Modulation of Nod-like Receptor Expression in the Thymus during Early Pregnancy in Ewes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. RNA Extraction and RT-qPCR Assay
2.3. Western Blot Analysis
2.4. Immunohistochemistry Analysis
2.5. Statistical Analysis
3. Results
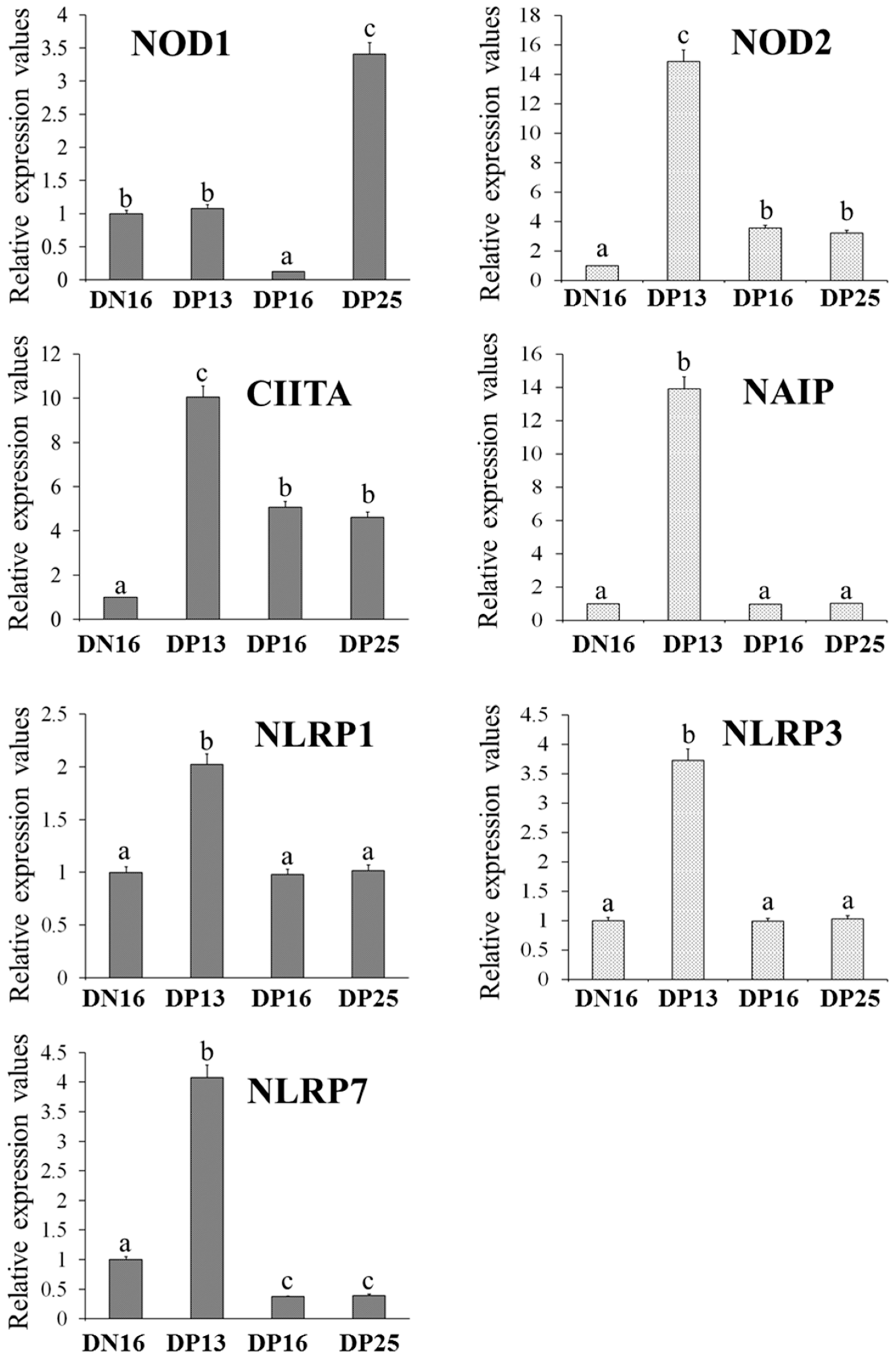
3.1. Relative Expression Levels of NOD1, NOD2, CIITA, NAIP, NLRP1, NLRP3 and NLRP7 mRNA in the Thymus
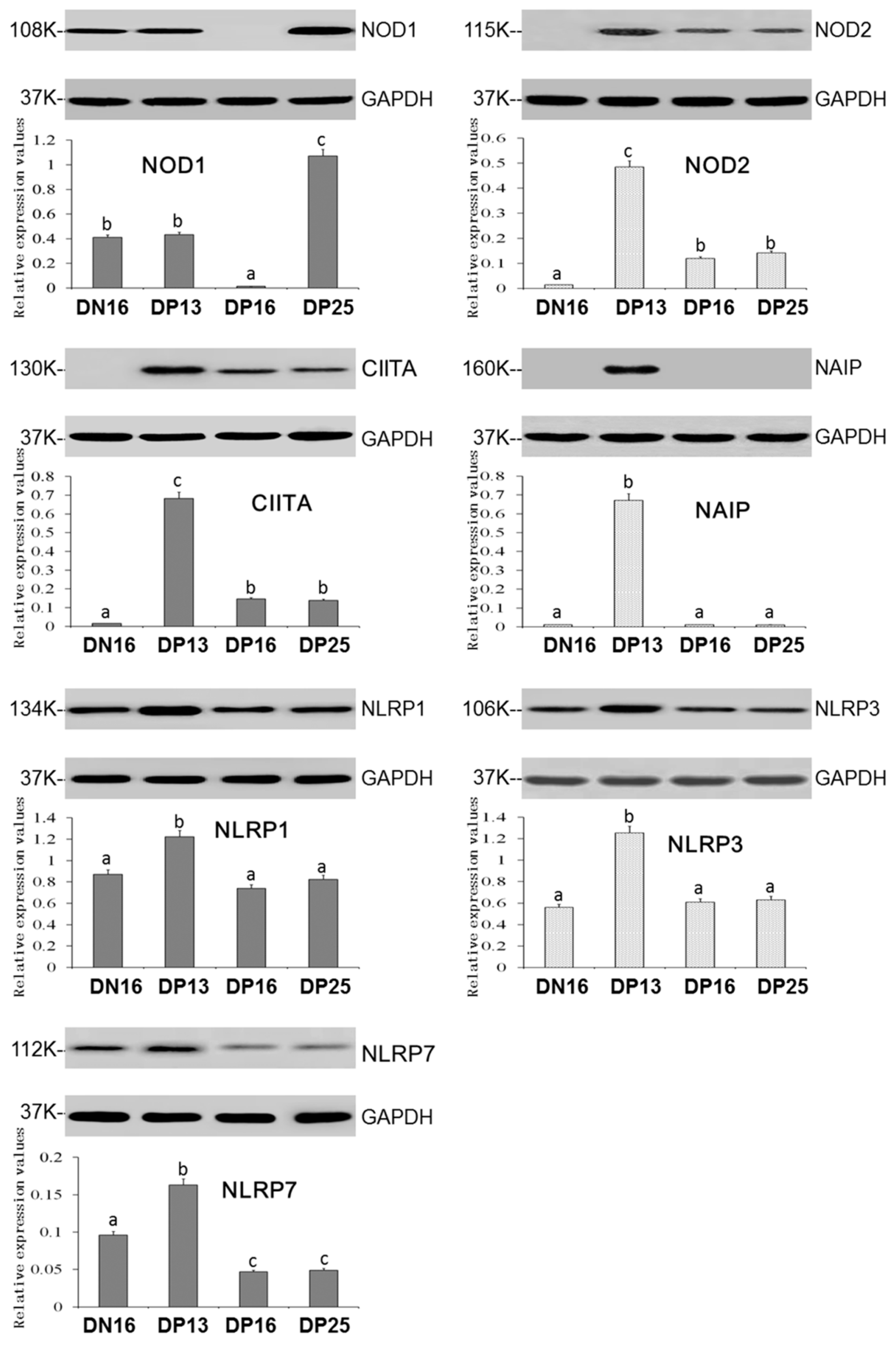
3.2. Expression of the NOD1, NOD2, CIITA, NAIP, NLRP1, NLRP3 and NLRP7 Proteins in the Thymus
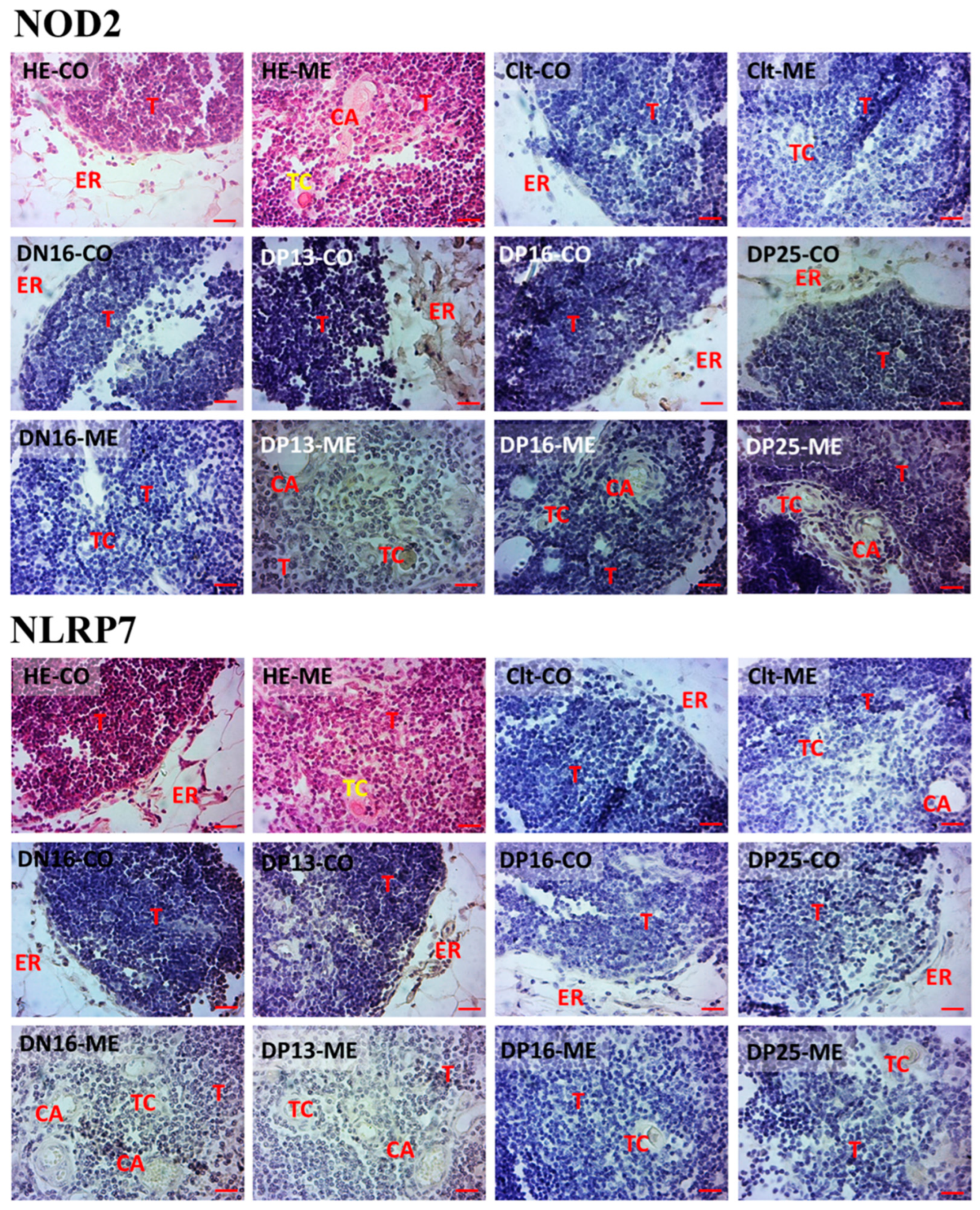
3.3. Immunohistochemistry for NOD2 and NLRP7 Proteins in the Thymus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Trindade, B.C.; Chen, G.Y. NOD1 and NOD2 in inflammatory and infectious diseases. Immunol. Rev. 2020, 297, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Tur, J.; Farrera, C.; Sánchez-Tilló, E.; Vico, T.; Guerrero-Gonzalez, P.; Fernandez-Elorduy, A.; Lloberas, J.; Celada, A. Induction of CIITA by IFN-γ in macrophages involves STAT1 activation by JAK and JNK. Immunobiology 2021, 226, 152114. [Google Scholar] [CrossRef] [PubMed]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) in innate immunity. TLRs, NLRs, and RLRs ligands as immunotherapeutic agents for hematopoietic diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef] [PubMed]
- Duéñez-Guzmán, E.A.; Haig, D. The evolution of reproduction-related NLRP genes. J. Mol. Evol. 2014, 78, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Lee, E.; Place, D.; Samir, P.; Mavuluri, J.; Sharma, B.R.; Balakrishnan, A.; Malireddi, R.K.S.; Geiger, R.; Zhu, Q.; et al. IRF8 regulates transcription of Naips for NLRC4 inflammasome activation. Cell 2018, 173, 920–933.e13. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, V.M. Pattern recognition at the maternal-fetal interface. Immunol. Investig. 2008, 37, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Burwick, R.M.; Lokki, A.I.; Fleming, S.D.; Regal, J.F. Editorial: Innate immunity in normal and adverse pregnancy. Front. Immunol. 2021, 12, 646596. [Google Scholar] [CrossRef]
- Rocha, C.C.; da Silveira, J.C.; Forde, N.; Binelli, M.; Pugliesi, G. Conceptus-modulated innate immune function during early pregnancy in ruminants: A review. Anim. Reprod. 2021, 18, e20200048. [Google Scholar] [CrossRef]
- Ott, T.L. Immunological detection of pregnancy: Evidence for systemic immune modulation during early pregnancy in ruminants. Theriogenology 2020, 150, 498–503. [Google Scholar] [CrossRef]
- Fiorenza, M.F.; Amaral, C.D.S.; da Anunciação, A.R.A.; Portela, V.V.M.; Marey, M.A.; Miyamoto, A.; Antoniazzi, A.Q. Possible impact of neutrophils on immune responses during early pregnancy in ruminants. Anim. Reprod. 2021, 18, e20210048. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, B.; Yan, X.; Zhang, L.; Gao, F.; Liu, Z. Expression of ISG15 in bone marrow during early pregnancy in ewes. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 767–772. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Mi, H.; Yan, J.K.; Yan, X.X.; Yang, L. Pregnancy-associated changes in expression of progesterone receptor and progesterone-induced blocking factor genes in bone marrow of ewes. Anim. Reprod. Sci. 2017, 186, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, J.; Wang, Q.; Lv, W.; Mi, H.; Liu, Y.; Yang, L. Changes in expression of ISG15, progesterone receptor and progesterone-induced blocking factor in ovine thymus during early pregnancy. Theriogenology 2018, 121, 153–159. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Wang, Y.; Li, N.; Cao, N.; Yang, L. Changes in expression of interferon-stimulated genes and ubiquitin activating enzyme E1-like in ovine thymus during early pregnancy. Anim. Reprod. 2020, 17, e20190134. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Lv, W.; Wang, P.; Wang, B.; Xue, J.; Zhang, L. Expression of interferon-stimulated gene 15-kDa protein, cyclooxygenase (COX) 1, COX-2, aldo-keto reductase family 1, member B1, and prostaglandin E synthase in the spleen during early pregnancy in sheep. Anim. Sci. J. 2018, 89, 1540–1548. [Google Scholar] [CrossRef]
- Yang, L.; Guo, R.; Yao, X.; Yan, J.; Bai, Y.; Zhang, L. Expression of progesterone receptor and progesterone-induced blocking factor in the spleen during early pregnancy in ewes. Livest. Sci. 2018, 209, 14–19. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Zhang, L.; Cao, N.; Cao, L.; Yang, L. Early pregnancy induces expression of STAT1, OAS1 and CXCL10 in ovine spleen. Animals 2019, 9, 882. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, L.; Yang, F.; Han, X.; Wang, Y.; Cao, N.; Yang, L. Relative abundance of interferon-stimulated genes STAT1, OAS1, CXCL10 and MX1 in ovine lymph nodes during early pregnancy. Anim. Reprod. Sci. 2020, 214, 106285. [Google Scholar] [CrossRef]
- Yang, L.; Zang, S.; Bai, Y.; Yao, X.; Zhang, L. Effect of early pregnancy on the expression of progesterone receptor and progesterone-induced blocking factor in ovine lymph node. Theriogenology 2017, 93, 78–83. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Q.; Liu, Y.; Zhang, L.; Lv, W.; Liu, B. Expression profiles of interferon-stimulated gene 15 and prostaglandin synthases in the ovine lymph nodes during early pregnancy. Mol. Reprod. Dev. 2019, 86, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F.A.P. The function of the thymus and its impact on modern medicine. Science 2020, 369, eaba2429. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, J.; Hidalgo, Y.; Sauma, D.; Rosemblatt, M.; Bono, M.R.; Núñez, S. The multifaceted roles of B cells in the thymus: From immune tolerance to autoimmunity. Front. Immunol. 2021, 12, 766698. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Koglgruber, R.; Cronin, S.J.F.; Uribesalgo, I.; Rauscher, E.; Harreiter, J.; Schuster, M.; Bancher-Todesca, D.; Pranjic, B.; Novatchkova, M.; et al. RANK links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy. Nature 2021, 589, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, S.; Mehta, R.B.; Forsberg, A.; Berg, G.; Brynhildsen, J.; Winqvist, O.; Jenmalm, M.C.; Ernerudh, J. Maintained thymic output of conventional and regulatory T cells during human pregnancy. J. Allergy Clin. Immunol. 2019, 143, 771–775.e7. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Mi, H.; Liu, B.; Wang, B.; Yang, L. Modulation of helper T cytokines in thymus during early pregnancy in ewes. Animals 2019, 9, 245. [Google Scholar] [CrossRef]
- Yang, L.; Lv, W.; Liu, Y.; Chen, K.; Xue, J.; Wang, Q.; Wang, B.; Zhang, L. Effect of early pregnancy on the expression of prostaglandin synthases in the ovine thymus. Theriogenology 2019, 136, 166–171. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, L.; Zhao, Z.; Li, N.; Wang, B.; Yang, L. Expression of melatonin receptors and CD4 in the ovine thymus, lymph node, spleen and liver during early pregnancy. Immunology 2020, 160, 52–63. [Google Scholar] [CrossRef]
- Cao, N.; Cao, L.; Gao, M.; Wang, H.; Zhang, L.; Yang, L. Changes in mRNA and protein levels of gonadotropin releasing hormone and receptor in ovine thymus, lymph node, spleen, and liver during early pregnancy. Domest. Anim. Endocrinol. 2021, 76, 106607. [Google Scholar] [CrossRef]
- Feng, P.; Wu, J.; Ren, Y.; Zhang, L.; Cao, J.; Yang, L. Early pregnancy regulates the expression of prolactin and its receptor in the thymus, the liver, the spleen and lymph nodes in sheep. Domest. Anim. Endocrinol. 2022, 81, 106731. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Cao, N.; Zhang, L.; Han, X.; Yang, L. Early pregnancy affects the expression of toll-like receptor pathway in ovine thymus. Reprod. Biol. 2020, 20, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Q.; Wang, H.; Feng, P.; Yang, G.; Yang, L. Effects of early pregnancy on the complement system in the ovine thymus. Vet. Res. Commun. 2022, 46, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Li, C.; Fu, S.; Yang, C.; Song, Y.; Liu, M.; Wang, Z.; Liang, P.; Zhang, J. NOD1 modulates decidual stromal cell function to maintain pregnancy in the early trimester. Cell. Biochem. Funct. 2019, 37, 464–473. [Google Scholar] [CrossRef]
- Ryu, B.J.; Han, J.W.; Kim, R.H.; Yun, S.; Kim, T.H.; Hur, S.E.; Kim, C.J.; Lee, S.K. Activation of NOD-1/JNK/IL-8 signal axis in decidual stromal cells facilitates trophoblast invasion. Am. J. Reprod. Immunol. 2017, 78, e12672. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, I.; Mulla, M.J.; Myrtolli, K.; Sfakianaki, A.K.; Norwitz, E.R.; Tadesse, S.; Guller, S.; Abrahams, V.M. Nod1 activation by bacterial iE-DAP induces maternal-fetal inflammation and preterm labor. J. Immunol. 2011, 187, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Gurugubelli Krishna, R.; Vishnu Bhat, B. Molecular mechanisms of intrauterine growth restriction. J. Matern. Fetal Neonatal. Med. 2018, 31, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Watanabe, T. NOD2, an intracellular innate immune sensor involved in host defense and Crohn’s disease. Mucosal Immunol. 2011, 4, 484–495. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Chen, H.; Sun, C.; Wang, H.Z.; Liu, M.L.; Li, Y.Y.; Nie, X.L.; Du, M.R.; Li, D.J.; Zhang, J.P. Expression and functional characterization of NOD2 in decidual stromal cells isolated during the first trimester of pregnancy. PLoS ONE 2014, 9, e99612. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Yang, C.; Fu, S.; Chen, X.; Zhang, S.; Li, Y.; Du, M.; Zhang, J. Different expression of NOD2 in decidual stromal cells between normal and unexplained recurrent spontaneous abortion women during first trimester gestation. Int. J. Clin. Exp. Pathol. 2014, 7, 8784–8790. [Google Scholar]
- Costello, M.J.; Joyce, S.K.; Abrahams, V.M. NOD protein expression and function in first trimester trophoblast cells. Am. J. Reprod. Immunol. 2007, 57, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Martinic, M.M.; Caminschi, I.; O’Keeffe, M.; Thinnes, T.C.; Grumont, R.; Gerondakis, S.; McKay, D.B.; Nemazee, D.; Gavin, A.L. The Bacterial Peptidoglycan-Sensing Molecules NOD1 and NOD2 Promote CD8+ Thymocyte Selection. J. Immunol. 2017, 198, 2649–2660. [Google Scholar] [CrossRef] [PubMed]
- LeibundGut-Landmann, S.; Waldburger, J.M.; Krawczyk, M.; Otten, L.A.; Suter, T.; Fontana, A.; Acha-Orbea, H.; Reith, W. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur. J. Immunol. 2004, 34, 1513–1525. [Google Scholar] [CrossRef]
- Yoo, I.; Kim, D.; Han, J.; Lee, S.; Hong, M.; Jeon, B.Y.; Kim, J.M.; Ka, H. Transcriptomic analysis of interferon-γ-regulated genes in endometrial explants and their possible role in regulating maternal endometrial immunity during the implantation period in pigs, a true epitheliochorial placentation species. Theriogenology 2020, 155, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, H.; Akizawa, H.; Sada, A.; Kishi, Y.; Yamanaka, K.; Takuma, T.; Sasaki, K.; Yamauchi, N.; Yanagawa, Y.; Nagano, M.; et al. Comparing spatial expression dynamics of bovine blastocyst under three different procedures: In-vivo, in-vitro derived, and somatic cell nuclear transfer embryos. Jpn. J. Vet. Res. 2015, 63, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.R.; Li, W.; Park, J.S.; Sofi, M.H.; Gourley, T.S.; Hangoc, G.; Kaplan, M.H.; Chang, C.H. Constitutive expression of CIITA directs CD4 T cells to produce Th2 cytokines in the thymus. Cell. Immunol. 2005, 233, 30–40. [Google Scholar] [CrossRef]
- Beug, S.T.; Cheung, H.H.; LaCasse, E.C.; Korneluk, R.G. Modulation of immune signalling by inhibitors of apoptosis. Trends Immunol. 2012, 33, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Rauch, I. The NAIP/NLRC4 inflammasome in infection and pathology. Mol. Aspects Med. 2020, 76, 100863. [Google Scholar] [CrossRef]
- Kofoed, E.M.; Vance, R.E. NAIPs: Building an innate immune barrier against bacterial pathogens. NAIPs function as sensors that initiate innate immunity by detection of bacterial proteins in the host cell cytosol. Bioessays 2012, 34, 589–598. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, X.; Ma, Y.; Li, J.; Liao, S.; Chen, J.; Wang, C. AK002210 promotes the proliferation, migration and invasion of trophoblast cell through regulating miR-590/NAIP signal axis. Arch. Biochem. Biophys. 2020, 688, 108366. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Sandstrom, A.; Vance, R.E. The NLRP1 inflammasome: New mechanistic insights and unresolved mysteries. Curr. Opin. Immunol. 2019, 60, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.P.; Leal, V.N.C.; Souza de Lima, D.; Reis, E.C.; Pontillo, A. Inflammasome genetics and complex diseases: A comprehensive review. Eur. J. Hum. Genet. 2020, 28, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.L.; Gomes, V.J.; Romao-Veiga, M.; Ribeiro, V.R.; Nunes, P.R.; Romagnoli, G.G.; Peracoli, J.C.; Peracoli, M.T.S. Silibinin downregulates the NF-κB pathway and NLRP1/NLRP3 inflammasomes in monocytes from pregnant women with preeclampsia. Molecules 2019, 24, 1548. [Google Scholar] [CrossRef] [PubMed]
- Meihe, L.; Shan, G.; Minchao, K.; Xiaoling, W.; Peng, A.; Xili, W.; Jin, Z.; Huimin, D. The ferroptosis-NLRP1 inflammasome: The vicious cycle of an adverse pregnancy. Front. Cell. Dev. Biol. 2021, 9, 707959. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the role of NLRP3 inflammasome in diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef]
- Mridha, A.R.; Wree, A.; Robertson, A.; Yeh, M.M.; Johnson, C.D.; Van Rooyen, D.M.; Haczeyni, F.; Teoh, N.C.; Savard, C.; Ioannou, G.N.; et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 2017, 66, 1037–1046. [Google Scholar] [CrossRef]
- Soczewski, E.; Grasso, E.; Gallino, L.; Hauk, V.; Fernández, L.; Gori, S.; Paparini, D.; Perez Leirós, C.; Ramhorst, R. Immunoregulation of the decidualization program: Focus on the endoplasmic reticulum stress. Reproduction 2020, 159, R203–R211. [Google Scholar] [CrossRef]
- Mulla, M.J.; Myrtolli, K.; Potter, J.; Boeras, C.; Kavathas, P.B.; Sfakianaki, A.K.; Tadesse, S.; Norwitz, E.R.; Guller, S.; Abrahams, V.M. Uric acid induces trophoblast IL-1β production via the inflammasome: Implications for the pathogenesis of preeclampsia. Am. J. Reprod. Immunol. 2011, 65, 542–548. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Q.; James, J.L.; Stone, P.R.; Chamley, L.W. IL-1 beta but not the NALP3 inflammasome is an important determinant of endothelial cell responses to necrotic/dangerous trophoblastic debris. Placenta 2015, 36, 1385–1392. [Google Scholar] [CrossRef]
- Yin, Y.; Yan, Y.; Jiang, X.; Mai, J.; Chen, N.C.; Wang, H.; Yang, X.F. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int. J. Immunopathol. Pharmacol. 2009, 22, 311–322. [Google Scholar] [CrossRef]
- Carriere, J.; Dorfleutner, A.; Stehlik, C. NLRP7: From inflammasome regulation to human disease. Immunology 2021, 163, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Abi Nahed, R.; Elkhoury Mikhael, M.; Reynaud, D.; Collet, C.; Lemaitre, N.; Michy, T.; Hoffmann, P.; Sergent, F.; Marquette, C.; Murthi, P.; et al. Role of NLRP7 in normal and malignant trophoblast cells. Biomedicines 2022, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Yu, P.H.; Li, Y.C.; Kuo, P.L. NLRP7 contributes to in vitro decidualization of endometrial stromal cells. Reprod. Biol. Endocrinol. 2017, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.Y.; Chen, K.R.; Li, Y.C.; Kuo, P.L. NLRP7 is involved in the differentiation of the decidual macrophages. Int. J. Mol. Sci. 2019, 20, 5994. [Google Scholar] [CrossRef]
- Radian, A.D.; de Almeida, L.; Dorfleutner, A.; Stehlik, C. NLRP7 and related inflammasome activating pattern recognition receptors and their function in host defense and disease. Microbes Infect. 2013, 15, 630–639. [Google Scholar] [CrossRef]
| Gene | Primer | Sequence | Size (bp) | Accession Numbers |
|---|---|---|---|---|
| NOD1 | Forward | CCTTGGCTGTCAGAGATTGGCTTC | 94 | XM_042248630.1 |
| Reverse | GCTTCTGGCTGTATCTGCTCACTG | |||
| NOD2 | Forward | TGCCATCCTCGCTCAGACATCTC | 117 | XM_042231601.1 |
| Reverse | CAGCCACACTGCCCTCTTTGC | |||
| CIITA | Forward | GCACCTCCTTCCAGTTCCTTGTTG | 119 | XM_042239890.1 |
| Reverse | CCTGTCCCAGTCCCTGAGATCG | |||
| NAIP | Forward | TTGTCCAGCAGTGTCAGCATCTTC | 82 | XM_012096791.3 |
| Reverse | ATTTCCACCACGCTGTCATCATCC | |||
| NLRP1 | Forward | AAGGAGGTGACCGAGATGCTGAG | 143 | XM_012185551.4 |
| Reverse | TGCCGCTTGAGTGAGGATGTATTG | |||
| NLRP3 | Forward | CTCTGGTTGGTCAGTTGCTGTCTC | 81 | XM_042250402.1 |
| Reverse | GGTCAGGGAATGGTTGGTGCTTAG | |||
| NLRP7 | Forward | GCCTGCTACTCGTTCATCCATCTC | 90 | XM_004015893.5 |
| Reverse | CCCTTCCTCCTCCTGCTCTTCC | |||
| GAPDH | Forward | GGGTCATCATCTCTGCACCT | 176 | NM_001190390.1 |
| Reverse | GGTCATAAGTCCCTCCACGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, Y.; Zhao, Z.; Cai, J.; Zhao, S.; Yang, L. Modulation of Nod-like Receptor Expression in the Thymus during Early Pregnancy in Ewes. Vaccines 2022, 10, 2128. https://doi.org/10.3390/vaccines10122128
Zhang L, Li Y, Zhao Z, Cai J, Zhao S, Yang L. Modulation of Nod-like Receptor Expression in the Thymus during Early Pregnancy in Ewes. Vaccines. 2022; 10(12):2128. https://doi.org/10.3390/vaccines10122128
Chicago/Turabian StyleZhang, Leying, Yuanjing Li, Zhenyang Zhao, Jiabao Cai, Shuxin Zhao, and Ling Yang. 2022. "Modulation of Nod-like Receptor Expression in the Thymus during Early Pregnancy in Ewes" Vaccines 10, no. 12: 2128. https://doi.org/10.3390/vaccines10122128
APA StyleZhang, L., Li, Y., Zhao, Z., Cai, J., Zhao, S., & Yang, L. (2022). Modulation of Nod-like Receptor Expression in the Thymus during Early Pregnancy in Ewes. Vaccines, 10(12), 2128. https://doi.org/10.3390/vaccines10122128





