Changes in HPV16/18 Prevalence among Unvaccinated Women with Cervical Intraepithelial Neoplasia in Japan: Assessment of Herd Effects following the HPV Vaccination Program
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. HPV Genotyping Procedures
2.3. Statistical Methods
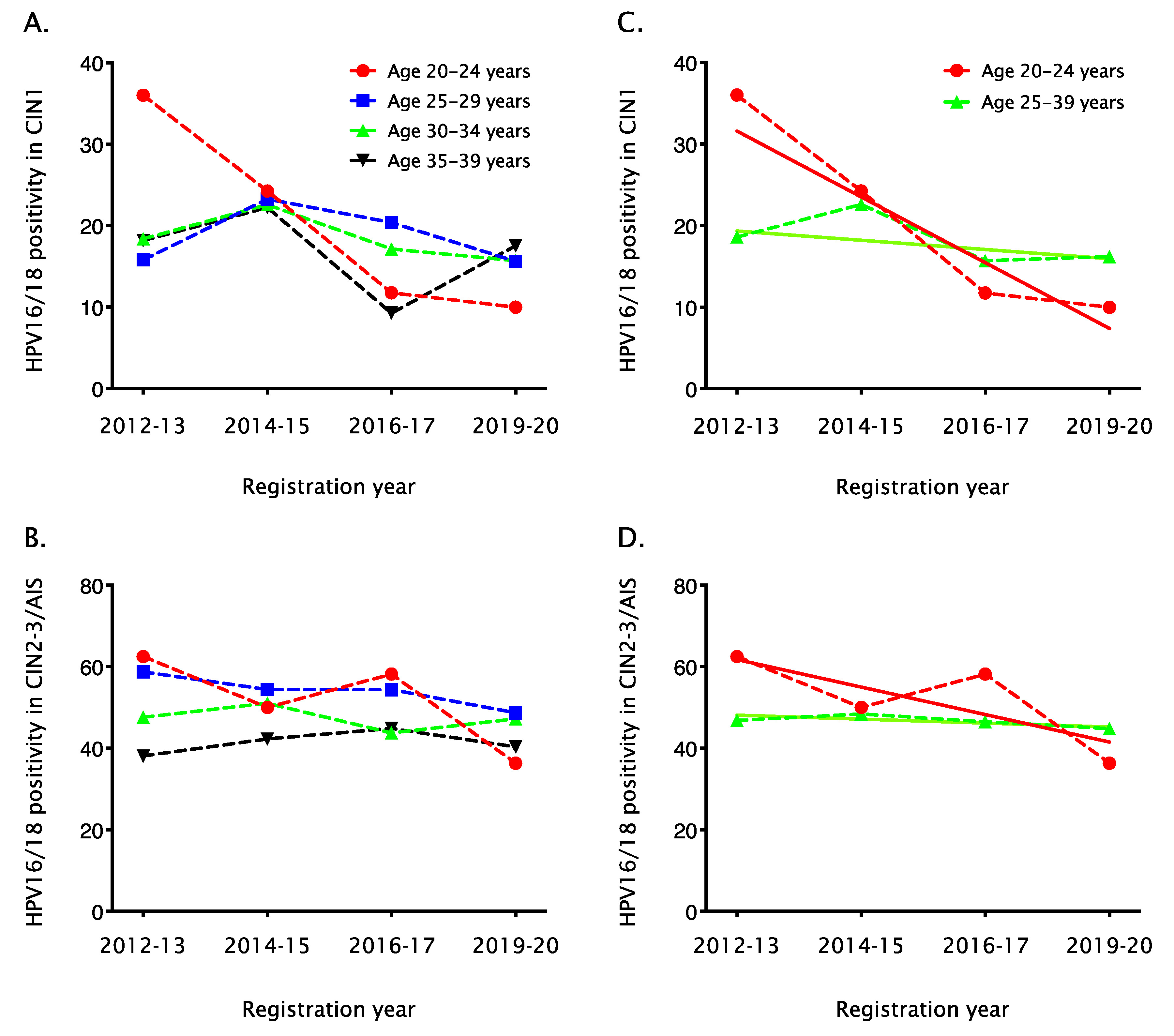
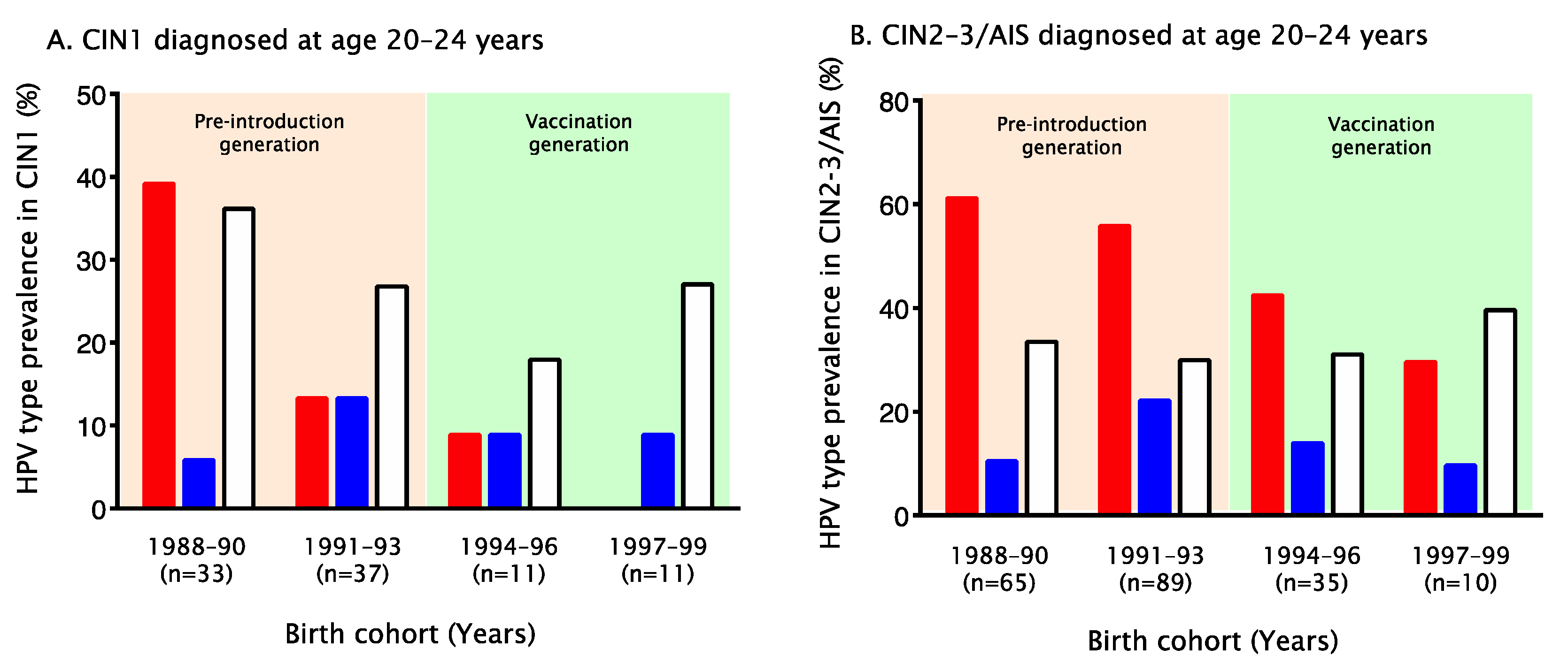
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yagi, A.; Ueda, Y.; Egawa-Takata, T.; Tanaka, Y.; Nakae, R.; Morimoto, A.; Terai, Y.; Ohmichi, M.; Ichimura, T.; Sumi, T.; et al. Realistic fear of cervical cancer risk in Japan depending on birth year. Hum. Vaccines Immunother. 2017, 13, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Hanley, S.J.; Yoshioka, E.; Ito, Y.; Kishi, R. HPV vaccination crisis in Japan. Lancet 2015, 385, 2571. [Google Scholar] [CrossRef]
- Ozawa, N.; Ito, K.; Tase, T.; Metoki, H.; Yaegashi, N. Beneficial Effects of Human Papillomavirus Vaccine for Prevention of Cervical Abnormalities in Miyagi, Japan. Tohoku J. Exp. Med. 2016, 240, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Shirasawa, H.; Shimizu, D.; Sato, N.; Ooyama, N.; Takahashi, O.; Terada, Y. Preventive effect of human papillomavirus vaccination on the development of uterine cervical lesions in young Japanese women. J. Obstet. Gynaecol. Res. 2017, 43, 1597–1601. [Google Scholar] [CrossRef]
- Ueda, Y.; Yagi, A.; Nakayama, T.; Hirai, K.; Ikeda, S.; Sekine, M.; Miyagi, E.; Enomoto, T. Dynamic changes in Japan’s prevalence of abnormal findings in cervical cervical cytology depending on birth year. Sci. Rep. 2018, 8, 5612. [Google Scholar] [CrossRef]
- Konno, R.; Konishi, H.; Sauvaget, C.; Ohashi, Y.; Kakizoe, T. Effectiveness of HPV vaccination against high grade cervical lesions in Japan. Vaccine 2018, 36, 7913–7915. [Google Scholar] [CrossRef]
- Ikeda, S.; Ueda, Y.; Hara, M.; Yagi, A.; Kitamura, T.; Kitamura, Y.; Konishi, H.; Kakizoe, T.; Sekine, M.; Enomoto, T.; et al. Human papillomavirus vaccine to prevent cervical intraepithelial neoplasia in Japan: A nationwide case-control study. Cancer Sci. 2021, 112, 839–846. [Google Scholar] [CrossRef]
- Yagi, A.; Ueda, Y.; Nakagawa, S.; Masuda, T.; Miyatake, T.; Ikeda, S.; Abe, H.; Hirai, K.; Sekine, M.; Miyagi, E.; et al. A nationwide birth year-by-year analysis of effectiveness of HPV vaccine in Japan. Cancer Sci. 2021, 112, 3691–3698. [Google Scholar] [CrossRef]
- Kudo, R.; Yamaguchi, M.; Sekine, M.; Adachi, S.; Ueda, Y.; Miyagi, E.; Hara, M.; Hanley, S.J.B.; Enomoto, T. Bivalent human papillomavirus vaccine effectiveness in a Japanese population: High vaccine-type-specific effectiveness and evidence of cross-protection. J. Infect. Dis. 2019, 219, 382–390. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Tabrizi, S.N.; Brotherton, J.M.; Kaldor, J.M.; Skinner, S.R.; Liu, B.; Bateson, D.; McNamee, K.; Garefalakis, M.; Phillips, S.; Cummins, E.; et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: A repeat cross-sectional study. Lancet Infect. Dis. 2014, 14, 958–966. [Google Scholar] [CrossRef]
- Oliver, S.E.; Unger, E.R.; Lewis, R.; McDaniel, D.; Gargano, J.W.; Steinau, M.; Markowitz, L.E. Prevalence of Human Papillomavirus Among Females After Vaccine Introduction–National Health and Nutrition Examination Survey, United States, 2003–2014. J. Infect. Dis. 2017, 216, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Hoes, J.; Woestenberg, P.J.; Bogaards, J.A.; King, A.J.; de Melker, H.E.; Berkhof, J.; Hoebe, C.J.P.A.; van der Sande, M.A.B.; van Benthem, B.H.B. Medical Microbiological Laboratories and Public Health Services. Population Impact of Girls-Only Human Papillomavirus 16/18 Vaccination in The Netherlands: Cross-Protective and Second-Order Herd Effects. Clin. Infect. Dis. 2021, 72, e103–e111. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yaegashi, N.; Iwata, T.; Ariyoshi, K.; Fujiwara, K.; Shiroyama, Y.; Usami, T.; Kawano, Y.; Horie, K.; Kawano, K.; et al. Monitoring the impact of a national HPV vaccination program in Japan (MINT Study): Rationale, design and methods. Jpn. J. Clin. Oncol. 2014, 44, 1000–1003. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumoto, K.; Yaegashi, N.; Iwata, T.; Yamamoto, K.; Nagashima, M.; Saito, T.; Ushijima, K.; Takahashi, F.; Noda, K.; Yoshikawa, H. Early impact of the Japanese immunization program implemented before the HPV vaccination crisis. Int. J. Cancer 2017, 141, 1704–1706. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yaegashi, N.; Iwata, T.; Yamamoto, K.; Aoki, Y.; Okadome, M.; Ushijima, K.; Kamiura, S.; Takehara, K.; Horie, K.; et al. Reduction in HPV16/18 prevalence among young women with high-grade cervical lesions following the Japanese HPV vaccination program. Cancer Sci. 2019, 110, 3811–3820. [Google Scholar] [CrossRef]
- Onuki, M.; Matsumoto, K.; Iwata, T.; Yamamoto, K.; Aoki, Y.; Maenohara, S.; Tsuda, N.; Kamiura, S.; Takehara, K.; Horie, K.; et al. Human papillomavirus genotype contribution to cervical cancer and precancer: Implications for screening and vaccination in Japan. Cancer Sci. 2020, 111, 2546–2557. [Google Scholar] [CrossRef]
- Gravitt, P.E.; Peyton, C.L.; Alessi, T.Q.; Alessi, T.Q.; Wheeler, C.M.; Coutlée, F.; Hildesheim, A.; Schiffman, M.H.; Scott, D.R.; Apple, R.J. Improved amplification of genital human papillomaviruses. J. Clin. Microbiol. 2000, 38, 357–361. [Google Scholar] [CrossRef]
- Kukimoto, I.; Matsumoto, K.; Takahashi, F.; Iwata, T.; Tanaka, K.; Yamaguchi-Naka, M.; Yamamoto, K.; Yahata, H.; Nakabayashi, M.; Kato, H.; et al. Human Papillomavirus (HPV) Genotyping Assay Suitable for Monitoring the Impact of the 9-Valent HPV Vaccine. Tohoku J. Exp. Med. 2020, 251, 287–294. [Google Scholar] [CrossRef]
- Woestenberg, P.J.; Bogaards, J.A.; King, A.J.; Leussink, S.; van der Sande, M.A.; Hoebe, C.J.; van Benthem, B.H. Assessment of herd effects among women and heterosexual men after girls-only HPV16/18 vaccination in the Netherlands: A repeated cross-sectional study. Int. J. Cancer. 2019, 144, 2718–2727. [Google Scholar] [CrossRef]
- Onuki, M.; Matsumoto, K.; Satoh, T.; Oki, A.; Okada, S.; Minaguchi, T.; Ochi, H.; Nakao, S.; Someya, K.; Yamada, N.; et al. Human papillomavirus infections among Japanese women: Age-related prevalence and type-specific risk for cervical cancer. Cancer Sci. 2009, 100, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Woestenberg, P.J.; King, A.J.; van Benthem, B.H.B.; Donken, R.; Leussink, S.; van der Klis, F.R.M.; de Melker, H.E.; van der Sande, M.A.B.; Hoebe, C.J.P.A.; Bogaards, J.A.; et al. Bivalent Vaccine Effectiveness Against Type-Specific HPV Positivity: Evidence for Cross-Protection Against Oncogenic Types Among Dutch STI Clinic Visitors. J. Infect. Dis. 2018, 217, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Sekine, M.; Kudo, R.; Adachi, S.; Ueda, Y.; Miyagi, E.; Hara, M.; Hanley, S.J.B.; Enomoto, T. Differential misclassification between self-reported status and official HPV vaccination records in Japan: Implications for evaluating vaccine safety and effectiveness. Papillomavirus Res. 2018, 6, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Rolnick, S.J.; Parker, E.D.; Nordin, J.D.; Hedblom, B.D.; Wei, F.; Kerby, T.; Jackson, J.M.; Crain, A.L.; Euler, G. Self-report compared to electronic medical record across eight adult vaccines: Do results vary by demographic factors? Vaccine 2013, 31, 3928–3935. [Google Scholar] [CrossRef]
| CIN1 | CIN2–3 or AIS | |
|---|---|---|
| (N = 754) | (N = 4297) | |
| Registration Year | ||
| 2012 | 58 | 172 |
| 2013 | 98 | 586 |
| 2014 | 94 | 602 |
| 2015 | 98 | 599 |
| 2016 | 95 | 642 |
| 2017 | 100 | 612 |
| 2019 | 21 | 78 |
| 2020 | 125 | 638 |
| 2021 | 65 | 368 |
| Age at Registration (Years) | ||
| 20–24 | 95 | 210 |
| 25–29 | 199 | 923 |
| 30–34 | 251 | 1596 |
| 35–39 | 209 | 1568 |
| Birth Cohort | ||
| 1973–1975 | 43 | 266 |
| 1976–1978 | 83 | 636 |
| 1979–1981 | 114 | 800 |
| 1982–1984 | 139 | 971 |
| 1985–1987 | 133 | 732 |
| 1988–1990 | 114 | 514 |
| 1991–1993 | 97 | 287 |
| 1994–1996 | 17 | 70 |
| 1997–1999 | 11 | 10 |
| 2000– | 3 | 11 |
| HPV Genotypes | ||
| Oncogenic * | 527 | 3932 |
| HPV16 | 101 | 1753 |
| HPV18 | 42 | 323 |
| Non-oncogenic | 118 | 159 |
| Negative | 109 | 206 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onuki, M.; Yamamoto, K.; Yahata, H.; Kanao, H.; Horie, K.; Konnai, K.; Nio, A.; Takehara, K.; Kamiura, S.; Tsuda, N.; et al. Changes in HPV16/18 Prevalence among Unvaccinated Women with Cervical Intraepithelial Neoplasia in Japan: Assessment of Herd Effects following the HPV Vaccination Program. Vaccines 2022, 10, 188. https://doi.org/10.3390/vaccines10020188
Onuki M, Yamamoto K, Yahata H, Kanao H, Horie K, Konnai K, Nio A, Takehara K, Kamiura S, Tsuda N, et al. Changes in HPV16/18 Prevalence among Unvaccinated Women with Cervical Intraepithelial Neoplasia in Japan: Assessment of Herd Effects following the HPV Vaccination Program. Vaccines. 2022; 10(2):188. https://doi.org/10.3390/vaccines10020188
Chicago/Turabian StyleOnuki, Mamiko, Kasumi Yamamoto, Hideaki Yahata, Hiroyuki Kanao, Koji Horie, Katsuyuki Konnai, Ai Nio, Kazuhiro Takehara, Shoji Kamiura, Naotake Tsuda, and et al. 2022. "Changes in HPV16/18 Prevalence among Unvaccinated Women with Cervical Intraepithelial Neoplasia in Japan: Assessment of Herd Effects following the HPV Vaccination Program" Vaccines 10, no. 2: 188. https://doi.org/10.3390/vaccines10020188
APA StyleOnuki, M., Yamamoto, K., Yahata, H., Kanao, H., Horie, K., Konnai, K., Nio, A., Takehara, K., Kamiura, S., Tsuda, N., Takei, Y., Shigeta, S., Nakai, H., Yoshida, H., Motohara, T., Kato, T., Nakamura, K., Hamanishi, J., Tasaka, N., ... for the MINT Study Group. (2022). Changes in HPV16/18 Prevalence among Unvaccinated Women with Cervical Intraepithelial Neoplasia in Japan: Assessment of Herd Effects following the HPV Vaccination Program. Vaccines, 10(2), 188. https://doi.org/10.3390/vaccines10020188






