The Subunit AEC/BC02 Vaccine Combined with Antibiotics Provides Protection in Mycobacterium tuberculosis-Infected Guinea Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Bacteria
2.2. Antibiotics and Vaccines
2.3. Study Design
2.4. Guinea Pig Infection
2.5. Chemotherapy
2.6. Vaccination
2.7. Observation of General Condition
2.8. Gross Pathological Score and Bacterial Loads
2.9. Histopathological Examination
2.10. Drug Sensitivity Testing of Phenotypes
2.11. Drug Sensitivity Testing of Genotypes
2.12. Statistical Analysis
3. Results
3.1. Effects of Immunotherapy on the General Condition of Guinea Pigs
3.2. Effects of Different Immunotherapy Procedures on the Gross Pathological Score and Bacterial Loads
3.2.1. Effect of Different Administration Cycles
3.2.2. Effect of Different Immunization Times
3.3. Effects of Different Immunotherapy Procedures on Histological Changes
3.4. Effect of Short-Term Chemotherapy on Bacterial Resistance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2022; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 27 October 2022).
- Roberts, L. How COVID is derailing the fight against HIV, TB and malaria. Nature 2021, 597, 314. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, L.; Gong, W. In silico analysis of peptide-based biomarkers for the diagnosis and prevention of latent tuberculosis infection. Front. Microbiol. 2022, 13, 947852. [Google Scholar] [CrossRef]
- Carranza, C.; Pedraza-Sanchez, S.; De Oyarzabal-Mendez, E.; Torres, M. Diagnosis for latent tuberculosis infection: New alternatives. Front. Immunol. 2020, 11, 2006. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Wu, X. Differential diagnosis of latent tuberculosis infection and active tuberculosis: A key to a successful tuberculosis control strategy. Front. Microbiol. 2021, 12, 745592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P. A Perspective on the success and failure of BCG. Front. Immunol. 2021, 12, 778028. [Google Scholar] [CrossRef] [PubMed]
- Dockrell, H.M.; Smith, S.G. What have we learnt about BCG vaccination in the last 20 years? Front. Immunol. 2017, 8, 1134. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, C.R., Jr.; Barry, C.E., 3rd; Lange, C. Treatment of tuberculosis. N. Engl. J. Med. 2015, 373, 2149–2160. [Google Scholar] [CrossRef]
- Mitchison, D.; Davies, G. The chemotherapy of tuberculosis: Past, present and future. Int. J. Tuberc. Lung Dis. 2012, 16, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Hermans, S.M.; Zinyakatira, N.; Caldwell, J.; Cobelens, F.G.J.; Boulle, A.; Wood, R. High rates of recurrent tuberculosis disease: A population-level cohort study. Clin. Infect. Dis. 2021, 72, 1919–1926. [Google Scholar] [CrossRef]
- Hatherill, M.; White, R.G.; Hawn, T.R. Clinical development of new TB vaccines: Recent advances and next steps. Front. Microbiol. 2019, 10, 3154. [Google Scholar] [CrossRef]
- Vekemans, J.; Brennan, M.J.; Hatherill, M.; Schrager, L.; Fritzell, B.; Rutkowski, K.; de Vos, B.; Zignol, M.; Thiry, G.; Ginsberg, A.M.; et al. Preferred product characteristics for therapeutic vaccines to improve tuberculosis treatment outcomes: Key considerations from World Health Organization consultations. Vaccine 2020, 38, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bouzeyen, R.; Javid, B. Therapeutic vaccines for tuberculosis: An overview. Front. Immunol. 2022, 13, 878471. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Preferred Product Characteristics for Therapeutic Vaccines to Improve Tuberculosis Treatment Outcomes; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/WHO-IVB-19-05 (accessed on 14 June 2019).
- Coler, R.N.; Bertholet, S.; Pine, S.O.; Orr, M.; Reese, V.; Windish, H.P.; Davis, C.; Kahn, M.; Baldwin, S.L.; Reed, S.G. Therapeutic immunization against Mycobacterium tuberculosis is an effective adjunct to antibiotic treatment. J. Infect. Dis. 2013, 207, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Chahar, M.; Rawat, K.D.; Reddy, P.; Gupta, U.D.; Natrajan, M.; Chauhan, D.S.; Katoch, K.; Prasad, G.B.; Katoch, V.M. Potential of adjunctive Mycobacterium w (MIP) immunotherapy in reducing the duration of standard chemotherapy against tuberculosis. Indian J. Tuberc. 2018, 65, 335–344. [Google Scholar] [CrossRef]
- Aagaard, C.; Hoang, T.; Dietrich, J.; Cardona, P.-J.; Izzo, A.; Dolganov, G.; Schoolnik, G.K.; Cassidy, J.P.; Billeskov, R.; Andersen, P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 2011, 17, 189–194. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 15 October 2020).
- Lu, J.B.; Chen, B.W.; Wang, G.Z.; Fu, L.L.; Shen, X.B.; Su, C.; Du, W.X.; Yang, L.; Xu, M. Recombinant tuberculosis vaccine AEC/BC02 induces antigen-specific cellular responses in mice and protects guinea pigs in a model of latent infection. J. Microbiol. Immunol. Infect. 2015, 48, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, M.; Wang, Z.Y.; Chen, B.W.; Du, W.X.; Su, C.; Shen, X.; Zhao, A.; Dong, N.; Wang, Y.; et al. The development and preliminary evaluation of a new Mycobacterium tuberculosis vaccine comprising Ag85b, HspX and CFP-10:ESAT-6 fusion protein with CpG DNA and aluminum hydroxide adjuvants. FEMS Immunol. Med. Microbiol. 2010, 59, 42–52. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/i/item/9789241514842 (accessed on 23 October 2018).
- World Health Organization. Guidance for the Surveillance of Drug Resistance in Tuberculosis, 6th ed.; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240018020 (accessed on 19 April 2021).
- Trollfors, B.; Sigurdsson, V.; Dahlgren-Aronsson, A. Prevalence of latent TB and effectiveness of BCG vaccination against latent tuberculosis: An observational study. Int. J. Infect. Dis. 2021, 109, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Cardona, P.-J.; Amat, I.; Gordillo, S.; Arcos, V.; Guirado, E.; Díaz, J.; Vilaplana, C.; Tapia, G.; Ausina, V. Immunotherapy with fragmented Mycobacterium tuberculosis cells increases the effectiveness of chemotherapy against a chronical infection in a murine model of tuberculosis. Vaccine 2005, 23, 1393–1398. [Google Scholar] [CrossRef]
- Cardona, P.J. The progress of therapeutic vaccination with regard to tuberculosis. Front. Microbiol. 2016, 7, 1536. [Google Scholar] [CrossRef]
- Guirado, E.; Gil, O.; Cáceres, N.; Singh, M.; Vilaplana, C.; Cardona, P.-J. Induction of a specific strong polyantigenic cellular immune response after short-term chemotherapy controls bacillary reactivation in murine and guinea pig experimental models of tuberculosis. Clin. Vaccine Immunol. 2008, 15, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, S.A.; Painter, H.; Zelmer, A.; Smith, S.G.; Seifert, K.; Amat, M.; Cardona, P.J.; Fletcher, H.A. RUTI vaccination enhances inhibition of mycobacterial growth ex vivo and induces a shift of monocyte phenotype in mice. Front. Immunol. 2019, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, C.; Montané, E.; Pinto, S.; Barriocanal, A.M.; Domenech, G.; Torres, F.; Cardona, P.J.; Costa, J. Double-blind, randomized, placebo-controlled Phase I Clinical Trial of the therapeutical antituberculous vaccine RUTI. Vaccine 2010, 28, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Nell, A.S.; D’lom, E.; Bouic, P.; Sabaté, M.; Bosser, R.; Picas, J.; Amat, M.; Churchyard, G.; Cardona, P.J. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: Randomized, placebo-controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS ONE 2014, 9, e89612. [Google Scholar] [CrossRef]
- Cardona, P.J. RUTI: A new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis 2006, 86, 273–289. [Google Scholar] [CrossRef]
- Cardona, P.J.; Amat, I. Origin and development of RUTI, a new therapeutic vaccine against Mycobacterium tuberculosis infection. Arch. Bronconeumol. 2006, 42, 25–32. [Google Scholar] [CrossRef]
- Lu, J.; Guo, X.; Wang, C.; Du, W.; Shen, X.; Su, C.; Wu, Y.; Xu, M. Therapeutic effect of subunit vaccine AEC/BC02 on Mycobacterium tuberculosis post-chemotherapy relapse using a latent infection murine model. Vaccines 2022, 10, 825. [Google Scholar] [CrossRef]
- Islam, M.; Hameed, H.A.; Mugweru, J.; Chhotaray, C.; Wang, C.; Tan, Y.; Liu, J.; Li, X.; Tan, S.; Ojima, I.; et al. Drug resistance mechanisms and novel drug targets for tuberculosis therapy. J. Genet. Genom. 2017, 44, 21–37. [Google Scholar] [CrossRef]
- Gonzalez-Juarrero, M. Immunity to TB and targets for immunotherapy. Immunotherapy 2012, 4, 187–199. [Google Scholar] [CrossRef]



| Group Number | Group | n |
|---|---|---|
| 1 | INH-RFP (2 w) + AEC/BC02 (3 doses) | 6 |
| 2 | INH-RFP (4 w) + AEC/BC02 (3 doses) | 6 |
| 3 | INH-RFP (2 w) + AEC/BC02 (6 doses) | 6 |
| 4 | INH-RFP (4 w) + AEC/BC02 (6 doses) | 6 |
| 5 | INH-RFP (2 w) | 6 |
| 6 | INH-RFP (4 w) | 6 |
| 7 | AEC/BC02 (3 doses) | 6 |
| 8 | AEC/BC02 (6 doses) | 6 |
| 9 | NS | 6 |
| Degree of Lesion | Liver | Spleen | Lung |
|---|---|---|---|
| None | 0 | 0 | 0 |
| Mild | 10 | 10 | 10 |
| Moderate | 20 | 20 | 20 |
| Severe | 25 | 35 | 30 |
| Group | Spleen | Lung | Liver |
|---|---|---|---|
| INH-RFP (2 w) + AEC/BC02 (3 doses) |  Arrows indicate granulomatous lesions |  Arrows indicate granulomatous lesions |  Arrows indicate granulomatous lesions |
| INH-RFP (4 w) + AEC/BC02 (3 doses) |  No granulomatous lesions |  Arrows indicate granulomatous lesions |  Arrows indicate granulomatous lesions |
| INH-RFP (2 w) + AEC/BC02 (6 doses) | 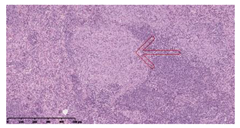 Arrows indicate granulomatous lesions |  Arrows indicate granulomatous lesions |  Arrows indicate granulomatous lesions |
| INH-RFP (4 w) + AEC/BC02 (6 doses) |  Arrows indicate granulomatous lesions |  Arrows indicate granulomatous lesions |  Arrows indicate granulomatous lesions |
| INH-RFP (2 w) |  Extensive granulomatous lesions |  Extensive granulomatous lesions |  Arrows indicate granulomatous lesions |
| INH-RFP (4 w) |  Granulomatous lesions in the middle |  Extensive granulomatous lesions |  Arrows indicate granulomatous lesions |
| AEC/BC02 (3 doses) |  Extensive granulomatous lesions |  Large granulomas lesions on both sides |  Extensive granulomatous lesions |
| AEC/BC02 (6 doses) |  Extensive granulomatous lesions |  Extensive granulomatous lesions |  Extensive granulomatous lesions |
| NS |  Extensive granulomatous lesions |  Extensive granulomatous lesions |  Extensive granulomatous lesions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Lu, J.; Li, J.; Du, W.; Shen, X.; Su, C.; Wu, Y.; Zhao, A.; Xu, M. The Subunit AEC/BC02 Vaccine Combined with Antibiotics Provides Protection in Mycobacterium tuberculosis-Infected Guinea Pigs. Vaccines 2022, 10, 2164. https://doi.org/10.3390/vaccines10122164
Guo X, Lu J, Li J, Du W, Shen X, Su C, Wu Y, Zhao A, Xu M. The Subunit AEC/BC02 Vaccine Combined with Antibiotics Provides Protection in Mycobacterium tuberculosis-Infected Guinea Pigs. Vaccines. 2022; 10(12):2164. https://doi.org/10.3390/vaccines10122164
Chicago/Turabian StyleGuo, Xiaonan, Jinbiao Lu, Junli Li, Weixin Du, Xiaobing Shen, Cheng Su, Yongge Wu, Aihua Zhao, and Miao Xu. 2022. "The Subunit AEC/BC02 Vaccine Combined with Antibiotics Provides Protection in Mycobacterium tuberculosis-Infected Guinea Pigs" Vaccines 10, no. 12: 2164. https://doi.org/10.3390/vaccines10122164
APA StyleGuo, X., Lu, J., Li, J., Du, W., Shen, X., Su, C., Wu, Y., Zhao, A., & Xu, M. (2022). The Subunit AEC/BC02 Vaccine Combined with Antibiotics Provides Protection in Mycobacterium tuberculosis-Infected Guinea Pigs. Vaccines, 10(12), 2164. https://doi.org/10.3390/vaccines10122164






