M.A.D.; Zhu, G.; Carrim, N.; Yu, R.; Gupta, S.; Marshall, J.; Rotstein, O.; Peng, J.; et al. GPIbα is required for platelet-mediated hepatic thrombopoietin generation. Blood 2018, 132, 622–634. [Google Scholar] [CrossRef]
- Karakas, D.; Xu, M.; Ni, H. GPIba is the driving force of hepatic thrombopoietin generation. Res. Pract. Thromb. Haemost. 2021, 5, e12506. [Google Scholar] [CrossRef]
- Hoffmeister, K.M. TPO-logy accepted. Blood 2018, 132, 555–557. [Google Scholar] [CrossRef]
- Ng, A.P.; Kauppi, M.; Metcalf, D.; Hyland, C.D.; Josefsson, E.C.; Lebois, M.; Zhang, J.G.; Baldwin, T.M.; Di Rago, L.; Hilton, D.J.; et al. Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proc. Natl. Acad. Sci. USA 2014, 111, 5884–5889. [Google Scholar] [CrossRef]
- Nishimura, S.; Nagasaki, M.; Kunishima, S.; Sawaguchi, A.; Sakata, A.; Sakaguchi, H.; Ohmori, T.; Manabe, I.; Italiano, J.E., Jr.; Ryu, T.; et al. IL-1α induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J. Cell. Biol. 2015, 209, 453–466. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunderlich, F.; Delic, D.; Gerovska, D.; Araúzo-Bravo, M.J. Vaccination Accelerates Liver-Intrinsic Expression of Megakaryocyte-Related Genes in Response to Blood-Stage Malaria. Vaccines 2022, 10, 287. https://doi.org/10.3390/vaccines10020287
Wunderlich F, Delic D, Gerovska D, Araúzo-Bravo MJ. Vaccination Accelerates Liver-Intrinsic Expression of Megakaryocyte-Related Genes in Response to Blood-Stage Malaria. Vaccines. 2022; 10(2):287. https://doi.org/10.3390/vaccines10020287
Chicago/Turabian StyleWunderlich, Frank, Denis Delic, Daniela Gerovska, and Marcos J. Araúzo-Bravo. 2022. "Vaccination Accelerates Liver-Intrinsic Expression of Megakaryocyte-Related Genes in Response to Blood-Stage Malaria" Vaccines 10, no. 2: 287. https://doi.org/10.3390/vaccines10020287
APA StyleWunderlich, F., Delic, D., Gerovska, D., & Araúzo-Bravo, M. J. (2022). Vaccination Accelerates Liver-Intrinsic Expression of Megakaryocyte-Related Genes in Response to Blood-Stage Malaria. Vaccines, 10(2), 287. https://doi.org/10.3390/vaccines10020287





