Immune Response to SARS-CoV-2 mRNA Vaccines in an Open-Label Multicenter Study in Participants with Relapsing Multiple Sclerosis Treated with Ofatumumab
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Participants, and Treatments
2.2. Outcomes and Assessments
2.3. Administrative Procedures
2.4. Statistical Methods
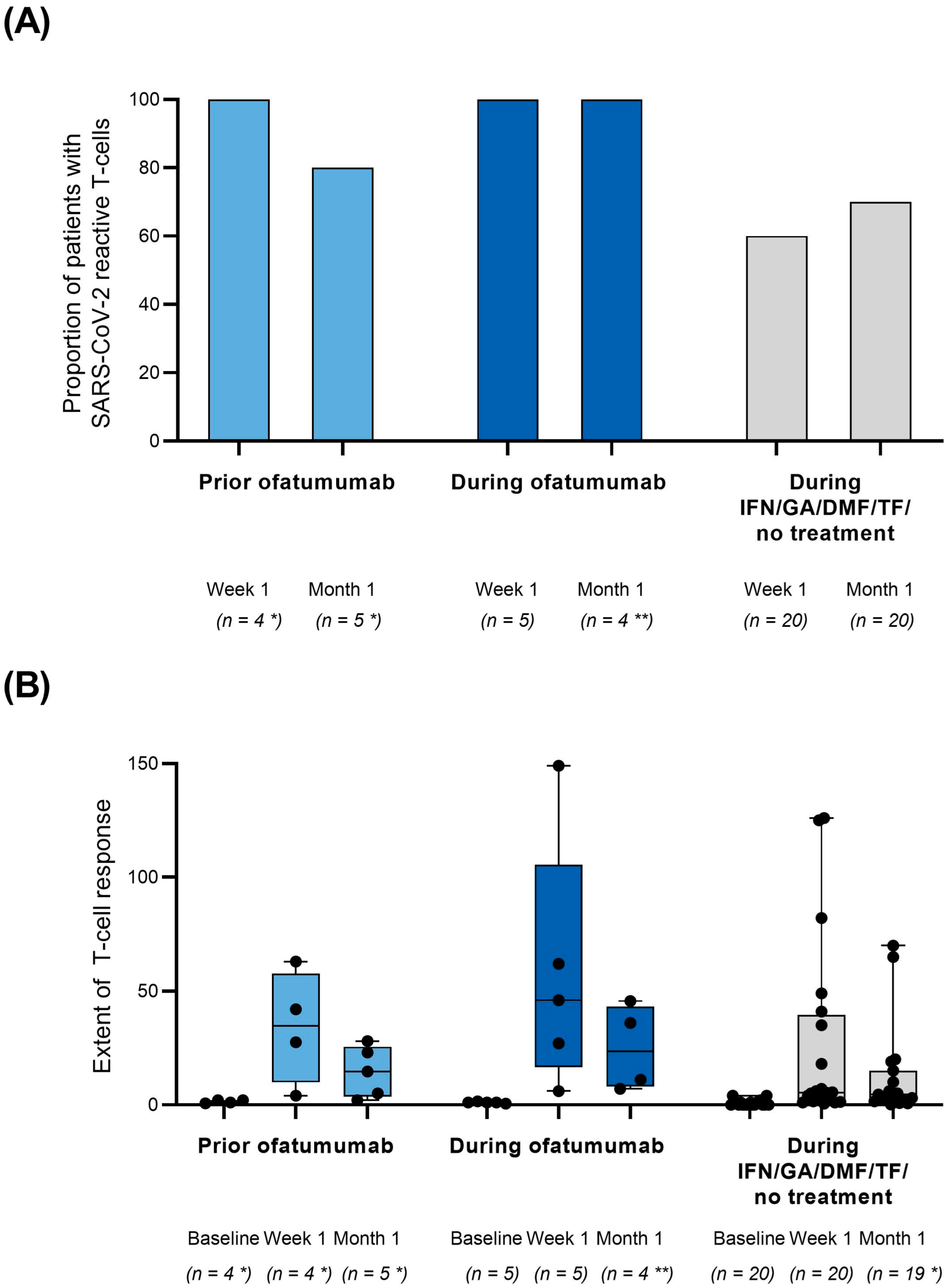
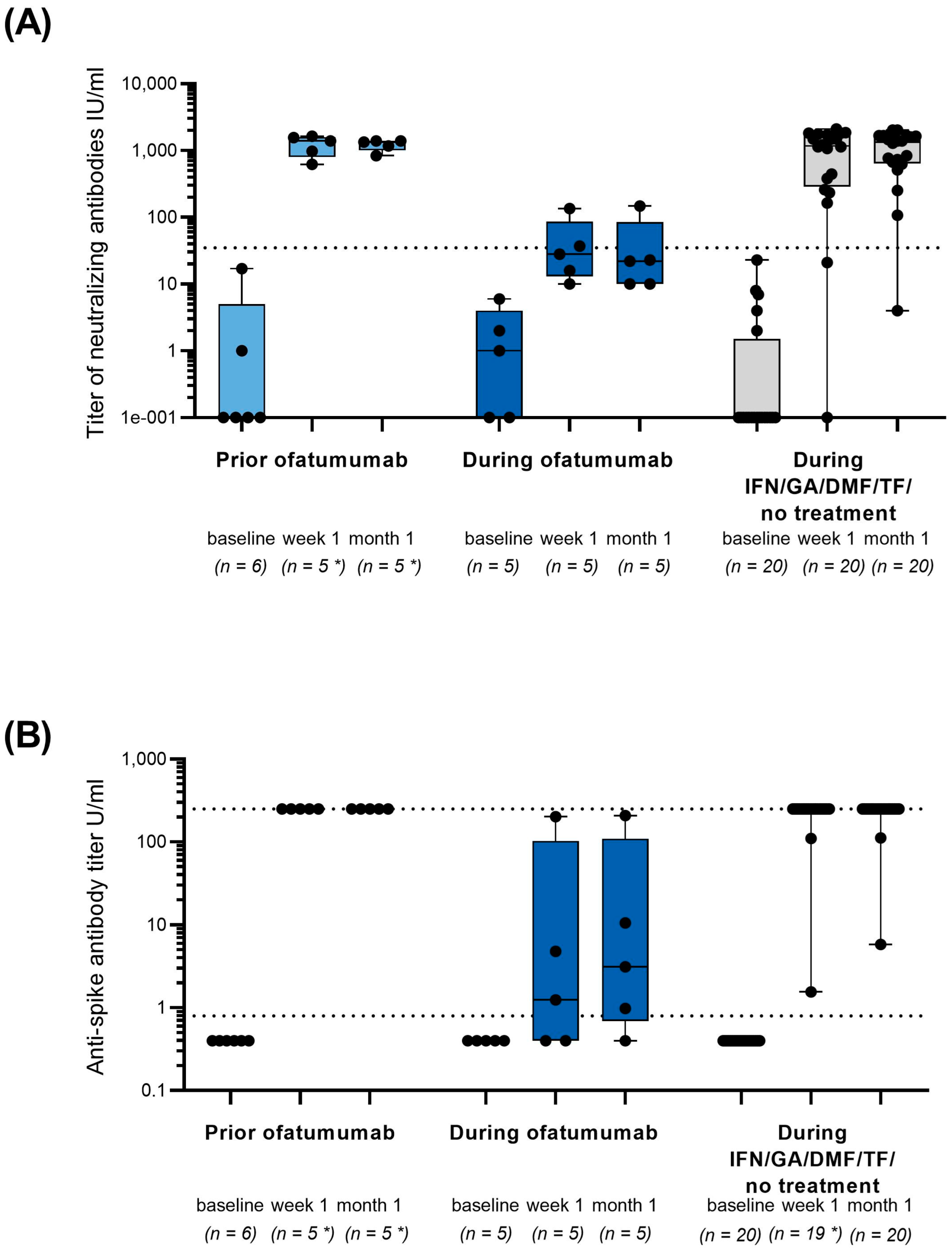
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gram, M.A.; Emborg, H.-D.; Schelde, A.B.; Friis, N.U.; Nielsen, K.F.; Moustsen-Helms, I.R.; Legarth, R.; Lam, J.U.H.; Chaine, M.; Malik, A.Z.; et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: A nationwide Danish cohort study. PLoS Med. 2022, 19, e1003992. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.W.; Rojas, O.L.; Gommerman, J.L. B cell depletion therapies in autoimmune disease: Advances and mechanistic insights. Nat. Rev. Drug Discov. 2021, 20, 179–199. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of Product Characteristics Kesimpta; European Medicines Agency: Amsterdam, The Netherlands, 2022.
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Calkwood, J.C.; Chognot, C.; Evershed, J.; Fox, E.J.; Herman, A.; Manfrini, M.; McNamara, J.; Robertson, D.S.; Stokmaier, D.; et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology 2020, 95, e1999–e2008. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cross, A.H.; Winthrop, K.; Wiendl, H.; Nicholas, J.; Meuth, S.G.; Giacomini, P.S.; Sacca, F.; Mancione, L.; Zielman, R.; et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult. Scler. 2022, 28, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Brochet, B.; Naismith, R.T.; Traboulsee, A.; Wolinsky, J.S.; Belachew, S.; Koendgen, H.; et al. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology 2020, 95, e1854–e1867. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Akondy, R.S. Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunol. Cell Biol. 2011, 89, 340–345. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Gallais, F.; Velay, A.; Nazon, C.; Wendling, M.J.; Partisani, M.; Sibilia, J.; Candon, S.; Fafi-Kremer, S. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg. Infect. Dis. 2021, 27, 113. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, M. What are the roles of antibodies versus a durable, high quality T-cell response in protective immunity against SARS-CoV-2? Vaccine X 2020, 6, 100076. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Groth, M.; Rauser, B.; Bopp, T. Assessing the immune response to SARS-CoV-2 mRNA vaccines in siponimod-treated patients: A nonrandomized controlled clinical trial (AMA-VACC). Ther. Adv. Neurol. Disord. 2022, 15, 17562864221135305. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Shen, L.; Tang, K. Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: A meta-analysis. EBioMedicine 2022, 81, 104102. [Google Scholar] [CrossRef]
- Toor, S.M.; Saleh, R.; Sasidharan Nair, V.; Taha, R.Z.; Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2021, 162, 30–43. [Google Scholar] [CrossRef]
- Woopen, C.; Dunsche, M.; Haase, R.; Raposo, C.; Pedotti, R.; Akgun, K.; Ziemssen, T. Timing of SARS-CoV-2 vaccination matters in people with multiple sclerosis on pulsed Anti-CD20 treatment. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200031. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Gutierrez, L.; Beckford, J.; Alachkar, H. Deciphering the TCR repertoire to solve the COVID-19 mystery. Trends Pharmacol. Sci. 2020, 41, 518–530. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Renia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Carrillo, J.; Izquierdo-Useros, N.; Avila-Nieto, C.; Pradenas, E.; Clotet, B.; Blanco, J. Humoral immune responses and neutralizing antibodies against SARS-CoV-2; implications in pathogenesis and protective immunity. Biochem. Biophys. Res. Commun. 2021, 538, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.Y.-L.; Pang, A.S.-R.; Chow, V.T.; Wang, D.-Y. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil. Med. Res. 2021, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Woopen, C.; Schleussner, K.; Akgun, K.; Ziemssen, T. Approach to SARS-CoV-2 vaccination in patients with multiple sclerosis. Front. Immunol. 2021, 12, 701752. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.H.; Delgado, S.; Habek, M.; Davydovskaya, M.; Ward, B.J.; Cree, B.A.C.; Totolyan, N.; Pingili, R.; Mancione, L.; Hu, X.; et al. COVID-19 outcomes and vaccination in people with relapsing multiple sclerosis treated with ofatumumab. Neurol. Ther. 2022, 11, 741–758. [Google Scholar] [CrossRef]
- Robert Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19). 23.09.2021–AKTUALISIERTER STAND FÜR DEUTSCHLAND; Robert Koch-Institut: Berlin, Germany, 2021.
- Robert Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19). 07.04.2022–AKTUALISIERTER STAND FÜR DEUTSCHLAND; Robert Koch-Institut: Berlin, Germany, 2022.
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- Adamec, I.; Rogic, D.; Penz, M.G.; Braun, C.; Habek, M. Humoral and cellular immunity in convalescent COVID-19 people with multiple sclerosis treated with ofatumumab. J. Neuroimmunol. 2022, 362, 577788. [Google Scholar] [CrossRef]
- Deutsche Multiple Sklerose Gesellschaft Bundesverband e.V. Update der Empfehlungen für Multiple-Sklerose-Erkrankte inklusive Update der Empfehlungen zur Corona-Schutzimpfung. Available online: https://www.dmsg.de/corona-virus-und-ms/multiple-sklerose-und-corona-virus-update-der-empfehlungen-des-dmsg-bundesverbandes (accessed on 10 October 2022).
| Variable * |
Cohort 1 Vaccination Prior to Treatment |
Cohort 2 Vaccination during Stable Treatment |
Cohort 3 Vaccination during IFN/GA/DMF/TF/ No DMT |
|---|---|---|---|
| N | 6 | 5 | 20 |
| Age, years | 32.5 (8.1) | 32.4 (7.7) | 48.6 (12.9) |
| Sex, female, n (%) | 5 (83.3) | 4 (80.0) | 16 (80.0) |
| Time since diagnosis, years | 2.7 (4.9) | 1.5 (1.9) | 13.99 (10.43) |
| Number of prior DMTs | 1.0 | 0.4 | 1.6 |
| Number of DMTs prior to ofatumumab, n (%) | |||
| 0 | 4 (66.7) | 3 (60.0) | n.a. |
| ≥1 | 2 (33.3) a | 2 (40.0) b | n.a. |
| Variable * |
Cohort 1 Vaccination Prior to Treatment |
Cohort 2 Vaccination during Stable Treatment |
Cohort 3 Vaccination during IFN/GA/DMF/TF/No DMT |
|---|---|---|---|
| N | 6 | 5 | 20 |
| Vaccination, n (%) | |||
| 1st (BioNTech/Pfizer | Moderna) | 5 (83.3) | 1 (16.7) | 5 (100.0) | 0 (0) | 19 (95.0) | 1 (5.0) |
| 2nd (BioNTech/Pfizer | Moderna) | 5 (83.3) | 1 (16.7) | 5 (100.0) | 0 (0) | 19 (95.0) | 1 (5.0) |
| Vaccination time interval, mean (SD) | |||
| 1st to 2nd vaccination, weeks/days | 3.0 (0.1) weeks | 3.4 (0.6) weeks | 36.8 (9.0) days |
| Adverse Events, n (%) | Cohort 1 Vaccination Prior to Treatment (n = 6) | Cohort 2 Vaccination during Stable Treatment (N = 5) |
|---|---|---|
| Adverse events (AEs) | 6 (100.0) | 5 (100.0) |
| General disorders and administration site conditions | 5 (83.3) | 3 (60.0) |
| Nervous system disorders | 5 (83.3) | 4 (80.0) |
| Musculoskeletal and connective tissue disorders | 1 (16.7) | 2 (40.0) |
| Blood and lymphatic system disorders | 1 (16.7) | 0 (0.0) |
| Infections and infestations | 3 (50.0) | 4 (80.0) |
| Ear and labyrinth disorders | 0 (0.0) | 0 (0.0) |
| Gastrointestinal disorders | 0 (0.0) | 1 (20.0) |
| Injury, poisoning, and procedural complications | 0 (0.0) | 0 (0.0) |
| Metabolism and nutrition disorders | 1 (16.7) | 0 (0.0) |
| Psychiatric disorders | 0 (0.0) | 0 (0.0) |
| Reproductive system and breast disorders | 0 (0.0) | 0 (0.0) |
| Respiratory, thoracic and mediastinal disorders | 2 (33.3) | 1 (20.0) |
| Skin and subcutaneous tissue disorders | 0 (0.0) | 1 (20.0) |
| Vascular disorders | 0 (0.0) | 0 (0.0) |
| Not coded | 0 (0.0) | 0 (0.0) |
| AEs related to DMTs | 2 (33.3) | 3 (60.0) |
| AEs related to SARS-CoV-2 vaccine | 3 (50.0) | 2 (40.0) |
| AEs leading to permanent discontinuation of study medication | 0 (0.0) | 0 (0.0) |
| AEs leading to temporary interruption of study medication | 0 (0.0) | 1 (20.0) |
| Serious adverse events | 0 (0.0) | 1 (20.0) |
| MS relapse | 0 (0.0) | 1 (20.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziemssen, T.; Groth, M.; Ettle, B.; Bopp, T. Immune Response to SARS-CoV-2 mRNA Vaccines in an Open-Label Multicenter Study in Participants with Relapsing Multiple Sclerosis Treated with Ofatumumab. Vaccines 2022, 10, 2167. https://doi.org/10.3390/vaccines10122167
Ziemssen T, Groth M, Ettle B, Bopp T. Immune Response to SARS-CoV-2 mRNA Vaccines in an Open-Label Multicenter Study in Participants with Relapsing Multiple Sclerosis Treated with Ofatumumab. Vaccines. 2022; 10(12):2167. https://doi.org/10.3390/vaccines10122167
Chicago/Turabian StyleZiemssen, Tjalf, Marie Groth, Benjamin Ettle, and Tobias Bopp. 2022. "Immune Response to SARS-CoV-2 mRNA Vaccines in an Open-Label Multicenter Study in Participants with Relapsing Multiple Sclerosis Treated with Ofatumumab" Vaccines 10, no. 12: 2167. https://doi.org/10.3390/vaccines10122167
APA StyleZiemssen, T., Groth, M., Ettle, B., & Bopp, T. (2022). Immune Response to SARS-CoV-2 mRNA Vaccines in an Open-Label Multicenter Study in Participants with Relapsing Multiple Sclerosis Treated with Ofatumumab. Vaccines, 10(12), 2167. https://doi.org/10.3390/vaccines10122167






