Prospective Evaluation of Side-Effects Following the First Dose of Oxford/AstraZeneca COVID-19 Vaccine among Healthcare Workers in Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Settings
2.2. Sample Size
2.3. Instrument and Data Collection
2.4. Data Analysis
3. Results
3.1. General Characteristics of Participants
3.2. Reported COVID-19 Vaccine Side-Effects
3.3. Associations of the Reported COVID-19 Vaccine Side-Effects
3.4. Multivariate Analyses of Factors Associated with the Reported COVID-19 Vaccine Side-Effects
3.5. Duration and Management of the Reported COVID-19 Vaccine Side-Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. Atenei Parm. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Algaissi, A.A.; Alharbi, N.K.; Hassanain, M.; Hashem, A.M. Preparedness and response to COVID-19 in Saudi Arabia: Building on MERS experience. J. Infect. Public Health 2020, 13, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.A.T.A.; Alshamsan, A.; Al-jedai, A. Current COVID-19 vaccine candidates: Implications in the Saudi population. Saudi Pharm. J. 2020, 28, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- FDA US. Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. Maryland: The United States Food and Drug Administration. 2021. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-COVID-19/comirnaty-and-pfizer-biontech-COVID-19-vaccine (accessed on 15 October 2021).
- WHO. Coronavirus Disease (COVID-19): COVID-19 Vaccines; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/COVID-19-vaccines (accessed on 10 November 2021).
- El-Shitany, N.A.; Harakeh, S.; Badr-Eldin, S.M.; Bagher, A.M.; Eid, B.; Almukadi, H.; Alghamdi, B.S.; Alahmadi, A.A.; Hassan, N.A.; Sindi, N.; et al. Minor to moderate side effects of Pfizer-Biontech COVID-19 vaccine among Saudi residents: A retrospective cross-sectional study. Int. J. Gen. Med. 2021, 14, 1389–1401. [Google Scholar] [CrossRef]
- Cavaleri, M.; Enzmann, H.; Straus, S.; Cooke, E. The European Medicines Agency’s EU conditional marketing authorisations for COVID-19 vaccines. Lancet 2021, 397, 355–357. [Google Scholar] [CrossRef]
- Mallapaty, S. China’s COVID vaccines have been crucial—now immunity is waning. Nature 2021, 598, 398–399. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- WHO. COVID-19 Advice for the Public: Getting Vaccinated; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/COVID-19-vaccines/advice (accessed on 2 November 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Kaur, R.J.; Dutta, S.; Bhardwaj, P.; Charan, J.; Dhingra, S.; Mitra, P.; Singh, K.; Yadav, D.; Sharma, P.; Misra, S. Adverse events reported from COVID-19 vaccine trials: A systematic review. Indian J. Clin. Biochem. 2021, 36, 427–439. [Google Scholar] [CrossRef]
- Abu-Halaweh, S.; Alqassieh, R.; Suleiman, A.; Al-Sabbagh, M.Q.; AbuHalaweh, M.; AlKhader, D.; Abu-Nejem, R.; Nabulsi, R.; Al-Tamimi, M.; Alwreikat, M.; et al. Qualitative assessment of early adverse effects of Pfizer-BioNTech and Sinopharm COVID-19 vaccines by telephone interviews. Vaccines 2021, 9, 950. [Google Scholar] [CrossRef]
- Klugar, M.; Riad, A.; Mekhemar, M.; Conrad, J.; Buchbender, M.; Howaldt, H.P.; Attia, S. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology 2021, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Saeed, B.Q.; Al-Shahrabi, R.; Alhaj, S.S.; Alkokhardi, Z.M.; Adrees, A.O. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int. J. Infect. Dis. 2021, 111, 219–226. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N., Jr.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021, 325, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Tobaiqy, M.; MacLure, K.; Elkout, H.; Stewart, D. Thrombotic adverse events reported for Moderna, Pfizer and Oxford-AstraZeneca COVID-19 vaccines: Comparison of occurrence and clinical outcomes in the eudravigilance database. Vaccines 2021, 9, 1326. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- MOH. MOH News. Riyadh: Ministry of Health. 2021. Available online: https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2021-10-15-001.aspx (accessed on 20 October 2021).
- Elharake, J.A.; Galal, B.; Alqahtani, S.A.; Kattan, R.F.; Barry, M.A.; Temsah, M.H.; Malik, A.A.; McFadden, S.M.; Yildirim, I.; Khoshnood, K.; et al. COVID-19 vaccine acceptance among health care workers in the Kingdom of Saudi Arabia. Int. J. Infect. Dis. 2021, 109, 286–293. [Google Scholar] [CrossRef]
- MOH. COVID-19 & Vaccine FAQs. Riyadh: Ministry of Health. 2021. Available online: https://www.moh.gov.sa/en/Ministry/HotTopics/Pages/COVID-19-Vaccine.aspx (accessed on 5 January 2022).
- Our World in Data. Saudi Arabia: Coronavirus Pandemic Country Profile. 2021. Available online: https://ourworldindata.org/coronavirus/country/saudi-arabia (accessed on 25 October 2021).
- Al-Mohaithef, M.; Padhi, B.K. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: A web-based national survey. J. Multidiscip. Healthc. 2020, 13, 1657–1663. [Google Scholar] [CrossRef]
- Barry, M.; Temsah, M.H.; Aljamaan, F.; Saddik, B.; Al-Eyadhy, A.; Alenezi, S.; Alamro, N.; Alhuzaimi, A.N.; Alhaboob, A.; Alhasan, K.; et al. COVID-19 vaccine uptake among healthcare workers in the fourth country to authorize BNT162b2 during the first month of rollout. Vaccine 2021, 39, 5762–5768. [Google Scholar] [CrossRef]
- Lwanga, S.K.; Lemeshow, S.; World Health Organization. Sample Size Determination in Health Studies: A Practical Manual; World Health Organization: Geneva, Switzerland, 1991. Available online: https://apps.who.int/iris/handle/10665/40062 (accessed on 15 October 2021).
- Israel, G.D. Determining Sample Size; Technical Report; University of Florida: Gainesville, FL, USA, 1992; Available online: https://www.tarleton.edu/academicassessment/documents/samplesize.pdf (accessed on 5 January 2022).
- Adam, M.; Gameraddin, M.; Alelyani, M.; Alshahrani, M.Y.; Gareeballah, A.; Ahmad, I.; Azzawi, A.; Komit, B.; Musa, A. Evaluation of post-vaccination symptoms of two common COVID-19 vaccines used in Abha, Aseer Region, Kingdom of Saudi Arabia. Patient Prefer. Adherence 2021, 15, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Bendel, R.B.; Afifi, A.A. Comparison of stopping rules in forward “stepwise” regression. J. Am. Stat. Assoc. 1977, 72, 46–53. [Google Scholar]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Gee, J.; Marquez, P.; Su, J.; Calvert, G.M.; Liu, R.; Myers, T.; Nair, N.; Martin, S.; Clark, T.; Markowitz, L.; et al. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020-January 13, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef]
- Alhazmi, A.; Alamer, E.; Daws, D.; Hakami, M.; Darraj, M.; Abdelwahab, S.; Maghfuri, A.; Algaissi, A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines 2021, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Al Bahrani, S.; Albarrak, A.; Alghamdi, O.A.; Alghamdi, M.A.; Hakami, F.H.; Al Abaadi, A.K.; Alkhrashi, S.A.; Alghamdi, M.Y.; Almershad, M.M.; Alenazi, M.M.; et al. Safety and reactogenicity of the ChAdOx1 (AZD1222) COVID-19 vaccine in Saudi Arabia. Int. J. Infect. Dis. 2021, 110, 359–362. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Alkazemi, A.; Alissa, A.; Alghamdi, I.; Alwarafi, G.; Waggas, H.A. Adverse events following AstraZeneca COVID-19 vaccine in Saudi Arabia: A cross-sectional study among healthcare and non-healthcare workers. Intervirology 2021. [Google Scholar] [CrossRef]
- Zaki, N.W.; Sidiq, M.; Qasim, M.; Aranas, B.; Hakamy, A.; Ruwais, N.; Alanezi, H.; Al Saudi, D.A.; Alshahrani, R.B.S.; Al-Thomali, A.B.A.; et al. Stress and psychological consequences of COVID-19 on health-care workers. J. Nat. Sci. Med. 2020, 3, 299–307. [Google Scholar] [CrossRef]
- Tissot, N.; Brunel, A.S.; Bozon, F.; Rosolen, B.; Chirouze, C.; Bouiller, K. Patients with history of COVID-19 had more side effects after the first dose of COVID-19 vaccine. Vaccine 2021, 39, 5087–5090. [Google Scholar] [CrossRef]
- Alghamdi, A.N.; Alotaibi, M.I.; Alqahtani, A.S.; Al Aboud, D.; Abdel-Moneim, A.S. BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front. Med. 2021, 8, 760047. [Google Scholar] [CrossRef]
- Abu-Hammad, O.; Alduraidi, H.; Abu-Hammad, S.; Alnazzawi, A.; Babkair, H.; Abu-Hammad, A.; Nourwali, I.; Qasem, F.; Dar-Odeh, N. Side effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines 2021, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Andrzejczak-Grządko, S.; Czudy, Z.; Donderska, M. Side effects after COVID-19 vaccinations among residents of Poland. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4418–4421. [Google Scholar] [CrossRef] [PubMed]
- Jayadevan, R.; Shenoy, R.S.; Anithadevi, T.S. Survey of symptoms following COVID-19 vaccination in India. MedRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.02.08.21251366v1.full.pdf (accessed on 20 November 2021).
- Esba, L.; Al Jeraisy, M. Reported adverse effects following COVID-19 vaccination at a tertiary care hospital, focus on cerebral venous sinus thrombosis (CVST). Expert Rev. Vaccines 2021, 20, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Alfawaz, H.; Amer, O.E.; Aljumah, A.A.; Aldisi, D.A.; Enani, M.A.; Aljohani, N.J.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Khattak, M.; et al. Effects of home quarantine during COVID-19 lockdown on physical activity and dietary habits of adults in Saudi Arabia. Sci. Rep. 2021, 11, 5904. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.A.; Garout, R.M.; Wahid, S.; Ayub, F.; Leen, M.; ZinAlddin, F.; Sultan, I. A survey on the side effects of Pfizer/BioNTech COVID-19 vaccine among vaccinated adults in Saudi Arabia. Cureus 2021, 13, e19222. [Google Scholar] [CrossRef]
- Vassallo, A.; Shajahan, S.; Harris, K.; Hallam, L.; Hockham, C.; Womersley, K.; Woodward, M.; Sheel, M. Sex and gender in COVID-19 vaccine research: Substantial evidence gaps remain. Front. Glob. Women Health 2021, 2, 761511. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef]
- McCartney, P.R. Sex-based vaccine response in the context of COVID-19. J. Obstet. Gynecol. Neonatal Nurs. 2020, 49, 405–408. [Google Scholar] [CrossRef]
- Harris, T.; Nair, J.; Fediurek, J.; Deeks, S.L. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–2015. Vaccine 2017, 35, 2600–2604. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Hocková, B.; Kantorová, L.; Slávik, R.; Spurná, L.; Stebel, A.; Havriľak, M.; Klugar, M. Side effects of mRNA-based COVID-19 vaccine: Nationwide phase IV study among healthcare workers in Slovakia. Pharmaceuticals 2021, 14, 873. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.; Kim, J.; Oh, C.E.; Lee, J.Y. Adverse events following immunization associated with the first and second doses of the ChAdOx1 nCoV-19 vaccine among healthcare workers in Korea. Vaccines 2021, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
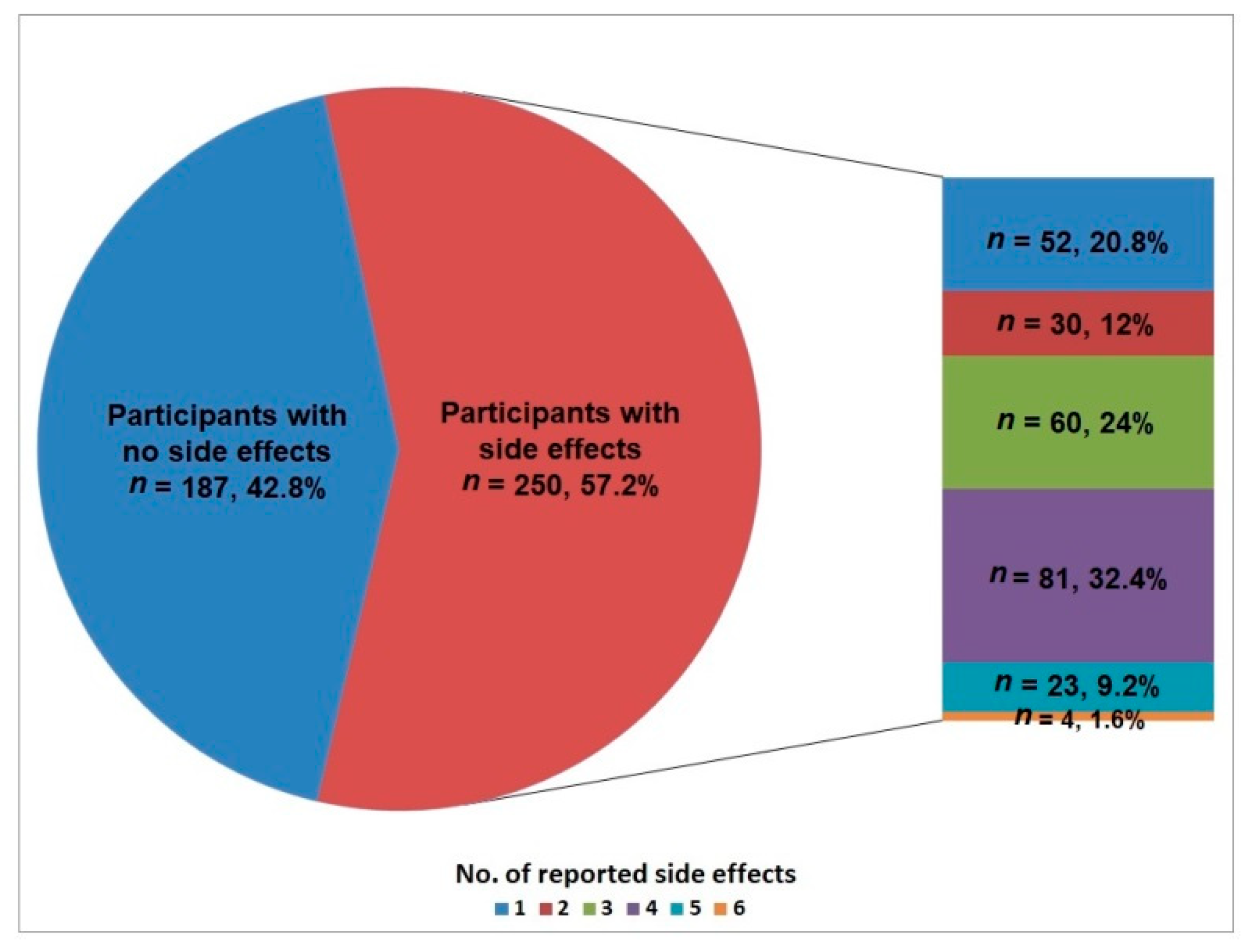
| Characteristics | n (%) | |
|---|---|---|
| Age group (years) | 20–30 | 169 (38.7) |
| 31–40 | 202 (46.2) | |
| 41–50 | 50 (11.4) | |
| >50 | 16 (3.7) | |
| Nationality | Saudi | 187 (42.8) |
| Non-Saudi | 250 (57.2) | |
| Gender | Females | 283 (64.8) |
| Males | 154 (35.2) | |
| Marital Status | Married | 250 (57.2) |
| Single | 187 (42.8) | |
| Residence | Jazan | 378 (86.5) |
| Sabia | 29 (6.6) | |
| Abu Arish | 30 (6.9) | |
| Occupation | Nurse | 167 (38.2) |
| Medical record professional | 61 (14.0) | |
| Administrative professional | 52 (11.9) | |
| Workers (such as cleaning staff, drivers, storekeepers) | 45 (10.3) | |
| Physician | 39 (8.9) | |
| Technician—medical | 37 (8.5) | |
| Security | 18 (4.1) | |
| Technician—nonmedical | 16 (3.7) | |
| Pharmacist | 2 (0.5) | |
| Diagnosed previously with COVID-19 | Yes | 55 (12.6) |
| No | 382 (87.4) | |
| History of chronic diseases | DM and hypertension | 3 (0.7) |
| No | 434 (99.3) | |
| Symptoms | Male (n = 90) | Female (n = 160) | Total (n = 250) | χ2 (p) |
|---|---|---|---|---|
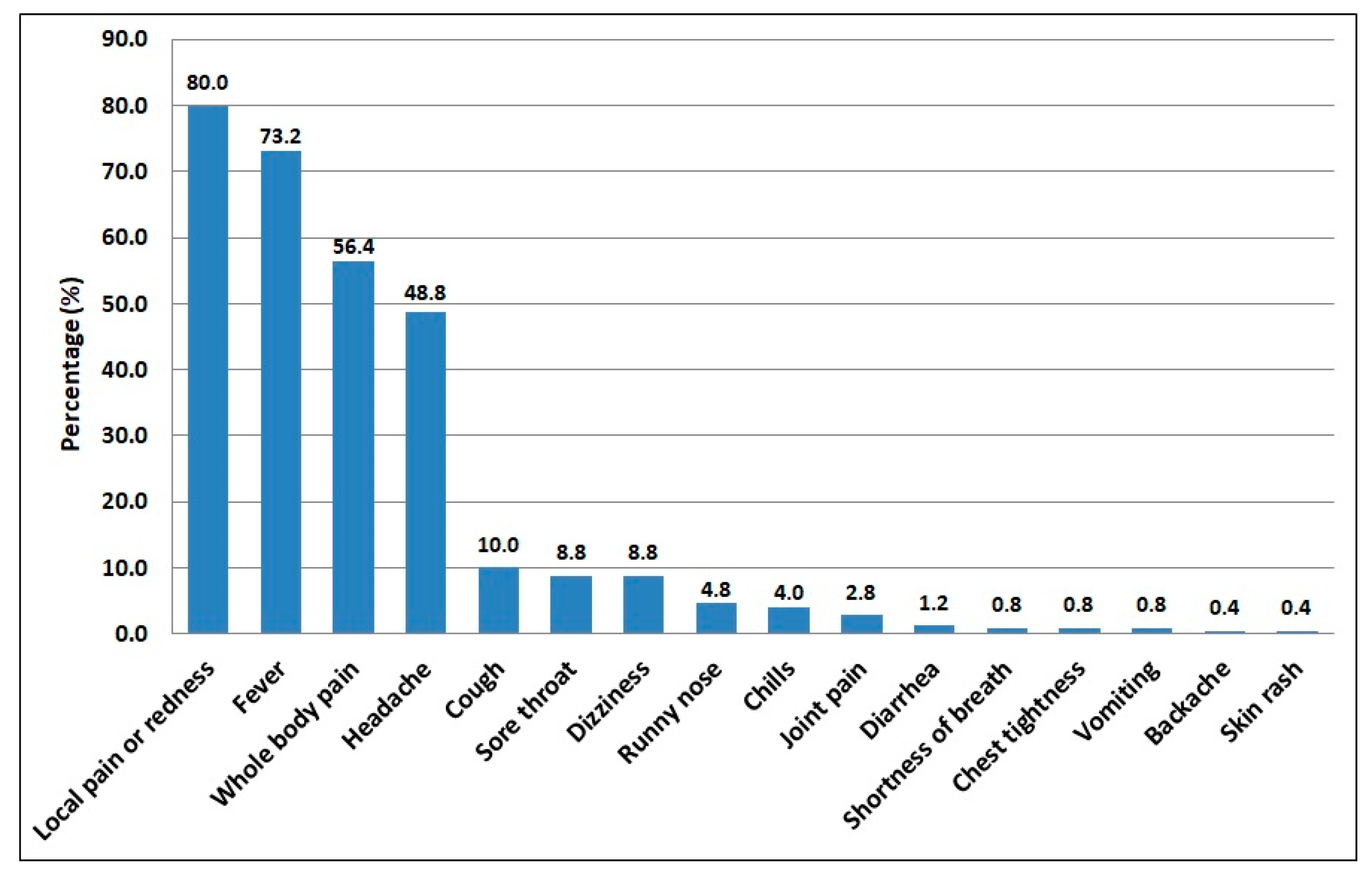
| Pain or redness at the site of injection | 72 (80.0) | 128 (80.0) | 200 (80.0) | 0.001 (0.999) |
| Fever | 65 (72.2) | 118 (73.8) | 183 (73.2) | 0.069 (0.793) |
| Whole body pain/fatigue | 61 (67.8) | 80 (50.0) | 141 (56.4) | 7.403 (0.007) |
| Headache | 51 (56.7) | 71 (44.4) | 122 (48.8) | 3.483 (0.062) |
| Cough | 13 (14.4) | 12 (7.5) | 25 (10.0) | 3.086 (0.079) |
| Sore throat | 12 (13.3) | 10 (6.2) | 22 (8.8) | 3.601 (0.058) |
| Dizziness | 3 (3.3) | 19 (11.9) | 22 (8.8) | 5.236 (0.022) |
| Runny nose | 6 (6.7) | 6 (3.8) | 12 (4.8) | 1.072 (0.301) † |
| Chills | 0 (0.0) | 10 (6.2) | 10 (4.0) | 5.859 (0.015) † |
| Joint pain | 2 (2.2) | 5 (3.1) | 7 (2.8) | 0.172 (0.587) † |
| Diarrhea | 1 (1.1) | 2 (1.2) | 3 (1.2) | 0.009 (0.735) † |
| Shortness of breath | 0 (0.0) | 2 (1.2) | 2 (0.8) | 1.134 (0.549) † |
| Chest tightness | 0 (0.0) | 2 (1.2) | 2 (0.8) | 1.134 (0.549) † |
| Vomiting | 0 (0.0) | 2 (1.2) | 2 (0.8) | 1.134 (0.549) † |
| Backache | 1 (1.1) | 0 (0.0) | 1 (0.4) | 1.785 (0.630) † |
| Skin rash | 0 (0.0) | 1 (0.6) | 1 (0.4) | 0.565 (0.710) † |
| Characteristic | Reported Side-Effects | OR (95% CI) | p | |
|---|---|---|---|---|
| Yes (n = 250) | No (n = 187) | |||
| Age group (years) | ||||
| 20–30 (n = 169) | 94 (55.6) | 75 (44.4) | 1 | |
| 31–40 (n = 202) | 115 (56.9) | 87 (43.1) | 1.06 (0.70, 1.60) | 0.801 |
| 41–50 (n = 50) | 29 (58.0) | 21 (42.0) | 1.10 (0.58, 2.09) | 0.766 |
| >50 (n = 16) | 12 (75.0) | 4 (25.0) | 2.39 (0.74, 7.72) | 0.134 |
| Gender | ||||
| Female (n = 283) | 160 (56.5) | 123 (43.5) | 0.93 (0.62, 1.38) | 0.701 |
| Male (n = 154) | 90 (58.4) | 64 (41.6) | 1 | |
| Nationality | ||||
| Saudi (n = 190) | 138 (73.8) | 49 (26.2) | 3.47 (2.30, 5.23) | <0.001 * |
| Non-Saudi (n = 254) | 112 (44.8) | 138 (55.2) | 1 | |
| Marital status | ||||
| Married (n = 255) | 145 (57.2) | 105 (42.0) | 1.08 (0.74, 1.58) | 0.699 |
| Single (n = 189) | 105 (56.1) | 82 (43.9) | 1 | |
| Residence | ||||
| Jazan (n = 378) | 205 (54.2) | 173 (45.8) | 0.36 (0.15, 0.86) | 0.017 |
| Sabia (n = 29) | 22 (75.9) | 7 (24.1) | 0.96 (0.29, 3.18) | 0.924 |
| Abu Arish (n = 30) | 23 (76.7) | 7 (23.3) | 1 | |
| Occupation | ||||
| Medical (n = 245) | 125 (51.0) | 120 (49.0) | 0.56 (0.38, 0.82) | 0.003 * |
| Nonmedical (n = 192) | 125 (65.1) | 67 (34.9) | 1 | |
| Diagnosed previously with COVID-19 | ||||
| Yes (n = 55) | 28 (50.9) | 27 (49.1) | 0.75 (0.43, 1.32) | 0.313 |
| No (n = 382) | 222 (58.1) | 160 (41.9) | 1 | |
| Characteristic | No. of Reported Side-Effects | Statistics | p |
|---|---|---|---|
| Median (IQR) | |||
| Age group (years) | H = 4.079 | 0.245 | |
| 20–30 (n = 94) | 3 (2, 4) | ||
| 31–40 (n = 115) | 3 (2, 4) | ||
| 41–50 (n = 29) | 3 (1, 4) | ||
| >50 (n = 12) | 2 (1, 3) | ||
| Gender | U = 6366 | 0.117 | |
| Female (n = 90) | 3 (2, 4) | ||
| Male (n = 160) | 3 (2, 4) | ||
| Nationality | U = 7643 | 0.878 | |
| Saudi (n = 138) | 2 (3, 4) | ||
| Non-Saudi (n = 112) | 2 (3, 4) | ||
| Marital status | U = 7475 | 0.802 | |
| Married (n = 145) | 3 (2, 4) | ||
| Single (n = 105) | 3 (2, 4) | ||
| Residence | H = 2.125 | 0.346 | |
| Jazan (n = 205) | 3 (2, 4) | ||
| Sabia (n = 22) | 3 (2, 4) | ||
| Abu Arish (n = 23) | 4 (3, 4) | ||
| Occupation | U = 5874 | <0.001 * | |
| Medical (n = 125) | 3 (1, 4) | ||
| Nonmedical (n = 125) | 4 (3, 4) | ||
| Diagnosed previously with COVID-19 | U = 3062 | 0.896 | |
| Yes (n = 28) | 3 (3, 4) | ||
| No (n = 222) | 3 (2, 4) |
| Variable | aOR | 95% CI | p |
|---|---|---|---|
| Nationality (Saudi) | 3.65 | 2.40, 5.55 | <0.001 * |
| Residence (Jazan) | 0.38 | 0.15, 0.95 | 0.038 * |
| Occupation (medical) | 0.67 | 0.43, 1.02 | 0.062 |
| Variable | aOR | 95% CI | p |
|---|---|---|---|
| Age (year) | 0.97 | 0.94, 1.49 | 0.093 |
| Gender (female) | 0.61 | 0.38, 0.97 | 0.038 * |
| Occupation (medical) | 0.42 | 0.26, 0.66 | <0.001 * |
| Onset and Duration of Side-Effects | Male (n = 90) | Female (n = 160) | Total (n = 250) | χ2 (p) |
|---|---|---|---|---|
| Onset | 0.172 (0.918) | |||
| Day 0 | 84 (93.3) | 150 (93.8) | 234 (93.6) | |
| Day 1 | 5 (5.6) | 9 (5.6) | 14 (5.6) | |
| Day 2 | 1 (1.1) | 1 (0.6) | 2 (0.8) | |
| Duration of symptoms | 5.196 (0.268) | |||
| One day | 8 (8.9) | 20 (12.5) | 28 (11.2) | |
| 2–3 days | 46 (51.1) | 87 (54.4) | 133 (53.2) | |
| 4–5 days | 30 (33.3) | 35 (21.9) | 65 (26.0) | |
| 6–7 days | 4 (4.4) | 14 (8.8) | 18 (7.2) | |
| More than 7 days | 2 (2.2) | 4 (2.5) | 6 (2.4) | |
| Medication taken for side-effects | 62 (68.9) | 104 (65.0) | 166 (66.4) | 0.390 (0.532) |
| Doctor’s visit due to side-effects | 10 (11.1) | 21 (13.1) | 31 (12.4) | 0.215 (0.643) |
| Hospitalization due to side-effects | 0 (0.0) | 1 (0.6) | 1 (0.4) | 0.565 (0.641) † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darraj, M.A.; Al-Mekhlafi, H.M. Prospective Evaluation of Side-Effects Following the First Dose of Oxford/AstraZeneca COVID-19 Vaccine among Healthcare Workers in Saudi Arabia. Vaccines 2022, 10, 223. https://doi.org/10.3390/vaccines10020223
Darraj MA, Al-Mekhlafi HM. Prospective Evaluation of Side-Effects Following the First Dose of Oxford/AstraZeneca COVID-19 Vaccine among Healthcare Workers in Saudi Arabia. Vaccines. 2022; 10(2):223. https://doi.org/10.3390/vaccines10020223
Chicago/Turabian StyleDarraj, Majid A., and Hesham M. Al-Mekhlafi. 2022. "Prospective Evaluation of Side-Effects Following the First Dose of Oxford/AstraZeneca COVID-19 Vaccine among Healthcare Workers in Saudi Arabia" Vaccines 10, no. 2: 223. https://doi.org/10.3390/vaccines10020223
APA StyleDarraj, M. A., & Al-Mekhlafi, H. M. (2022). Prospective Evaluation of Side-Effects Following the First Dose of Oxford/AstraZeneca COVID-19 Vaccine among Healthcare Workers in Saudi Arabia. Vaccines, 10(2), 223. https://doi.org/10.3390/vaccines10020223





