Abstract
Metabolomics is emerging as a promising tool to understand the effect of immunometabolism for the development of novel host-directed alternative therapies. Immunometabolism can modulate both innate and adaptive immunity in response to pathogens and vaccinations. For instance, infections can affect lipid and amino acid metabolism while vaccines can trigger bile acid and carbohydrate pathways. Metabolomics as a vaccinomics tool, can provide a broader picture of vaccine-induced biochemical changes and pave a path to potentiate the vaccine efficacy. Its integration with other systems biology tools or treatment modes can enhance the cure, response rate, and control over the emergence of drug-resistant strains. Mycobacterium tuberculosis (Mtb) infection can remodel the host metabolism for its survival, while there are many biochemical pathways that the host adjusts to combat the infection. Similarly, the anti-TB vaccine, Bacillus Calmette-Guerin (BCG), was also found to affect the host metabolic pathways thus modulating immune responses. In this review, we highlight the metabolomic schema of the anti-TB vaccine and its therapeutic applications. Rewiring of immune metabolism upon BCG vaccination induces different signaling pathways which lead to epigenetic modifications underlying trained immunity. Metabolic pathways such as glycolysis, central carbon metabolism, and cholesterol synthesis play an important role in these aspects of immunity. Trained immunity and its applications are increasing day by day and it can be used to develop the next generation of vaccines to treat various other infections and orphan diseases. Our goal is to provide fresh insight into this direction and connect various dots to develop a conceptual framework.
1. Introduction
Before the COVID-19 pandemic, Tuberculosis (TB) an infection caused by Mycobacterium tuberculosis (Mtb) was the leading cause of death from a single infectious agent ranking above both Human Immunodeficiency Virus (HIV) and malaria. The situation has become even worse amid the COVID-19 pandemic which has disrupted the global health infrastructure; causing the number of deaths due to TB to rise for the first time in over 20 years [1,2]. Bacterial drug resistance is a growing concern as even newly introduced anti-TB drugs such as bedaquiline and delaminid are failing to fight the infection [3]. Long treatment regimens, rising cases of HIV-TB coinfection, and the rapid emergence of drug-resistant strains underscore the necessity for newer prophylactic and therapeutic modalities with less or no dependency on antibiotics. Inhibiting the transmission cycle of TB with novel effective vaccines have the potential to achieve the World Health Organization’s (WHO) ambitious goal of ending the TB epidemic by 2035 [4].
To date Bacillus Calmette-Guerin (BCG) is the only licensed vaccine against TB. First used in 1921, it was orally administered to a child in Paris by Dr. Benjamin Weill-Halle [5]. Although BCG vaccination in infants is moderately effective in preventing severe, extrapulmonary TB, it has shown varied efficacy in preventing TB in adolescents and adults in different clinical trials [6]. It is unclear what different physiological and metabolic factors account for these variable immune responses to the BCG vaccine.
Several studies have revealed extensive alterations in the host metabolism upon Mtb infection [7,8] and subsequent treatment [9,10] (Table 1). These changes represent not only the state or progression of the diseases but also the success of the therapeutic interventions. Analysis of host-directed metabolomics can profile the non-genomic and non-transcriptional aspects of these by providing a snapshot of the metabolites released in body fluids such as blood or urine. It has been used to screen specific metabolic identifiers of hypoxic metabolism and inflammation during Mtb infection [11,12,13,14,15].
A distinct metabolic reprogramming is observed in response to demands caused by various bacterial or viral infections as well as vaccinations [16]. Glycolysis, fatty acid biosynthesis, bile acid metabolism, sphingolipid, and sphingomyelin production, different amino acid metabolism and catabolism, creatine metabolism, and glutaminolysis are common metabolic pathways essential for the immune system’s response against Mtb, E. coli, influenza, SARS-CoV-2, chikungunya, and dengue (Figure 1) [15].
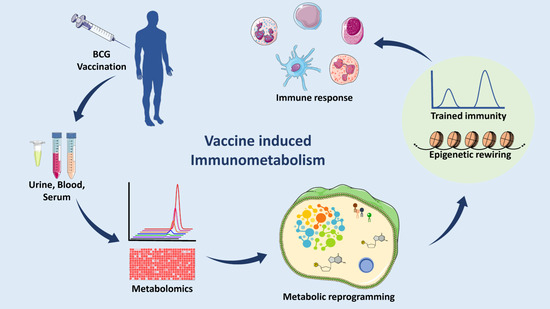
Figure 1.
Analyzing Immunometabolomic effects of TB vaccination. In a clinical setup, body fluids (such as blood serum or plasma and urine) would be collected and processed to run on a GC-MS or LC-MS to profile the levels of the metabolites and then analyzed various bioinformatic tools. Quantitative analysis shows the changes in different pathways as compared to placebo subjects. A correlation has been shown between metabolic remodeling, trained immunity, and epigenetics. These may potentiate the effect of the vaccine to mount a better immune response. (Abbreviations: GC-MS: Gas chromatography-mass spectrometry, LC-MS: Liquid chromatography-mass spectrometry.) (The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license).
It is well known that our immune system develops cellular memory upon infection and vaccination. However, the process by which this memory helps to protect against future exposures to unrelated pathogens is not well understood. A recent study observed the activation of innate immunity in an experiment where β-glucan (a component of C. albicans cell wall) challenged human monocytes and showed an ex vivo increase in cytokine synthesis in response to an unrelated stimulation [17,18]. There are some studies describing similar relationships of BCG vaccination with non-specific protection against different causes of mortality. This process of innate immune system adaptation was found to be associated with epigenetic changes in immune cells and is known as trained immunity [19,20]. A few studies have illuminated the effect of the BCG vaccine on host metabolism and its relationship with trained immunity. Specifically, changes in central carbon metabolism and lipid metabolic pathways have been linked to histone modification enzymes. In this review, we summarize the metabolic reprogramming that occurs due to the BCG vaccination, as well as its relationship with trained immunity, and strategies for the development of novel vaccines and applications. This discussion aims to describe TB pathogenesis and provide a condensed set of understandings of the host immunometabolism upon Mtb infection and vaccine administration (Figure 1).

Table 1.
Metabolic changes upon Mtb infection and BCG vaccine administration.
Table 1.
Metabolic changes upon Mtb infection and BCG vaccine administration.
| Metabolic Modulator | Sample Type | Metabolomics Technique Used | Affected Metabolites or Pathways | References |
|---|---|---|---|---|
| Mtb Infection | Blood serum, plasma, and urine | Gas chromatography-mass spectrometry, Liquid chromatography-mass spectrometry, Flow injection analysis-tandem mass spectrometry | Urea, sphingolipid, sphingosine-1-phosphate, sulfoxymethionine, fatty acid metabolism, sphingomyelins, phosphatidylcholines, lysophosphatidylcholines, amino-acyl tRNA, lysosome pathways, mannose metabolism, pyruvate, citrate, protein digestion pathways, asparagine, aspartate, citrulline, cysteine, lysine, leucine, methylamine, gamma-glutamylglutamine, glutamate, formate, glutamine, histidine, inosine, methionine, tryptophan, kynurenine, lactate, fatty acid beta-oxidation, itaconate, mycolic acids, phthiocerol dimycocerosate, glycerophosphocholine, nicotinate. | [7,8,21,22,23,24,25,26,27] |
| BCG Vaccination | Blood serum | Liquid chromatography-mass spectrometry | Purine biosynthesis, N6-carbomoyltheronyladenosine, glucose metabolism, alpha-ketobutyrate, 1,5-anhydroglucitol, methylguanine, fumarate, glutamate, glutamine, acetyl-CoA, lactate, glucose, nicotinamide adenine dinucleotide, 2-sulfotrehalose, trehalose 6-phosphate, mycobactin, 1-tuberculosynadenosine, conjugate-mycothiolhexadecanoyl-sn-glycero-3-phospho-(1′-myo-inositol), Hypoxanthine, para-aminobenzoic acid, lysophosphatidylcholines, lysophosphatidylethanolamines, sphingolipid metabolism, docosahexaenoic acid. | [9,10,14,28,29] |
2. Mtb: A Smart Pathogen
Mtb has coexisted with human hosts since our early hominid ancestors over 3 million years ago. The long period of co-evolution between Mtb and humans has led to the development of complex molecular interactions between the pathogen and the host’s immune system [30]. Mtb is admitted into alveolar macrophages via host C-type lectin, CD91, and membrane Toll-like receptors [TLRs], which recognize the mycobacterial molecular patterns, such as cell wall lipoglycans and lipoarabinomannan [31]. Mtb not only evades the body’s primary defenses against pathogen entry but also survives within-host immune cells. Inside macrophages, Mtb prevents normal processes, such as phagolysosome formation and antigen presentation [32], by secreting virulence proteins that affect host pathways like the endosomal sorting complex. Mtb virulence factors (such as ESAT-6, phenolic glycolipid, and phthiocerol dimycocerosates) further drive macrophage polarization [33]. Upon stimulation by pathogen exposure macrophages shift metabolic pathways, allocating recourses to attack the pathogen. Understanding the molecular and metabolic reprogramming occurring in Mtb and the host cells can enhance our understanding of Mtb survival techniques; and improve therapeutic development. Mtb employs multiple strategies to survive inside the macrophage. It first avoids phagosome digestion by interfering with phagosomal maturation markers, such as Rab GTPase, involved in membrane fusion events [34,35,36]. This can also prevent the acidification required for the activity of lysosomal enzymes [37,38,39]. Studies report that Mtb not only survives inside the macrophage but ensures contact with the cytosol using the ESX-1/T7S and phthiocerol dimycocerosates (DIM/PDIM) systems [40,41,42,43,44]. This cytosolic accessibility is an important aspect of the complex host-pathogen interaction as it provides the access to essential nutrients [45,46]. A better understanding of this cytosolic exposure mechanism of the pathogen can help to improve the protective efficacy of vaccine candidates. Mtb’s interference with lysosomal enzymes and host metabolism may also impact host cell apoptotic and necrotic pathways, further contributing to Mtb survival, replication, and dissemination [44,47,48,49].
Mtb infection can result in granuloma formation, wherein the bacterium is sequestered and prevented from causing infection, but it is also protected from host immune destruction [50]. Mtb displays self-regulated epigenetic changes when entering the latent stage, allowing for adjustment of metabolism and survival in low pH [51]; permitting Mtb to persist within the granuloma. Each site of granuloma formation provides a microenvironment that induces phenotypic and metabolic heterogeneity. Cavitation, when a granuloma penetrates the underlying tissue, can allow the pathogen to access oxygen and a privileged environment [52]. Relative adaptation to these stringent environments results in increased pathogenicity during secondary reactivation [53]. It has been reasoned that this plasticity is a result of Mtb’s long period of co-evolution with humans. Through the duration of the latent infection, CD4+ T-cells specifically produce a Th1 cytokine reaction that functions to maintain granuloma integrity. Later during TB infection, a more significant role is played by CD8+ T-cells, which regulate the lysis of infected cells. T-cells also activate macrophages by releasing Tumor Necrosis Factor Alpha (TNF-α) and Interferon gamma (IFN-γ) [54].
3. Host Metabolic Adaptation upon Mtb Infection
Along its timeline of evolution, Mtb has learned to survive within the human host through fine-tuning metabolism and counteracting the host immune system. Gluconeogenesis is the primary source of carbon for the bacterium in the host. Phosphoenolpyruvate carboxykinase (PEPCK) was found to play an essential role in bacterial survival during macrophage infection [55]. With the help of isotope labeling and metabolomic profiling, it was found that Mtb can simultaneously utilize multiple types of carbon substrates (such as glycerol, acetate, and dextrose) for sustained growth [21] (Table 1). Mtb favors fatty acid metabolism during the infection but can rely on many metabolic pathways [56,57]. The serum metabolome shows higher amounts of aspartate, sulfoxy methionine, and glutamate and lower levels of asparagine, methionine, and glutamine in patients with active TB as compared to patients with latent infections or healthy subjects [8]. Macrophages overexpress HIF-1α (hypoxia-inducible factor-1) which upregulates lactate dehydrogenase-A (LDH) and lowers pyruvate levels, thus reducing the availability of glucose for Mtb survival [7] (Table 1). Targeted metabolomics of human plasma has revealed that both active and latent Mtb enhance the catabolism of tryptophan to kynurenine, and it is reversed with the anti-TB treatment [22]. Increased tryptophan metabolism was linked to a CD4+ T cell response promoting immune tolerance and protecting the host from inflammation bursts [58]. Host phospholipase D was also found to play a key role in controlling the Mtb infection via Sphingosine 1-phosphate (S1P) metabolism [59] (Table 1).
4. BCG Vaccine: Immunometabolic Reprogramming and Trained Immunity
Calmette and Guerin first used a live-attenuated Mycobacterium bovis strain along with beef bile and found this resulted in protection against TB in bovine and rodent animal models [60,61]. Genomic studies further revealed that knocking out the region of deletion 1 (RD1) was associated with loss of Mtb virulence. This attenuated strain was termed BCG. RD1 is a 10.7 kb fragment containing 9 Open Reading Frames (ORFs) normally present in virulent Mtb and M. bovis strains. Genes present in the RD1 region encode for two major proteins of Mtb, ESAT-6, and CPF-10, parts of ESX1 secretion system. Although the non-virulent phenotype is predominantly associated with the deletion of the RD1 region, however, its reintroduction does not completely restore the virulence suggesting the role of other factors [62].
Heterogeneity between individuals causes some variation in the trained immune system and responses to BCG vaccination, however, the basis for these differences has not been well defined [63,64]. The composition of circulating metabolites impacts both innate and adaptive immune components and might contribute significantly towards variations observed in the immune responses to vaccines among individuals.
Many metabolites are associated with trained immunity. Particularly BCG vaccination led to an increase in glycolysis and glutamine metabolism (Figure 2, Table 1). Conversely, inhibiting glutaminolysis reduces the trained immune responses [65]. Fumarate is another key metabolite that accumulates in the primed cells following glutaminolysis. Specifically, it has been shown to facilitate trained immunity by inhibiting KDM5 histone demethylase activity and enhancing H3K4me3 at the promoters of immune genes [66]. BCG-induced trained immunity has been implicated in modulating the plasma concentration of succinate and malate, key intermediates of the TCA cycle, and glutamine and glutamate metabolism [10] (Table 1). Furthermore, succinate is implicated in innate immune signaling and promoting increased production of IL-1β in macrophages [67]. Overall, these studies highlight the critical role of metabolites in regulating the trained immune responses following BCG vaccination (Figure 2).
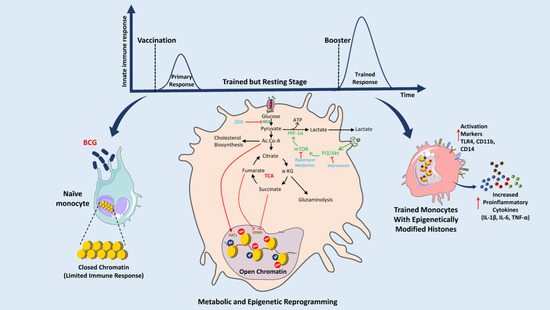
Figure 2.
Mechanism of BCG-induced metabolic reprogramming and its role in the activation of trained immunity through epigenetic modifications of histones. BCG stimulates innate immune cells such as naïve monocytes, macrophages, and natural killer (NK) cells. Phagolysosomal digestion of BCG induces PI3/Akt and mTOR signaling pathways which directly affect the expression of HIF1α thus modifying the glycolysis and the TCA cycle. Acetyl-CoA (Ac.CoA), fumarate, and succinate can affect the activity of HATs and KDMs and induce epigenetic modifications of histones. These modifications of histones result in better access to promoters of proinflammatory cytokines (e.g., IL-1β, IL-6, TNF-α) and program immune cells for the next encounter. Booster or second exposure results in an excessive amount of cytokines and activation of different markers (TLR4, CD11b, and CD14). This trained immunity can protect against many diseases like sepsis, type 1 diabetes mellitus, pneumonia, asthma, allergic rhinitis, melanoma, etc. (Abbreviations: BCG: Bacillus Calmette-Guérin, PI3: Phosphatidylinositol 3-kinase, Akt: Serine/Threonine protein kinase B, mTOR: mammalian Target of Rapamycin, HIF1α: Hypoxia Inducible Factor 1 Subunit Alpha, ATP: Adenosine triphosphate, TCA: Tricarboxylic Acid cycle, IL-1β: Interleukin-1 beta, IL-6: Interleukin 6, TNF-α: Tumor necrosis factor alpha, TLR4: Toll-Like Receptor 4, CD11b: Cluster of Differentiation molecule 11B, CD14: Cluster of Differentiation 14, 2DG: 2-Deoxy-d-glucose, Ac.CoA: Acetyl coenzyme A, Me: Methylation, HAT: Histone Acetyltransferases, KDMs: Lysine Demethylases.) (The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.).
The rapid protection by BCG in newborns suggests it might be mediated by the induction of innate immune responses. The different mechanisms involved in BCG-induced protection are being actively investigated [68,69,70]. In a recent study involving 6544 high-risk neonates, it was found that BCG induces granulocyte colony-stimulating factor (G-CSF) to promote granulopoiesis resulting in the enhanced generation of neutrophils that protect from neonatal sepsis [71].
One potential mechanism for BCG-induced heterologous protection is the epigenetic reprogramming of innate immune cells, a process termed trained immunity (Figure 2) [19,20,72]. Through this process, innate immune cells such as macrophages, monocytes, and natural killer (NK) cells, are prepared for rechallenge with heterologous stimuli later in life (Figure 2) [19,20,72]. The changes induced by BCG exposure affect immune cell responsiveness, resulting in their enhanced effector functions, such as the release of cytokines and reactive oxygen species upon encountering non-related infections. BCG-associated protective effects might be driven by the modulation of metabolites [73]. These metabolites may serve as cofactors for enzymes involved in the modification of chromatin and DNA to train the immune cells [72]. The active metabolites needed for maintaining trained immune responses are derived from the modulation of glucose, glutamine, and cholesterol metabolic pathways [74]. Diverse factors including physiological or pathological states of individuals and environmental factors such as nutrition, impact the human metabolome [75,76].
The differences in active or dysregulated metabolic pathways identified in healthy and diseased individuals are being extensively categorized. Compared to other basic research, the mechanistic insights yielded through a metabolomic approach focus more on the gene- interactome intersection. By nature, these are key regulatory points with high therapeutic potential [76,77]. There is a reciprocal relationship between plasma metabolite levels and the immune response. Plasma composition can modulate immune activation, while systematic immune responses can affect metabolism and thus plasma metabolite levels. Hematologic and other modes of metabolite quantification are emerging as novel tools in disease staging and possibly prediction of pathologic progression [15,78].
As discussed above the BCG vaccine is generally given earlier in life as it shows less protective effects when administered in adolescence and adulthood. In infants, several metabolites in monocytes and macrophages, including fatty acids, acetyl-coenzyme A, and succinate, regulate the epigenetic modulations required for trained immunity [64,79,80,81]. In recent years, mass spectroscopy-based metabolomics has been utilized to examine neonatal plasma [82] however, few studies have used this technique to examine the immune response to vaccines in infants [83,84,85]. In another clinical study (n = 100), metabolomic profiling of infant plasma demonstrated that BCG vaccination induces a metabolic shift in lysolipid pathways, including lysophosphatidylcholines [9] (Table 1). Significantly increased levels of some monoacylglycerols, sphingolipids, steroids, and lipoprotein lipase metabolites along with decreased biosynthesis of progestin steroids and palmitoylglycerols, were also observed upon BCG vaccination [9]. While recent research has shed light on the complexity of metabolic shifts during the immune response, more investigation toward understanding neonatal immunometabolism is required [86].
5. Can BCG Be Used for Other Diseases?
In addition to protecting against tuberculosis, BCG vaccination is associated with enhanced protection from some unrelated infections and leads to reduced mortality. Interestingly, upon BCG administration, healthy human subjects showed a phenomenon similar to the cytokine storm, which persisted for almost 3 months after the vaccination [19,87]. BCG can also trigger heterologous immunity (antigen-independent) characterized by IL-17 and INF-γ productions from T-cells [88]. Studies have demonstrated that the presence of a BCG scar or PPD (purified protein derivative) is associated with a significant reduction in infant mortality [89,90]. Further research using randomized trials concluded the reduction of neonatal mortality associated with early BCG-vaccination may be a result of decreased incidence of respiratory infections and sepsis [91,92,93]. BCG vaccination was also found to have protective effects against lethal candidiasis in regular mice [94], and severe combined immunodeficient (SCID) mice, suggesting a role of B and T cell-independent immune response [19].
A study by Arts, R.J.W. et al. revealed a significant association of BCG with non-specific production of IL-1β and protection against yellow fever viremia [64]. Subsequently, it is known to offer protection against the Influenza virus [95], Candida albicans [19], Leishmania species [96,97], malaria [63], human immunodeficiency virus (HIV) [98], and hepatitis C virus (HCV) [99] infections. Additionally, this may occur due to BCG-mediated modulation of T-cell-directed autoimmunity [100]. Moreover, BCG was found to be effective in bladder cancer. Various clinical trials show that intravesical injection of BCG can control the recurrence of superficial bladder tumors by provoking local immune activity [101]. Since BCG affects the host glucose metabolism, its effect on Type 1 diabetes mellitus (T1DM) was also studied by many groups. Shehadeh et al. administered 17 T1DM patients with a single dose of BCG and found that 65% of subjects show clinical remission by 4th week [102]. While many other clinical trials failed to establish the correlations between BCG and T1DM [13], they all were done before the year 2000, when metabolomics was not well developed. Because BCG affects the host-trained immunity and metabolism, it has also been proposed as a treatment for asthma, allergic rhinitis, and atopic dermatitis. When administered via the intranasal route, BCG significantly attenuated allergy-related inflammation and hypersensitivity [103,104]. However, enough significant epidemiological and metabolomic data are not yet available to establish a relationship between BCG and atopy.
Novel TB Vaccines
The target of WHO’s ‘End TB’ program to achieve a 95% reduction in deaths from TB and a 90% reduction in TB incidence by 2035, requires multisector efforts incorporating socio-economic identity, novel diagnostic and therapeutic interventions, and effective vaccine development. Vaccines have shown their potential in controlling and even eradiating many life-threatening diseases. For this reason, the End TB program has an immediate need for a vaccine candidate that is effective in all age groups, for preventing infection, improving antibiotic treatment outcomes, and reducing relapse events. Current TB vaccine candidates are grouped into several categories including viral vector vaccines, attenuated live vaccines, inactivated whole cell vaccines, and adjuvanted protein subunit vaccines, among other formulations.
Examples of recombinant viral vector vaccines include the Ad5 Ag85A, MVA85A, ChAdOx1 85A, and TB/FLU-04L engineered strains (Table 2). The Ad5 Ag85A vaccine in particular is designed as a replication-deficient adenoviral serotype 5 (Ad5) vector containing the Ag85A antigen from Mtb (Table 2). Typically, this strain is incorporated in the booster vaccine given after BCG priming [105,106,107,108]. MVA85A (recombinant modified vaccinia virus Ankara containing antigen 85A from Mtb) was reported to induce a robust Ag85A-specific CD4+ and CD8+ response. In animal cell models incubated with low-dose aerosol infections, this immunologic modem displayed increased protection compared to those induced with BCG [109]. These same studies have described the chimpanzee adenovirus [110] as expressing the Ag85A antigen of Mtb (ChAdOx1 85A) [111]. The corresponding mucosal vector vaccine composed of a replication-deficient attenuated influenza virus expresses Ag85A and ESAT-6 Mtb antigens (TB/FLU-04L), the protective efficacy of BCG (Table 2) [112].
VPM1002 and MTBVAC are viable whole-cell vaccines that can carry multiple antigens (Table 2). Like BCG, these strains also have complex and individualized immune reactions. As observed in the case of BCG, pre-sensitization by these nontuberculous mycobacterial strains reduces the pathologic consequences of subsequent mycobacterial infections. VPM1002 is developed as a recombinant BCG vaccine with improved efficacy. The immunogenicity of this vaccine was tempered with genetic engineering techniques, modulating the release of mycobacterial antigens into the host cell cytosol. Specifically, the gene encoding the urease C (UreC enzyme) was replaced with the Listeriolysin O (LLO) encoding sequences (Hly) of Listeria monocytogenes (Table 2) [113,114]. Normally the urease C enzyme inhibits phagocytic lysosome maturation and improves the survival of Mtb in macrophages [115,116]. The other possible vaccine candidate, MTBVAC is a live, attenuated Mtb strain derived from an Mtb clinical isolate belonging to modern lineage 4 which retains the T cell epitopes described in tuberculous mycobacterial strains, as well as the ESAT6 and CFP10 antigens. In preclinical evaluation, MTBVAC showed improved efficacy as compared to BCG (Table 2) [117]. Attenuation was achieved by the deletion of two genes, phoP, and fadD26, a crucial modulator of virulence [117]. Currently, the MTBVAC formulation is being adapted for use as a preventive vaccine as well as a booster vaccine for BCG-primed adults.
RUTI, Vaccae, DAR-901, and Immuvac are vaccine candidates based on inactivated whole-cell derivatives (Table 2). RUTI is a multi-antigen vaccine derived from the cell wall of Mtb cultivated in hypoxic conditions and is formulated as a liposomal suspension (Table 2) [118]. Vaccae is derived from heat-killed Mycobacterium vaccae, a non-pathogenic environmental mycobacterium. It was reported to complement the effectiveness of BCG vaccination, resulting in an enhanced immune response against TB (Table 2) [119]. Similarly, DAR 901 is derived from heat-killed non-tuberculous mycobacteria identified from the SRL-172 master cell bank [120]. Clinical trials examining the efficacy and safety of the DAR-901 vaccine in BCG-immunized adults determined that it effectively induced the formation of cellular and humoral immune responses while displaying no adverse effects after three doses (Table 2) [121,122,123]. Immuvac, is a vaccine derived from heat-killed Mycobacterium indicus pranii a non-pathogenic mycobacterium closely related to M. avium [124]. It is currently approved by the Central Drugs Standard Control Organization, India (CDSCO) and the FDA as an immunotherapeutic and immunoprophylactic agent for treating multibacillary leprosy [125,126,127].
Multiple protein subunit vaccines have also been developed including AEC/BC02 (using Ag85B and ESAT6-CFP10 antigens with BC02 compound adjuvant) [128]; H56:IC31 (using Ag85B, ESAT-6, and Rv2660c antigens with IC31 adjuvant) [129]; ID93 + GLA-SE (using Rv2608, Rv3619, Rv3620 and Rv1813 antigens with GLA-SE adjuvant) [130], and M72/AS01E (using Mtb32 and Mtb39 antigens with AS01B, AS02A, or AS01E adjuvant) [131,132,133,134,135] (Table 2). Protein subunit vaccines are primarily developed for use as booster vaccines in BCG-primed individuals to extend immune protection.
Despite all the efforts in this area the goal of ending the TB epidemic is still far away. During 2015–2021 the total incidence of TB infection and mortality was reduced by 10% and 5.9%, respectively. This is modest considering the 50% and 75% reduction milestones for infection and mortality set for 2025 by the WHO (Global tuberculosis report, WHO, 2022). The development and introduction of newer technologies are needed to fuel research in the TB diagnostic and therapeutic areas. While the whole genome sequence (WGS) of the Mtb (H37Rv) was uncovered in 1998 [136], recent developments in genomic techniques have provided a clear resolution at the nucleotide level. Whole genome analysis allows for screening clinically relevant genetic information, such as sequences related to drug resistance, and variations between bacterial species and strains [137]. Additionally, these techniques can help identify specific genetic regions responsible for antigenicity and virulence.
The TB vaccine development space can be improved by enhancing our understanding of the host immune response to TB infection and finding an effective route of antigen delivery (immunization) leading to sensitization [138]. Nanomaterial-based vaccine delivery systems have also shown potential in vaccine storage, improved stability of antigens in blood, and greater specificity in targeted delivery [139,140]. Rapidly emerging mRNA-based vaccination technology has shown its potential in vaccine development against many diseases including SARS-CoV-2 [141]. Current research in this area is focused on improving mRNA stability, optimizing delivery vectors, and enhancing control of protein expression. These advances have already led to the development of novel therapeutics such as self-amplifying RNA vaccines [142]. A recently adapted Mtb subunit vaccine, ID93, is composed of a self-replicating RNA molecule with a nanostructural carrier [143].

Table 2.
TB vaccine candidates with clinical trial status.
Table 2.
TB vaccine candidates with clinical trial status.
| Vaccine Category | Vaccine Candidate | Antigen and Formulation | Latest Clinical Trial Phase (Status) # | NCT Number (References) |
|---|---|---|---|---|
| Recombinant viral vector | Ad5 Ag85A | Ag85A antigen expressed in Adenovirus serotype 5 | I (Completed in 2021) | NCT02337270 [144,145,146] |
| MVA85A | Ag85A antigen expressed in modified Vaccinia virus Ankara | IIa (Completed in 2021) | NCT03681860 [109] | |
| ChAdOx1 85A | Ag85A antigen expressed in Chimpanzee adenovirus | I (Completed in 2021) | NCT03681860 [110,147] | |
| TB/FLU-04L | Ag85A & ESAT-6 antigens expressed in attenuated replication-deficient influenza virus vector | I (Completed in 2015) | NCT02501421 [148] | |
| Viable whole-cell | VPM1002 | Recombinant BCG vaccine | III (Ongoing) | NCT04351685 [149,150] |
| MTBVAC | Attenuated Mtb clinical isolate with ESAT6 & CFP10 and independent genetic deletions of phoP & fadD26 genes | II (Completed in 2022) | NCT03536117 [151,152] | |
| Inactivated whole-cell | RUTI | Polyantigenic liposomal formulation of detoxified, fragmented Mtb | II (Ongoing) | NCT04919239 [153,154,155] |
| Vaccae | Heat-killed M. vaccae | III (Completed in 2017) | NCT01979900 [156] | |
| DAR-901 | Heat killed nontuberculous mycobacteria | II (Completed in 2020) | NCT02712424 [121,122,157,158] | |
| MIP/Immuvac | Whole cell, heat inactivated Mycobacterium indicus pranii | III (Completed in 2012) | NCT00341328 [159] | |
| Protein subunit | AEC/BC02 | Ag85b, ESAT6-CFP10 fusion protein, with BC02 adjuvant | II (Ongoing) | NCT05284812 [128] |
| H56:IC31 | Fusion protein of Ag85B, ESAT-6 and Rv2660c with IC31 adjuvant | II (Ongoing) | NCT03512249 [160,161,162,163] | |
| ID93 + GLA-SE | Fusion protein of Rv1813, Rv2608, Rv3619, and Rv3620 with GLA-SE adjuvant | IIa (Unknown) | NCT03806686 [164,165,166] | |
| M72/AS01E | Fusion protein of Mtb32A and Mtb39A with AS01E adjuvant | II (Ongoing) | NCT04556981 [167,168] |
# Status of the respective clinical trial as shown on clinicaltrials.gov (accessed on 7 December 2022).
6. Key Challenges and Recommendations
With the increasing prevalence of infections by drug resistant Mtb strains, it is important to find alternative therapies. The integration of vaccinology and metabolomics has formed a key development pipeline. Due to its impact on immune and metabolic responses, BCG has been considered a therapeutic and prophylactic tool against various autoimmune and inflammatory diseases. However, it comes with many challenges. One outstanding problem is that metabolomic studies have mainly been limited to murine models, but the molecular mechanism of the actions of these effects are yet to be investigated. There are many enhancers like rapamycin (a modulator of the mTOR complex), which have been used along with BCG to boost immunogenicity in mice [169]. However, these need to be validated in clinical trials with bigger cohorts. Coadministration of some other metabolic modulators (for example Dichloroacetate, Metformin, Silybin, and 2-deoxyglucose) can also be useful to amplify the immune impact of the BCG vaccination [65].
Tuberculosis infection has heterogeneous pathology containing both active and latent bacilli. This makes complete eradication of the Mtb infection difficult [170]. Metabolomic understanding of the immune system is still in its infancy. In addition to genetics many non-heritable variables such as food habits, geographical location, previous disease or vaccination status, and other environmental factors can confound the observations of different clinical studies. To account for this, all therapeutic studies might be designed using a multifactorial approach and analysis [171]. Furthermore, combining other omics datasets with immunometabolomics information can reveal complex genomic, proteomic, or transcriptomic clusters, implicating their role in immune activity and vaccination.
Future Prospective
Tuberculosis is an old disease however, its cure remains a major challenge. Advanced systems biology approaches present solutions to understand the disease and account for the complex host-pathogen relationship. Novel metabolomic and chromatographic technologies such as hydrophilic interaction chromatography (HILIC), ion-exchange chromatography, chiral liquid chromatography, capillary electrophoresis mass spectrometry (CE-MS), ion-mobility separation (IMS), and liquid-liquid micro extraction (LLME) can be helpful to analyze a broad range of metabolites with great accuracy [172]. Machine learning (ML) and artificial intelligence (AI) based programs are facilitating next-generation solutions for high-resolution mass spectrometry (HRMS) with improved data quality, accurate metabolite annotations, and network analysis [173]. With the emergence of the COVID-19 pandemic, the landscape of infectious disease research has changed. Vaccines like BCG, which have an impact on trained immunity and metabolism, provide an adjunctive approach to pandemic preparedness. We strongly believe that the formation of global shared databases can aid in the development of precision models of personalized vaccination against several life-threatening diseases. This collaborative effort could also help to allocate vaccine resources and to predict possible epidemiologic events well in advance.
7. Conclusions
The human metabolome is vast and is under the influence of many pathological and physiological factors. With the development of high-quality mass spectrometry and chromatography, metabolomics is evolving as a tool to study diseases and therapies. In the last few years, various studies have shown that metabolism is associated with the functional state of the human immune system. Metabolic factors can affect the protective natural immune response against diseases as well as the efficacy of vaccines. Knowledge of immunometabolism in diseased and vaccinated individuals presents an immense unharnessed therapeutic potential to cure many autoinflammatory diseases, viral and bacterial infections, and metabolic disorders.
BCG is one of the oldest vaccines in human history, saving millions of lives every year. By highlighting metabolic aspects of the BCG vaccine pertaining to the human immune system, our review presents metabolomics as a promising vaccinomics tool for discovering novel avenues to cure difficult infections. BCG impacts glycolysis in immune cells and activates lactate biosynthesis through mTOR and HIF-1α signaling. It was also found to affect the levels of TCA cycle intermediates, hence affecting lipid biosynthesis and the activity of histone-modifying enzymes (such as HAT and KDMs) via fumarate and succinate. These lead to epigenetic modifications and induction of trained immunity in different innate immune cells such as monocytes, macrophages, and NK cells. Upon subsequent exposure, these cells increase the relative secretions of proinflammatory cytokines and provide protection against many non-specific diseases and infections for which no therapy yet exists. Many unanswered questions remain, such as what frequency of BCG vaccination is required to sustain the trained immunity. Greater analysis is needed to uncover the exact role of metabolic modulators in trained immunity and the impacts of many unknown metabolites (the dark matter of metabolism). As we enter the era of Big Data, novel analysis methods such as machine learning are required to implement a multi-omics approach to understanding immunity. This understanding can aid the development of novel anti-TB vaccines, leading to better immunogenic vectors with wider applications. Combining such vaccines with other modes of treatment can allow for targeted therapy of many diseases and reduce the emergence of drug resistance. Cross-discipline collaborations and global data integrations can accelerate the development of metabolomics applications in vaccinomics, to make informed decisions in clinical settings.
Author Contributions
V.S. conceptualized, designed, and edited the manuscript, V.S., B.S.R., D.K. and E.H.R. did the literature research and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
This article does not include or report any data collection at any stage.
Data Availability Statement
There are no supporting data associated with this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Bacillus Calmette-Guerin (BCG), Mycobacterium tuberculosis (Mtb), Tuberculosis (TB), toll-like receptors (TLRs), Phosphoenolpyruvate carboxykinase (PEPCK), lactate dehydrogenase-A (LDH), natural killer (NK), severe combined immunodeficient (SCID), Type 1 diabetes mellitus (T1DM).
References
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L. How COVID is derailing the fight against HIV, TB and malaria. Nature 2021, 597, 314. [Google Scholar] [CrossRef] [PubMed]
- de Vos, M.; Ley, S.D.; Wiggins, K.B.; Derendinger, B.; Dippenaar, A.; Grobbelaar, M.; Reuter, A.; Dolby, T.; Burns, S.; Schito, M.; et al. Bedaquiline Microheteroresistance after Cessation of Tuberculosis Treatment. N. Engl. J. Med. 2019, 380, 2178–2180. [Google Scholar] [CrossRef] [PubMed]
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G.; Getahun, H.; et al. WHO’s new end TB strategy. Lancet 2015, 385, 1799–1801. [Google Scholar] [CrossRef] [PubMed]
- Weill, J. Homage to Benjamin Weill-Halle on the 40th anniversary of bcg vaccination. La Presse Med. 1964, 72, 2420–2421. [Google Scholar]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.M.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG Vaccine Against Tuberculosis: A Systematic Review of Randomized Controlled Trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shim, D.; Kim, K.E.S.; Lee, W.; Shin, S.J. Understanding Metabolic Regulation Between Host and Pathogens: New Opportunities for the Development of Improved Therapeutic Strategies Against Mycobacterium tuberculosis Infection. Front. Cell. Infect. Microbiol. 2021, 11, 635335. [Google Scholar] [CrossRef]
- Cho, Y.; Park, Y.; Sim, B.; Kim, J.; Lee, H.; Cho, S.N.; Kang, Y.A.; Lee, S.G. Identification of serum biomarkers for active pulmonary tuberculosis using a targeted metabolomics approach. Sci. Rep. 2020, 10, 3825. [Google Scholar] [CrossRef]
- Diray-Arce, J.; Angelidou, A.; Jensen, K.J.; Conti, M.G.; Kelly, R.S.; Pettengill, M.A.; Liu, M.; van Haren, S.D.; McCulloch, S.D.; Michelloti, G.; et al. Bacille Calmette-Guérin vaccine reprograms human neonatal lipid metabolism in vivo and in vitro. Cell Rep. 2022, 39, 110772. [Google Scholar] [CrossRef]
- Koeken, V.; Qi, C.; Mourits, V.P.; de Bree, L.C.J.; Moorlag, S.; Sonawane, V.; Lemmers, H.; Dijkstra, H.; Joosten, L.A.B.; van Laarhoven, A.; et al. Plasma metabolome predicts trained immunity responses after antituberculosis BCG vaccination. PLoS Biol. 2022, 20, e3001765. [Google Scholar] [CrossRef]
- Weiner, J., 3rd; Parida, S.K.; Maertzdorf, J.; Black, G.F.; Repsilber, D.; Telaar, A.; Mohney, R.P.; Arndt-Sullivan, C.; Ganoza, C.A.; Fae, K.C.; et al. Biomarkers of inflammation, immunosuppression and stress with active disease are revealed by metabolomic profiling of tuberculosis patients. PLoS ONE 2012, 7, e40221. [Google Scholar] [CrossRef]
- Frediani, J.K.; Jones, D.P.; Tukvadze, N.; Uppal, K.; Sanikidze, E.; Kipiani, M.; Tran, V.T.; Hebbar, G.; Walker, D.I.; Kempker, R.R.; et al. Plasma metabolomics in human pulmonary tuberculosis disease: A pilot study. PLoS ONE 2014, 9, e108854. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, C.J.; Hsiao, Y.H.; Chang, Y.H.; Liu, S.J.; Hsu, H.Y. Therapeutic Effects of BCG Vaccination on Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2020, 2020, 8954125. [Google Scholar] [CrossRef] [PubMed]
- Kuhtreiber, W.M.; Tran, L.; Kim, T.; Dybala, M.; Nguyen, B.; Plager, S.; Huang, D.; Janes, S.; Defusco, A.; Baum, D.; et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: The value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 2018, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Diray-Arce, J.; Conti, M.G.; Petrova, B.; Kanarek, N.; Angelidou, A.; Levy, O. Integrative Metabolomics to Identify Molecular Signatures of Responses to Vaccines and Infections. Metabolites 2020, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Immunometabolism: Another Road to Sepsis and Its Therapeutic Targeting. Inflammation 2019, 42, 765–788. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- de Carvalho, L.P.; Fischer, S.M.; Marrero, J.; Nathan, C.; Ehrt, S.; Rhee, K.Y. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem. Biol. 2010, 17, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Siddiqa, A.; Jones, D.P.; Liu, K.; Kempker, R.R.; Nizam, A.; Shah, N.S.; Ismail, N.; Ouma, S.G.; Tukvadze, N.; et al. Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight 2020, 5, e137131. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J., 3rd; Maertzdorf, J.; Sutherland, J.S.; Duffy, F.J.; Thompson, E.; Suliman, S.; McEwen, G.; Thiel, B.; Parida, S.K.; Zyla, J.; et al. Metabolite changes in blood predict the onset of tuberculosis. Nat. Commun. 2018, 9, 5208. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Prakash, H. Sphingolipids Are Dual Specific Drug Targets for the Management of Pulmonary Infections: Perspective. Front. Immunol. 2017, 8, 378. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Weiner, J.R.; Maertzdorf, J. Accelerating tuberculosis vaccine trials with diagnostic and prognostic biomarkers. Expert Rev. Vaccines 2017, 16, 845–853. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Banoei, M.M.; Winston, B.W.; Schraufnagel, D.E. Metabolomics: Applications and Promise in Mycobacterial Disease. Ann. Am. Thorac. Soc. 2015, 12, 1278–1287. [Google Scholar] [CrossRef]
- Zhou, A.; Ni, J.; Xu, Z.; Wang, Y.; Lu, S.; Sha, W.; Karakousis, P.C.; Yao, Y.F. Application of (1)h NMR spectroscopy-based metabolomics to sera of tuberculosis patients. J. Proteome Res. 2013, 12, 4642–4649. [Google Scholar] [CrossRef]
- Diaz, C.; Perez Del Palacio, J.; Valero-Guillen, P.L.; Mena Garcia, P.; Perez, I.; Vicente, F.; Martin, C.; Genilloud, O.; Sanchez Pozo, A.; Gonzalo-Asensio, J. Comparative Metabolomics between Mycobacterium tuberculosis and the MTBVAC Vaccine Candidate. ACS Infect. Dis. 2019, 5, 1317–1326. [Google Scholar] [CrossRef]
- Magdalena, D.; Michal, S.; Marta, S.; Magdalena, K.P.; Anna, P.; Magdalena, G.; Rafal, S. Targeted metabolomics analysis of serum and Mycobacterium tuberculosis antigen-stimulated blood cultures of pediatric patients with active and latent tuberculosis. Sci. Rep. 2022, 12, 4131. [Google Scholar] [CrossRef]
- Scriba, T.J.; Coussens, A.K.; Fletcher, H.A. Human Immunology of Tuberculosis. Microbiol. Spectr. 2017, 5, 15. [Google Scholar] [CrossRef]
- Gupta, A. Protective efficacy of Mycobacterium indicus pranii against tuberculosis and underlying local lung immune responses in guinea pig model. Vaccine 2012, 30, 6198–6209. [Google Scholar] [CrossRef] [PubMed]
- Ravesloot-Chávez, M.M.; Van Dis, E.; Stanley, S.A. The Innate Immune Response to Mycobacterium tuberculosis Infection. Annu. Rev. Immunol. 2021, 39, 611–637. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Singh, V.K.; Hunter, R.L.; Jagannath, C. Macrophage heterogeneity and plasticity in tuberculosis. J. Leukoc. Biol. 2019, 106, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Via, L.E.; Deretic, D.; Ulmer, R.J.; Hibler, N.S.; Huber, L.A.; Deretic, V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 1997, 272, 13326–13331. [Google Scholar] [CrossRef] [PubMed]
- Tailleux, L.; Maeda, N.; Nigou, J.; Gicquel, B.; Neyrolles, O. How is the phagocyte lectin keyboard played? Master class lesson by Mycobacterium tuberculosis. Trends Microbiol. 2003, 11, 259–263. [Google Scholar] [CrossRef]
- Vergne, I.; Fratti, R.A.; Hill, P.J.; Chua, J.; Belisle, J.; Deretic, V. Mycobacterium tuberculosis phagosome maturation arrest: Mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol. Biol. Cell 2004, 15, 751–760. [Google Scholar] [CrossRef]
- Sturgill-Koszycki, S.; Schlesinger, P.H.; Chakraborty, P.; Haddix, P.L.; Collins, H.L.; Fok, A.K.; Allen, R.D.; Gluck, S.L.; Heuser, J.; Russell, D.G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 1994, 263, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Bach, H.; Sun, J.; Hmama, Z.; Av-Gay, Y. Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc. Natl. Acad. Sci. USA 2011, 108, 19371–19376. [Google Scholar] [CrossRef]
- Axelrod, S.; Oschkinat, H.; Enders, J.; Schlegel, B.; Brinkmann, V.; Kaufmann, S.H.; Haas, A.; Schaible, U.E. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell. Microbiol. 2008, 10, 1530–1545. [Google Scholar] [CrossRef]
- van der Wel, N.; Hava, D.; Houben, D.; Fluitsma, D.; van Zon, M.; Pierson, J.; Brenner, M.; Peters, P.J. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 2007, 129, 1287–1298. [Google Scholar] [CrossRef]
- Houben, D.; Demangel, C.; van Ingen, J.; Perez, J.; Baldeon, L.; Abdallah, A.M.; Caleechurn, L.; Bottai, D.; van Zon, M.; de Punder, K.; et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell. Microbiol. 2012, 14, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Simeone, R.; Bobard, A.; Lippmann, J.; Bitter, W.; Majlessi, L.; Brosch, R.; Enninga, J. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 2012, 8, e1002507. [Google Scholar] [CrossRef] [PubMed]
- Simeone, R.; Sayes, F.; Song, O.; Groschel, M.I.; Brodin, P.; Brosch, R.; Majlessi, L. Cytosolic access of Mycobacterium tuberculosis: Critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog. 2015, 11, e1004650. [Google Scholar] [CrossRef] [PubMed]
- Augenstreich, J.; Arbues, A.; Simeone, R.; Haanappel, E.; Wegener, A.; Sayes, F.; Le Chevalier, F.; Chalut, C.; Malaga, W.; Guilhot, C.; et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell. Microbiol. 2017, 19, e12726. [Google Scholar] [CrossRef]
- Singh, K.H.; Jha, B.; Dwivedy, A.; Choudhary, E.; N, A.G.; Ashraf, A.; Arora, D.; Agarwal, N.; Biswal, B.K. Characterization of a secretory hydrolase from Mycobacterium tuberculosis sheds critical insight into host lipid utilization by M. tuberculosis. J. Biol. Chem. 2017, 292, 11326–11335. [Google Scholar] [CrossRef]
- Dwivedy, A.; Ashraf, A.; Jha, B.; Kumar, D.; Agarwal, N.; Biswal, B.K. De novo histidine biosynthesis protects Mycobacterium tuberculosis from host IFN-gamma mediated histidine starvation. Commun. Biol. 2021, 4, 410. [Google Scholar] [CrossRef]
- Aguilo, J.I.; Alonso, H.; Uranga, S.; Marinova, D.; Arbues, A.; de Martino, A.; Anel, A.; Monzon, M.; Badiola, J.; Pardo, J.; et al. ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cell. Microbiol. 2013, 15, 1994–2005. [Google Scholar] [CrossRef]
- Dallenga, T.; Repnik, U.; Corleis, B.; Eich, J.; Reimer, R.; Griffiths, G.W.; Schaible, U.E. M. tuberculosis-Induced Necrosis of Infected Neutrophils Promotes Bacterial Growth Following Phagocytosis by Macrophages. Cell Host Microbe 2017, 22, 519–530.e513. [Google Scholar] [CrossRef]
- Lerner, T.R.; Borel, S.; Greenwood, D.J.; Repnik, U.; Russell, M.R.; Herbst, S.; Jones, M.L.; Collinson, L.M.; Griffiths, G.; Gutierrez, M.G. Mycobacterium tuberculosis replicates within necrotic human macrophages. J. Cell Biol. 2017, 216, 583–594. [Google Scholar] [CrossRef]
- Elkington, P.; Lerm, M.; Kapoor, N.; Mahon, R.; Pienaar, E.; Huh, D.; Kaushal, D.; Schlesinger, L.S. In Vitro Granuloma Models of Tuberculosis: Potential and Challenges. J. Infect. Dis. 2019, 219, 1858–1866. [Google Scholar] [CrossRef]
- Batista, L.A.F.; Silva, K.J.S.; da Costa, E.S.L.M.; de Moura, Y.F.; Zucchi, F.C.R. Tuberculosis: A granulomatous disease mediated by epigenetic factors. Tuberculosis 2020, 123, 101943. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.D.; Chiu, C.; Churchyard, G.J.; Esmail, H.; Lewinsohn, D.M.; Gandhi, N.R.; Fennelly, K.P. Tuberculosis Infectiousness and Host Susceptibility. J. Infect. Dis. 2017, 216, S636–S643. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Muniyan, R. Latent tuberculosis: Interaction of virulence factors in Mycobacterium tuberculosis. Mol. Biol. Rep. 2021, 48, 6181–6196. [Google Scholar] [CrossRef] [PubMed]
- Loxton, A.G.; van Rensburg, I.C. FasL regulatory B-cells during Mycobacterium tuberculosis infection and TB disease. J. Mol. Biol. 2021, 433, 166984. [Google Scholar] [CrossRef]
- Marrero, J.; Rhee, K.Y.; Schnappinger, D.; Pethe, K.; Ehrt, S. Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. USA 2010, 107, 9819–9824. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; VanderVen, B.C.; Fahey, R.J.; Russell, D.G. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 2013, 288, 6788–6800. [Google Scholar] [CrossRef]
- Kinsella, R.J.; Fitzpatrick, D.A.; Creevey, C.J.; McInerney, J.O. Fatty acid biosynthesis in Mycobacterium tuberculosis: Lateral gene transfer, adaptive evolution, and gene duplication. Proc. Natl. Acad. Sci. USA 2003, 100, 10320–10325. [Google Scholar] [CrossRef]
- Johnson, T.S.; Munn, D.H. Host indoleamine 2,3-dioxygenase: Contribution to systemic acquired tumor tolerance. Immunol. Investig. 2012, 41, 765–797. [Google Scholar] [CrossRef]
- Garg, S.K.; Volpe, E.; Palmieri, G.; Mattei, M.; Galati, D.; Martino, A.; Piccioni, M.S.; Valente, E.; Bonanno, E.; De Vito, P.; et al. Sphingosine 1-phosphate induces antimicrobial activity both in vitro and in vivo. J. Infect. Dis. 2004, 189, 2129–2138. [Google Scholar] [CrossRef]
- Dara, M.; Acosta, C.D.; Rusovich, V.; Zellweger, J.P.; Centis, R.; Migliori, G.B. Bacille Calmette-Guérin vaccination: The current situation in Europe. Eur. Respir. J. 2014, 43, 24–35. [Google Scholar] [CrossRef]
- Franco-Paredes, C.; Rouphael, N.; Del Rio, C.; Santos-Preciado, J.I. Vaccination strategies to prevent tuberculosis in the new millennium: From BCG to new vaccine candidates. Int. J. Infect. Dis. 2006, 10, 93–102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pym, A.S.; Brodin, P.; Majlessi, L.; Brosch, R.; Demangel, C.; Williams, A.; Griffiths, K.E.; Marchal, G.; Leclerc, C.; Cole, S.T. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 2003, 9, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Walk, J.; de Bree, L.C.J.; Graumans, W.; Stoter, R.; van Gemert, G.J.; van de Vegte-Bolmer, M.; Teelen, K.; Hermsen, C.C.; Arts, R.J.W.; Behet, M.C.; et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e105. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Gonçalves, L.G.; Belinha, A.; et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Zimmermann, P.; Donath, S.; Perrett, K.P.; Messina, N.L.; Ritz, N.; Netea, M.G.; Flanagan, K.L.; van der Klis, F.R.M.; Curtis, N. The influence of neonatal Bacille Calmette-Guérin (BCG) immunisation on heterologous vaccine responses in infants. Vaccine 2019, 37, 3735–3744. [Google Scholar] [CrossRef]
- Moorlag, S.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- Curtis, N.; Sparrow, A.; Ghebreyesus, T.A.; Netea, M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet 2020, 395, 1545–1546. [Google Scholar] [CrossRef]
- Brook, B.; Harbeson, D.J.; Shannon, C.P.; Cai, B.; He, D.; Ben-Othman, R.; Francis, F.; Huang, J.; Varankovich, N.; Liu, A.; et al. BCG vaccination-induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci. Transl. Med. 2020, 12, eaax4517. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Fok, E.T.; Davignon, L.; Fanucchi, S.; Mhlanga, M.M. The lncRNA Connection Between Cellular Metabolism and Epigenetics in Trained Immunity. Front. Immunol. 2018, 9, 3184. [Google Scholar] [CrossRef]
- Playdon, M.C.; Ziegler, R.G.; Sampson, J.N.; Stolzenberg-Solomon, R.; Thompson, H.J.; Irwin, M.L.; Mayne, S.T.; Hoover, R.N.; Moore, S.C. Nutritional metabolomics and breast cancer risk in a prospective study. Am. J. Clin. Nutr. 2017, 106, 637–649. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]
- Pettengill, M.A.; van Haren, S.D.; Levy, O. Soluble mediators regulating immunity in early life. Front. Immunol. 2014, 5, 457. [Google Scholar] [CrossRef]
- Reinke, S.N.; Walsh, B.H.; Boylan, G.B.; Sykes, B.D.; Kenny, L.C.; Murray, D.M.; Broadhurst, D.I. 1H NMR derived metabolomic profile of neonatal asphyxia in umbilical cord serum: Implications for hypoxic ischemic encephalopathy. J. Proteome Res. 2013, 12, 4230–4239. [Google Scholar] [CrossRef]
- Kan, B.; Michalski, C.; Fu, H.; Au, H.H.T.; Lee, K.; Marchant, E.A.; Cheng, M.F.; Anderson-Baucum, E.; Aharoni-Simon, M.; Tilley, P.; et al. Cellular metabolism constrains innate immune responses in early human ontogeny. Nat. Commun. 2018, 9, 4822. [Google Scholar] [CrossRef]
- Conti, M.G.; Angelidou, A.; Diray-Arce, J.; Smolen, K.K.; Lasky-Su, J.; De Curtis, M.; Levy, O. Immunometabolic approaches to prevent, detect, and treat neonatal sepsis. Pediatr. Res. 2020, 87, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Shannon, C.P.; Amenyogbe, N.; Bennike, T.B.; Diray-Arce, J.; Idoko, O.T.; Gill, E.E.; Ben-Othman, R.; Pomat, W.S.; van Haren, S.D.; et al. Dynamic molecular changes during the first week of human life follow a robust developmental trajectory. Nat. Commun. 2019, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Petrick, L.M.; Schiffman, C.; Edmands, W.M.B.; Yano, Y.; Perttula, K.; Whitehead, T.; Metayer, C.; Wheelock, C.E.; Arora, M.; Grigoryan, H.; et al. Metabolomics of neonatal blood spots reveal distinct phenotypes of pediatric acute lymphoblastic leukemia and potential effects of early-life nutrition. Cancer Lett. 2019, 452, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Nakaya, H.I.; Subramaniam, S.; Pulendran, B. Systems vaccinology: Enabling rational vaccine design with systems biological approaches. Vaccine 2015, 33, 5294–5301. [Google Scholar] [CrossRef]
- Amenyogbe, N.; Levy, O.; Kollmann, T.R. Systems vaccinology: A promise for the young and the poor. Philos. Trans. R. Soc. Lond B Biol. Sci. 2015, 370, 20140340. [Google Scholar] [CrossRef]
- Angelidou, A.; Diray-Arce, J.; Conti, M.G.; Smolen, K.K.; van Haren, S.D.; Dowling, D.J.; Husson, R.N.; Levy, O. BCG as a Case Study for Precision Vaccine Development: Lessons From Vaccine Heterogeneity, Trained Immunity, and Immune Ontogeny. Front. Microbiol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- Roth, A.; Gustafson, P.; Nhaga, A.; Djana, Q.; Poulsen, A.; Garly, M.L.; Jensen, H.; Sodemann, M.; Rodriques, A.; Aaby, P. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 2005, 34, 540–547. [Google Scholar] [CrossRef]
- Garly, M.L.; Martins, C.L.; Balé, C.; Baldé, M.A.; Hedegaard, K.L.; Gustafson, P.; Lisse, I.M.; Whittle, H.C.; Aaby, P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine 2003, 21, 2782–2790. [Google Scholar] [CrossRef]
- Kristensen, I.; Aaby, P.; Jensen, H. Routine vaccinations and child survival: Follow up study in Guinea-Bissau, West Africa. BMJ 2000, 321, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, S.; Aaby, P.; Napirna, B.M.; Roth, A.; Ravn, H.; Rodrigues, A.; Whittle, H.; Benn, C.S. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr. Infect. Dis. J. 2012, 31, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N.; et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Wout, J.W.; Poell, R.; van Furth, R. The role of BCG/PPD-activated macrophages in resistance against systemic candidiasis in mice. Scand. J. Immunol. 1992, 36, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, J.; Kox, M.; Stokman, R.; Gerretsen, J.; Diavatopoulos, D.A.; van Crevel, R.; Rimmelzwaan, G.F.; Pickkers, P.; Netea, M.G. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J. Infect. Dis. 2015, 212, 1930–1938. [Google Scholar] [CrossRef]
- Pereira, L.I.; Dorta, M.L.; Pereira, A.J.; Bastos, R.P.; Oliveira, M.A.; Pinto, S.A.; Galdino, H., Jr.; Mayrink, W.; Barcelos, W.; Toledo, V.P.; et al. Increase of NK cells and proinflammatory monocytes are associated with the clinical improvement of diffuse cutaneous leishmaniasis after immunochemotherapy with BCG/Leishmania antigens. Am. J. Trop. Med. Hyg. 2009, 81, 378–383. [Google Scholar] [CrossRef]
- Fortier, A.H.; Mock, B.A.; Meltzer, M.S.; Nacy, C.A. Mycobacterium bovis BCG-induced protection against cutaneous and systemic Leishmania major infections of mice. Infect. Immun. 1987, 55, 1707–1714. [Google Scholar] [CrossRef]
- Aldovini, A.; Young, R.A. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature 1991, 351, 479–482. [Google Scholar] [CrossRef]
- Uno-Furuta, S.; Matsuo, K.; Tamaki, S.; Takamura, S.; Kamei, A.; Kuromatsu, I.; Kaito, M.; Matsuura, Y.; Miyamura, T.; Adachi, Y.; et al. Immunization with recombinant Calmette-Guerin bacillus (BCG)-hepatitis C virus (HCV) elicits HCV-specific cytotoxic T lymphocytes in mice. Vaccine 2003, 21, 3149–3156. [Google Scholar] [CrossRef]
- Ristori, G.; Buzzi, M.G.; Sabatini, U.; Giugni, E.; Bastianello, S.; Viselli, F.; Buttinelli, C.; Ruggieri, S.; Colonnese, C.; Pozzilli, C.; et al. Use of Bacille Calmette-Guerin (BCG) in multiple sclerosis. Neurology 1999, 53, 1588–1589. [Google Scholar] [CrossRef]
- Herr, H.W.; Morales, A. History of bacillus Calmette-Guerin and bladder cancer: An immunotherapy success story. J. Urol. 2008, 179, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, N.; Calcinaro, F.; Bradley, B.J.; Bruchim, I.; Vardi, P.; Lafferty, K.J. Effect of adjuvant therapy on development of diabetes in mouse and man. Lancet 1994, 343, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Hopfenspirger, M.T.; Agrawal, D.K. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J. Immunol. 2002, 168, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Kowalewicz-Kulbat, M.; Locht, C. BCG for the prevention and treatment of allergic asthma. Vaccine 2021, 39, 7341–7352. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 2004, 173, 6357–6365. [Google Scholar] [CrossRef]
- Vordermeier, H.M. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 2009, 77, 3364–3373. [Google Scholar] [CrossRef]
- Dean, G. Comparison of the immunogenicity and protection against bovine tuberculosis following immunization by BCG-priming and boosting with adenovirus or protein based vaccines. Vaccine 2014, 32, 1304–1310. [Google Scholar] [CrossRef]
- Metcalfe, H.J. Ag85A-specific CD4+ T cell lines derived after boosting BCG-vaccinated cattle with Ad5-85A possess both mycobacterial growth inhibition and anti-inflammatory properties. Vaccine 2018, 36, 2850–2854. [Google Scholar] [CrossRef]
- Williams, A.; Hatch, G.J.; Clark, S.O.; Gooch, K.E.; Hatch, K.A.; Hall, G.A.; Huygen, K.; Ottenhoff, T.H.; Franken, K.L.; Andersen, P.; et al. Evaluation of vaccines in the EU TB Vaccine Cluster using a guinea pig aerosol infection model of tuberculosis. Tuberculosis 2005, 85, 29–38. [Google Scholar] [CrossRef]
- Stylianou, E. Improvement of BCG protective efficacy with a novel chimpanzee adenovirus and a modified vaccinia Ankara virus both expressing Ag85A. Vaccine 2015, 33, 6800–6808. [Google Scholar] [CrossRef]
- Hawkridge, T. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J. Infect. Dis. 2008, 198, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Dockrell, H.M. Towards new TB vaccines: What are the challenges? Pathog. Dis. 2016, 74, ftw016. [Google Scholar] [CrossRef] [PubMed]
- Spertini, F. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: A randomised, double-blind, controlled phase I trial. Lancet Respir. Med. 2015, 3, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E. The recombinant Bacille Calmette-Guerin vaccine VPM1002: Ready for clinical efficacy testing. Front. Immunol. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Reyrat, J.M.; Berthet, F.X.; Gicquel, B. The urease locus of Mycobacterium tuberculosis and its utilization for the demonstration of allelic exchange in Mycobacterium bovis bacillus Calmette-Guerin. Proc. Natl. Acad. Sci. USA 1995, 92, 8768–8772. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.H.; Hart, P.D.; Young, M.R. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature 1980, 286, 79–80. [Google Scholar] [CrossRef]
- Gonzalo-Asensio, J. MTBVAC: Attenuating the human pathogen of tuberculosis (TB) toward a promising vaccine against the TB epidemic. Front. Immunol. 2017, 8, 1803. [Google Scholar] [CrossRef]
- Cardona, P.J.; Amat, I. Origin and development of RUTI, a new therapeutic vaccine against Mycobacterium tuberculosis infection. Arch. Bronconeumol. 2006, 42, 25–32. [Google Scholar] [CrossRef]
- Johnson, J.L.; Kamya, R.M.; Okwera, A.; Loughlin, A.M.; Nyole, S.; Hom, D.L.; Wallis, R.S.; Hirsch, C.S.; Wolski, K.; Foulds, J.; et al. Randomized controlled trial of Mycobacterium vaccae immunotherapy in non-human immunodeficiency virus-infected ugandan adults with newly diagnosed pulmonary tuberculosis. The Uganda-Case Western Reserve University Research Collaboration. J. Infect. Dis. 2000, 181, 1304–1312. [Google Scholar] [CrossRef][Green Version]
- Reyn, C.F. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS 2010, 24, 675–685. [Google Scholar] [CrossRef]
- Reyn, C.F. Safety and immunogenicity of an inactivated whole cell tuberculosis vaccine booster in adults primed with BCG: A randomized, controlled trial of DAR-901. PLoS ONE 2017, 12, e0175215. [Google Scholar]
- Masonou, T. CD4+ T cell cytokine responses to the DAR-901 booster vaccine in BCG-primed adults: A randomized, placebo-controlled trial. PLoS ONE 2019, 14, e0217091. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.R. Altruism, scepticism, and collective decision-making in foreign-born U.S. residents in a tuberculosis vaccine trial. BMC Public Health 2018, 18, 1–12. [Google Scholar]
- Saini, V.; Raghuvanshi, S.; Talwar, G.P.; Ahmed, N.; Khurana, J.P.; Hasnain, S.E.; Tyagi, A.K.; Tyagi, A.K. Polyphasic taxonomic analysis establishes Mycobacterium indicus pranii as a distinct species. PLoS ONE 2009, 4, e6263. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Disabilities in multibacillary leprosy following multidrug therapy with and without immunotherapy with Mycobacterium w antileprosy vaccine. Int. J. Lepr. Other Mycobact. Dis. 1999, 67, 1–9. [Google Scholar]
- Sharma, P. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: Clinical field trials with a follow up of 8-10 years. Lepr. Rev. 2005, 76, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Mycobacterium w vaccine, a useful adjuvant to multidrug therapy in multibacillary leprosy: A report on hospital based immunotherapeutic clinical trials with a follow-up of 1-7 years after treatment. Lepr. Rev. 2000, 71, 179–192. [Google Scholar]
- Lu, J.B.; Chen, B.W.; Wang, G.Z.; Fu, L.L.; Shen, X.B.; Su, C.; Du, W.X.; Yang, L.; Xu, M. Recombinant tuberculosis vaccine AEC/BC02 induces antigen-specific cellular responses in mice and protects guinea pigs in a model of latent infection. J. Microbiol. Immunol. Infect. 2015, 48, 597–603. [Google Scholar] [CrossRef]
- Perez-Martinez, A.P. Conservation in gene encoding Mycobacterium tuberculosis antigen Rv2660 and a high predicted population coverage of H56 multistage vaccine in South Africa. Infect. Genet. Evol. 2017, 55, 244–250. [Google Scholar] [CrossRef]
- Bertholet, S.; Ireton, G.C.; Kahn, M.; Guderian, J.; Mohamath, R.; Stride, N.; Laughlin, E.M.; Baldwin, S.L.; Vedvick, T.S.; Coler, R.N.; et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J. Immunol. 2008, 181, 7948–7957. [Google Scholar] [CrossRef]
- Homolka, S.; Ubben, T.; Niemann, S. High sequence variability of the ppE18 gene of clinical Mycobacterium tuberculosis complex strains potentially impacts effectivity of vaccine candidate M72/AS01E. PLoS ONE 2016, 11, e0152200. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J. A randomized, controlled dose-finding phase II study of the M72/AS01 candidate tuberculosis vaccine in healthy PPD-positive adults. J. Clin. Immunol. 2013, 33, 1360–1375. [Google Scholar] [CrossRef]
- Skeiky, Y.A. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect. Immun. 1999, 67, 3998–4007. [Google Scholar] [CrossRef]
- Dillon, D.C. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 1999, 67, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Al-Attiyah, R. In vitro cellular immune responses to complex and newly defined recombinant antigens of Mycobacterium tuberculosis. Clin. Exp. Immunol. 2004, 138, 139–144. [Google Scholar] [CrossRef]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E., 3rd; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.A.; Manson, A.L.; Desjardins, C.A.; Abeel, T.; Earl, A.M. Deciphering drug resistance in Mycobacterium tuberculosis using whole-genome sequencing: Progress, promise, and challenges. Genome Med. 2019, 11, 45. [Google Scholar] [CrossRef]
- Garcia, J.I.; Allue-Guardia, A.; Tampi, R.P.; Restrepo, B.I.; Torrelles, J.B. New Developments and Insights in the Improvement of Mycobacterium tuberculosis Vaccines and Diagnostics Within the End TB Strategy. Curr. Epidemiol. Rep. 2021, 8, 33–45. [Google Scholar] [CrossRef]
- Diego-Gonzalez, L.; Crecente-Campo, J.; Paul, M.J.; Singh, M.; Reljic, R.; Alonso, M.J.; Gonzalez-Fernandez, A.; Simon-Vazquez, R. Design of Polymeric Nanocapsules for Intranasal Vaccination against Mycobacterium Tuberculosis: Influence of the Polymeric Shell and Antigen Positioning. Pharmaceutics 2020, 12, 489. [Google Scholar] [CrossRef]
- Gheibi Hayat, S.M.; Darroudi, M. Nanovaccine: A novel approach in immunization. J. Cell. Physiol. 2019, 234, 12530–12536. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Beissert, T.; Perkovic, M.; Vogel, A.; Erbar, S.; Walzer, K.C.; Hempel, T.; Brill, S.; Haefner, E.; Becker, R.; Tureci, O.; et al. A Trans-amplifying RNA Vaccine Strategy for Induction of Potent Protective Immunity. Mol. Ther. 2020, 28, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.L.; Reese, V.A.; Larsen, S.E.; Beebe, E.; Guderian, J.; Orr, M.T.; Fox, C.B.; Reed, S.G.; Coler, R.N. Prophylactic efficacy against Mycobacterium tuberculosis using ID93 and lipid-based adjuvant formulations in the mouse model. PLoS ONE 2021, 16, e0247990. [Google Scholar] [CrossRef] [PubMed]
- Smaill, F.; Xing, Z. Human type 5 adenovirus-based tuberculosis vaccine: Is the respiratory route of delivery the future? Expert Rev. Vaccines 2014, 13, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Smaill, F. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci. Transl. Med. 2013, 5, 205ra134. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M. Induction of an immune-protective T-cell repertoire with diverse genetic coverage by a novel viral-vectored tuberculosis vaccine in humans. J. Infect. Dis. 2016, 214, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, M.; Satti, I.; Minhinnick, A.; Harris, S.; Riste, M.; Ramon, R.L.; Sheehan, S.; Thomas, Z.M.; Wright, D.; Stockdale, L.; et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime—MVA85A boost in healthy UK adults. Vaccine 2020, 38, 779–789. [Google Scholar] [CrossRef]
- Stukova, M.; Khairullin, B.; Bekembaeva, G.; Erofeeva, M.; Shurygina, A.; Pisareva, M.; Buzitskaya, J.; Grudinin, M.; Kassenov, M.; Sandybaev, N. Randomized double-blind placebo-controlled phase I trial of intranasal TB/FLU-04L tuberculosis vaccine in BCG-vaccinated healthy adults aged 18–50 years. In Proceedings of the 4th Global Forum on TB Vaccines, Shanghai, China, 21–24 April 2015; pp. 21–24. [Google Scholar]
- Grode, L. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 2013, 31, 1340–1348. [Google Scholar] [CrossRef]
- Loxton, A.G.; Knaul, J.K.; Grode, L.; Gutschmidt, A.; Meller, C.; Eisele, B.; Johnstone, H.; van der Spuy, G.; Maertzdorf, J.; Kaufmann, S.H. Safety and immunogenicity of the recombinant Mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin. Vaccine Immunol. 2017, 24, e00439-16. [Google Scholar] [CrossRef]
- Tameris, M.; Mearns, H.; Penn-Nicholson, A.; Gregg, Y.; Bilek, N.; Mabwe, S.; Geldenhuys, H.; Shenje, J.; Luabeya, A.K.K.; Murillo, I.; et al. Live-attenuated Mycobacterium tuberculosis vaccine MTBVAC versus BCG in adults and neonates: A randomised controlled, double-blind dose-escalation trial. Lancet Respir. Med. 2019, 7, 757–770. [Google Scholar] [CrossRef]
- Marinova, D. MTBVAC from discovery to clinical trials in tuberculosis-endemic countries. Expert Rev. Vaccines 2017, 16, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Prabowo, S.A.; Painter, H.; Zelmer, A.; Smith, S.G.; Seifert, K.; Amat, M.; Cardona, P.J.; Fletcher, H.A. RUTI Vaccination Enhances Inhibition of Mycobacterial Growth ex vivo and Induces a Shift of Monocyte Phenotype in Mice. Front. Immunol. 2019, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, C. Double-blind, randomized, placebo-controlled phase I clinical trial of the therapeutical antituberculous vaccine RUTI. Vaccine 2010, 28, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Cardona, P.J. RUTI: A new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis 2006, 86, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Moran-Mendoza, O.; Marion, S.A.; Elwood, K.; Patrick, D.M.; FitzGerald, J.M. Tuberculin skin test size and risk of tuberculosis development: A large population-based study in contacts. Int. J. Tuberc. Lung Dis. 2007, 11, 1014–1020. [Google Scholar] [PubMed]
- Munseri, P.; Said, J.; Amour, M.; Magohe, A.; Matee, M.; Rees, C.A.; Mackenzie, T.; Tvaroha, S.; Bailey-Kellogg, C.; Maro, I.; et al. DAR-901 vaccine for the prevention of infection with Mycobacterium tuberculosis among BCG-immunized adolescents in Tanzania: A randomized controlled, double-blind phase 2b trial. Vaccine 2020, 38, 7239–7245. [Google Scholar] [CrossRef]
- Lahey, T. Immunogenicity and protective efficacy of the DAR-901 booster vaccine in a murine model of tuberculosis. PLoS ONE 2016, 11, e0168521. [Google Scholar] [CrossRef]
- Patel, N.; Trapathi, S.B. Improved cure rates in pulmonary tuberculosis category II (retreatment) with mycobacterium w. J. Indian Med. Assoc. 2003, 101, 680–682. [Google Scholar]
- Jenum, S.; Tonby, K.; Rueegg, C.S.; Ruhwald, M.; Kristiansen, M.P.; Bang, P.; Olsen, I.C.; Sellaeg, K.; Rostad, K.; Mustafa, T.; et al. A Phase I/II randomized trial of H56:IC31 vaccination and adjunctive cyclooxygenase-2-inhibitor treatment in tuberculosis patients. Nat. Commun. 2021, 12, 6774. [Google Scholar] [CrossRef]
- Bekker, L.G.; Dintwe, O.; Fiore-Gartland, A.; Middelkoop, K.; Hutter, J.; Williams, A.; Randhawa, A.K.; Ruhwald, M.; Kromann, I.; Andersen, P.L.; et al. A phase 1b randomized study of the safety and immunological responses to vaccination with H4:IC31, H56:IC31, and BCG revaccination in Mycobacterium tuberculosis-uninfected adolescents in Cape Town, South Africa. EClinicalMedicine 2020, 21, 100313. [Google Scholar] [CrossRef]
- Suliman, S. Dose optimization of H56:IC31 vaccine for tuberculosis-endemic populations. A double-blind, placebo-controlled, dose-selection trial. Am. J. Respir. Crit. Care Med. 2019, 199, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Luabeya, A.K. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine 2015, 33, 4130–4140. [Google Scholar] [CrossRef] [PubMed]
- Day, T.A.; Penn-Nicholson, A.; Luabeya, A.K.K.; Fiore-Gartland, A.; Du Plessis, N.; Loxton, A.G.; Vergara, J.; Rolf, T.A.; Reid, T.D.; Toefy, A.; et al. Safety and immunogenicity of the adjunct therapeutic vaccine ID93 + GLA-SE in adults who have completed treatment for tuberculosis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Respir. Med. 2021, 9, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Penn-Nicholson, A.; Tameris, M.; Smit, E.; Day, T.A.; Musvosvi, M.; Jayashankar, L.; Vergara, J.; Mabwe, S.; Bilek, N.; Geldenhuys, H.; et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: A randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir. Med. 2018, 6, 287–298. [Google Scholar] [CrossRef]
- Coler, R.N. The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: First-in-human trial. NPJ Vaccines 2018, 3, 34. [Google Scholar] [CrossRef]
- Tait, D.R.; Hatherill, M.; Van Der Meeren, O.; Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.J.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef]
- Meeren, O. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 2018, 379, 1621–1634. [Google Scholar] [CrossRef]
- Jagannath, C.; Bakhru, P. Rapamycin-induced enhancement of vaccine efficacy in mice. Methods Mol. Biol 2012, 821, 295–303. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Weiner, J.; von Reyn, C.F. Novel approaches to tuberculosis vaccine development. Int J. Infect. Dis. 2017, 56, 263–267. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.G.; Palese, P.; Poland, G.A. Current Challenges in Vaccinology. Front. Immunol. 2020, 11, 1181. [Google Scholar] [CrossRef]
- Miggiels, P.; Wouters, B.; van Westen, G.J.P.; Dubbelman, A.-C.; Hankemeier, T. Novel technologies for metabolomics: More for less. TrAC Trends Anal. Chem. 2019, 120, 115323. [Google Scholar] [CrossRef]
- Petrick, L.M.; Shomron, N. AI/ML-driven advances in untargeted metabolomics and exposomics for biomedical applications. Cell Rep. Phys. Sci. 2022, 3, 100978. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).