Assessment of the Neutralizing Antibody Response of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Naïve and Previously Infected Individuals: A Comparative Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethical Approval and Sample Collection
2.2. Serology Testing
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristic
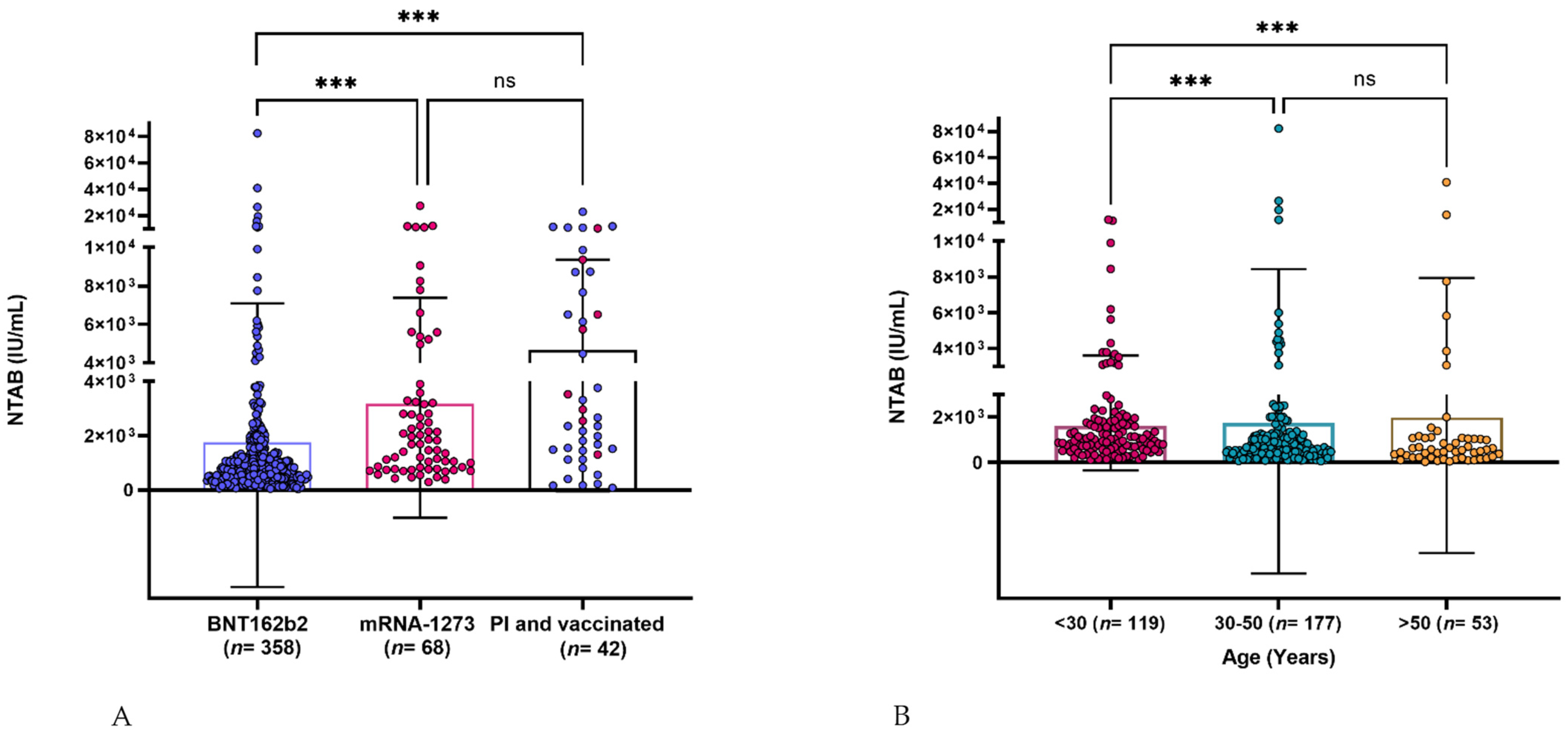
3.2. Neutralizing Antibody Response Assessment
3.3. Age Effect on Neutralizing Antibody Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johns Hopkins Coronavirus Resource Center. COVID-19 Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 16 September 2021).
- WHO. Available online: https://covid19.who.int/region/emro/country/qa (accessed on 18 January 2022).
- Yap, C.; Ali, A.; Prabhakar, A.; Prabhakar, A.; Pal, A.; Lim, Y.Y.; Kakodkar, P. Comprehensive literature review on COVID-19 vaccines and role of SARS-CoV-2 variants in the pandemic. Ther. Adv. Vaccines Immunother. 2021, 9, 25151355211059791. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadeq, D.; Shurrab, F.; Ismail, A.; Amanullah, F.H.; Thomas, S.; Aldewik, N.; Yassine, H.M.; Rahim, H.A.; Abu-Raddad, L.J.; Nasrallah, G.K. Comparison of antibody immune responses between BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in naïve and previously infected individuals. J. Travel Med. 2021, 28, taab190. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2021, 3, e52–e61. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Nasrallah, G.K.; Dargham, S.R.; Shurrab, F.; Al-Sadeq, D.W.; Al-Jighefee, H.; Chemaitelly, H.; Al Kanaani, Z.; Al Khal, A.; Al Kuwari, E.; Coyle, P. Analytic comparison between three high-throughput commercial SARS-CoV-2 antibody assays reveals minor discrepancies in a high-incidence population. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Younes, S.; Al-Jighefee, H.; Shurrab, F.; Al-Sadeq, D.; Younes, N.; Dargham, S.; Al-Dewik, N.; Qotba, H.; Syed, M.; Alnuaimi, A.; et al. Diagnostic Efficiency of Three Fully Automated Serology Assays and Their Correlation with a Novel Surrogate Virus Neutralization Test in Symptomatic and Asymptomatic SARS-COV-2 Individuals. Microorganisms 2021, 9, 245. [Google Scholar] [CrossRef]
- Ismail, A.; Shurrab, F.M.; Al-Jighefee, H.T.; Al-Sadeq, D.W.; Qotba, H.; Al-Shaar, I.A.; Yassine, H.M.; Abu-Raddad, L.J.; Nasrallah, G.K. Can commercial automated immunoassays be utilized to predict neutralizing antibodies after SARS-CoV-2 infection? A comparative study between three different assays. Front. Biosci. (Landmark Ed.) 2021, 26, 198–206. [Google Scholar] [CrossRef]
- Anichini, G.; Terrosi, C.; Gandolfo, C.; Gori Savellini, G.; Fabrizi, S.; Miceli, G.B.; Cusi, M.G. SARS-CoV-2 antibody response in persons with past natural infection. N. Engl. J. Med. 2021, 385, 90–92. [Google Scholar] [CrossRef]
- Vicenti, I.; Basso, M.; Gatti, F.; Scaggiante, R.; Boccuto, A.; Zago, D.; Modolo, E.; Dragoni, F.; Parisi, S.G.; Zazzi, M. Faster decay of neutralizing antibodies in never infected than previously infected healthcare workers three months after the second BNT162b2 mRNA COVID-19 vaccine dose. Int. J. Infect. Dis. 2021, 112, 40–44. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Sokal, A.; Barba-Spaeth, G.; Fernández, I.; Broketa, M.; Azzaoui, I.; de La Selle, A.; Vandenberghe, A.; Fourati, S.; Roeser, A.; Meola, A.; et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity 2021, 54, 2893–2907. [Google Scholar] [CrossRef]
- Castro, A.; Ozturk, K.; Zanetti, M.; Carter, H. In silico analysis suggests less effective MHC-II presentation of SARS-CoV-2 RBM peptides: Implication for neutralizing antibody responses. PLoS ONE 2021, 16, e0246731. [Google Scholar] [CrossRef]
- Tyner, H.L.; Thompson, M.G.; Burgess, J.L.; Grant, L.; Gaglani, M.; Kuntz, J.L.; Naleway, A.L.; Thornburg, N.J.; Caban-Martinez, A.J.; Yoon, S.K.; et al. Neutralizing Antibody Response to Pseudotype SARS-CoV-2 Differs between mRNA-1273 and BNT162b2 COVID-19 Vaccines and by History of SARS-CoV-2 Infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Ponticelli, D.; Antonazzo, I.C.; Caci, G.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; Ferrara, P. Dynamics of antibody response to BNT162b2 mRNA COVID-19 vaccine after 6 months. J. Travel Med. 2021, 28, taab173. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health-Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. medRxiv 2021. [Google Scholar]
- Jalkanen, P.; Kolehmainen, P.; Häkkinen, H.; Huttunen, M.; Tähtinen, P.; Lundberg, R.; Maljanen, S.; Reinholm, A.; Tauriainen, S.; Pakkanen, S. COVID-19 mRNA vaccine induced antibody responses and neutralizing antibodies against three SARS-CoV-2 variants. Nat. Commun. 2021, 12, 3991. [Google Scholar] [CrossRef]
- Abe, K.T.; Hu, Q.; Mozafarihashjin, M.; Samson, R.; Manguiat, K.; Robinson, A.; Rathod, B.; Wang, J.H.; Iskilova, M.; Pasculescu, A. Neutralizing antibody responses to SARS-CoV-2 variants in vaccinated Ontario long-term care home residents and workers. medRxiv 2021. [Google Scholar]
- Collier, D.A.; Ferreira, I.A.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; Elmer, A.; Kingston, N.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
| Characteristic | BNT162b2 N (%) | mRNA-1273 N (%) | |||
|---|---|---|---|---|---|
| Gender | Naïve | PI | Naïve | PI | |
| Male | 170 (47.5) | 10 (29.4) | 27 (39.7) | 3 (37.5) | |
| Female | 175 (48.9) | 15 (44.1) | 41 (60.3) | 5 (62.5) | |
| Unknown | 13 (3.6) | 9 (26.4) | - | - | |
| Total | 358 | 34 | 68 | 8 | |
| Age (years) | >30 | 119 (33.2) | 15 (44.1) | 41 (60.3) | 3 (37.5) |
| 30–50 | 177 (49.4) | 17 (50) | 25 (36.8) | 5 (62.5) | |
| >50 | 53 (14.8) | 2 (5.8) | 2 (2.9) | - | |
| Unknown | 9 (2.5) | - | - | - | |
| Total | 358 | 34 | 68 | 8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurrab, F.M.; Al-Sadeq, D.W.; Abou-Saleh, H.; Al-Dewik, N.; Elsharafi, A.E.; Hamaydeh, F.M.; Halawa, B.Y.A.; Jamaleddin, T.M.; Hameed, H.M.A.; Nizamuddin, P.B.; et al. Assessment of the Neutralizing Antibody Response of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Naïve and Previously Infected Individuals: A Comparative Study. Vaccines 2022, 10, 191. https://doi.org/10.3390/vaccines10020191
Shurrab FM, Al-Sadeq DW, Abou-Saleh H, Al-Dewik N, Elsharafi AE, Hamaydeh FM, Halawa BYA, Jamaleddin TM, Hameed HMA, Nizamuddin PB, et al. Assessment of the Neutralizing Antibody Response of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Naïve and Previously Infected Individuals: A Comparative Study. Vaccines. 2022; 10(2):191. https://doi.org/10.3390/vaccines10020191
Chicago/Turabian StyleShurrab, Farah M., Duaa W. Al-Sadeq, Haissam Abou-Saleh, Nader Al-Dewik, Amira E. Elsharafi, Fatima M. Hamaydeh, Bushra Y. Abo Halawa, Tala M. Jamaleddin, Huda M. Abdul Hameed, Parveen B. Nizamuddin, and et al. 2022. "Assessment of the Neutralizing Antibody Response of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Naïve and Previously Infected Individuals: A Comparative Study" Vaccines 10, no. 2: 191. https://doi.org/10.3390/vaccines10020191
APA StyleShurrab, F. M., Al-Sadeq, D. W., Abou-Saleh, H., Al-Dewik, N., Elsharafi, A. E., Hamaydeh, F. M., Halawa, B. Y. A., Jamaleddin, T. M., Hameed, H. M. A., Nizamuddin, P. B., Amanullah, F. H., Daas, H. I., Abu-Raddad, L. J., & Nasrallah, G. K. (2022). Assessment of the Neutralizing Antibody Response of BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines in Naïve and Previously Infected Individuals: A Comparative Study. Vaccines, 10(2), 191. https://doi.org/10.3390/vaccines10020191










