Determinants of the Level of Anti-SARS-CoV-2 IgG ANTibodiEs after Vaccination (DANTE-SIRIO 7) Study in a Large Cohort of Healthcare Workers
Abstract
1. Introduction
2. Material and Methods
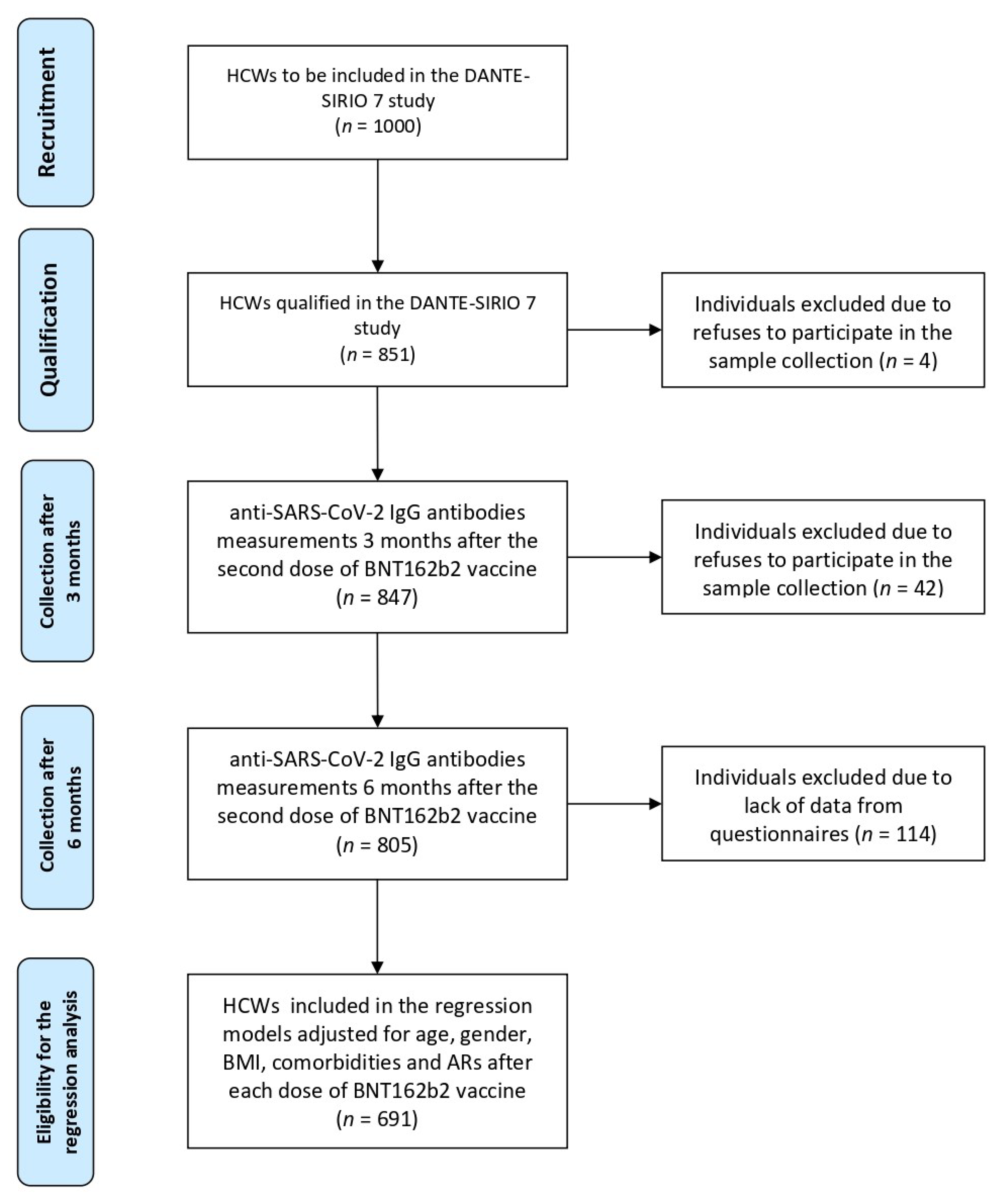
2.1. Study Design and Participants
2.2. Questionnaire
2.3. SARS-CoV-2 IgG Antibody Testing
2.4. Statistical Methods and Data Evaluation
3. Results
3.1. Characteristics of Participants
3.2. Characteristics of Post-Vaccine Adverse Effects after the First and the Second Dose of Vaccine
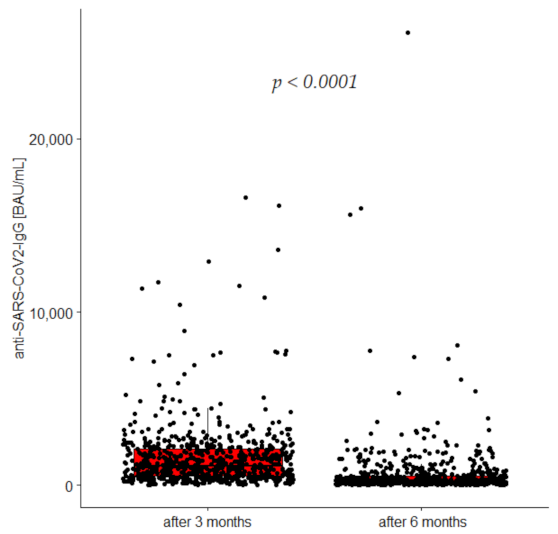
3.3. Anti-SARS-CoV-2 IgG Concentrations 3 and 6 Months after Vaccination
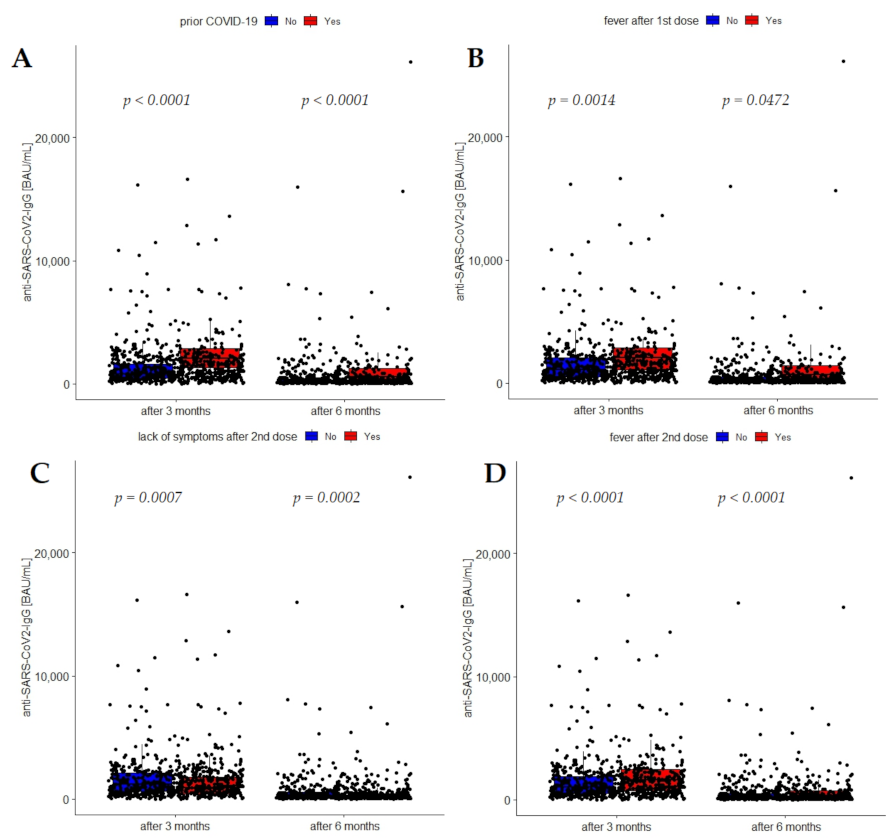
3.4. Association of Prior-COVID-19 Infection with Anti-SARS-CoV-2 IgG Concentrations after Vaccination
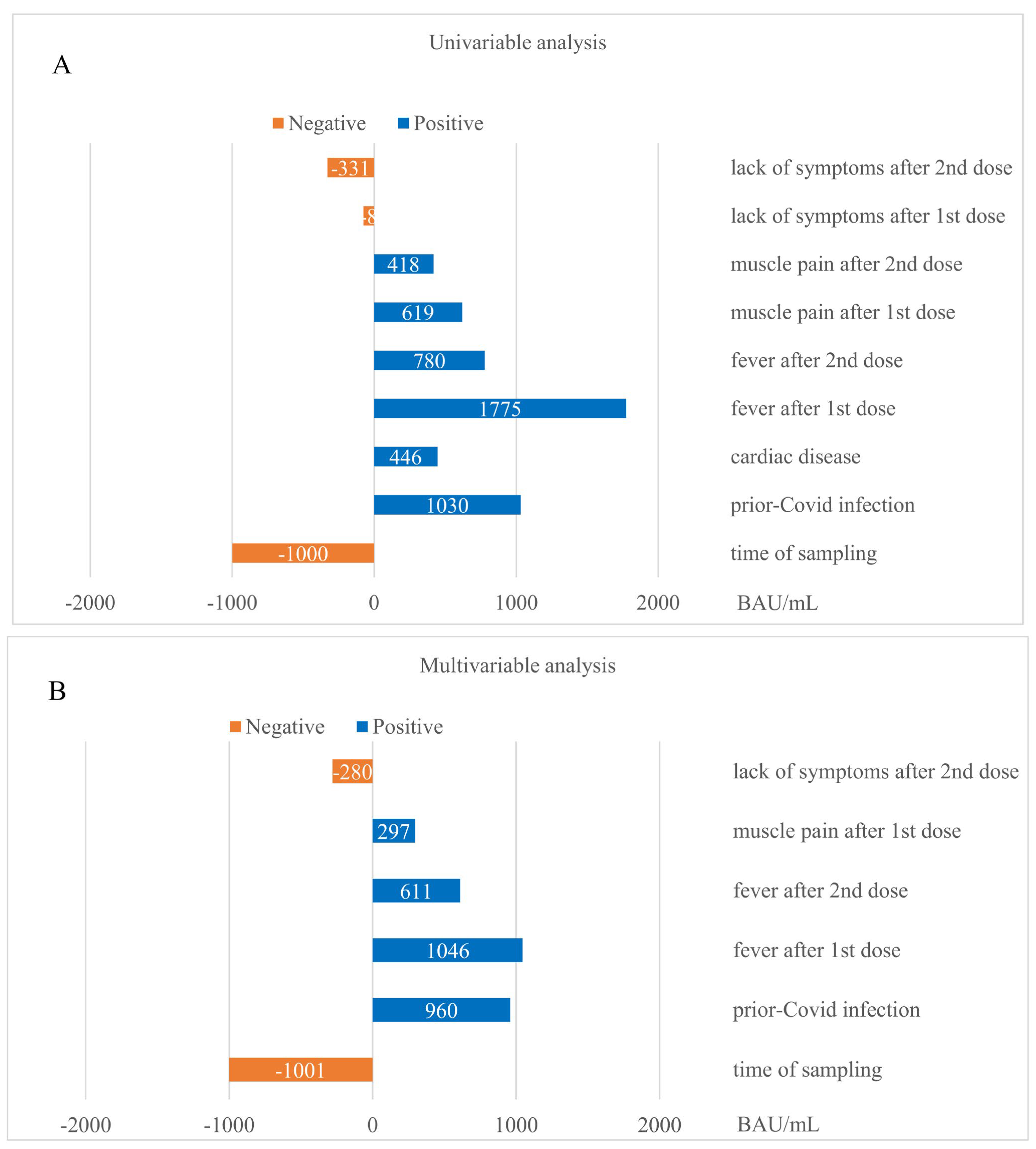
3.5. Determinants of Anti-SARS-CoV-2 IgG Levels Post Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Eng. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Fireman, B. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly Rep. 2022, 71, 255–263. [Google Scholar] [PubMed]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Hopkins, S. Protection against SARS-CoV-2 after COVID-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Interim Guidelines for COVID-19 Antibody Testing in Clinical and Public Health Settings. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html (accessed on 30 June 2022).
- Kubica, J.; Wlodarczyk, Z.; Stolarek, W.; Wojtal, E.; Buszko, K.; Grzelakowska, K.; Navarese, E.P. Determinants of the level of anti-SARS-CoV- 2 IgG ANTibodiEs after vaccination (DANTE-SIRIO 7) study. A rationale and protocol of the study. Med. Res. J. 2021, 6, 312–315. [Google Scholar] [CrossRef]
- Rzymski, P.; Pazgan-Simon, M.; Kamerys, J.; Moniuszko-Malinowska, A.; Sikorska, K.; Wernik, J.; Zarębska-Michaluk, D.; Supronowicz, L.; Sobala-Szczygieł, B.; Skrzat-Klapaczyńska, A.; et al. Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines 2022, 10, 557. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Eng. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Eng. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.A.; Laing, E.D.; Olsen, C.H.; Goguet, E.; Moser, M.; Jackson-Thompson, B.M.; Samuels, E.C.; Pollett, S.D.; Tribble, D.R.; Davies, J.; et al. Adverse Effects and Antibody Titers in Response to the BNT162b2 mRNA COVID-19 Vaccine in a Prospective Study of Healthcare Workers. Open Forum. Infect. Dis. 2021, 9, ofab575. [Google Scholar] [CrossRef] [PubMed]
- Kanizsai, A.; Molnar, T.; Varnai, R.; Zavori, L.; Tőkés-Füzesi, M.; Szalai, C.; Csecsei, P. Fever after Vaccination against SARS-CoV-2 with mRNA-Based Vaccine Associated with Higher Antibody Levels during 6 Months Follow-Up. Vaccines 2022, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Eng. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Patel, M.K. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Eng. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Eng. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Haahr, S.; Mogensen, S. Function of fever in infectious disease. Biomedicine 1978, 28, 305–307. [Google Scholar]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Mace, T.A.; Zhong, L.; Kilpatrick, C.; Zynda, E.; Lee, C.-T.; Capitano, M.; Minderman, H.; Repasky, E.A. Differentiation of CD8+ T cells into effector cells is enhanced by physiological range hyperthermia. J. Leukoc. Biol. 2011, 90, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Higa, Y.; Esaki, A.; Nabeshima, Y.; Nakazono, A. Does reactogenicity after a second injection of the BNT162b2 vaccine predict spike IgG antibody levels in healthy Japanese subjects? PLoS ONE 2021, 16, e0257668. [Google Scholar] [CrossRef] [PubMed]
- Uwamino, Y.; Kurafuji, T.; Sato, Y.; Tomita, Y.; Shibata, A.; Tanabe, A.; Murata, M. Young age, female sex, and presence of systemic adverse reactions are associated with high post-vaccination antibody titer after two doses of BNT162b2 mRNA SARS-CoV-2 vaccination: An observational study of 646 Japanese healthcare workers and university staff. Vaccine 2022, 40, 1019–1025. [Google Scholar] [PubMed]
- Otani, J.; Ohta, R.; Sano, C. Association between Immunoglobulin G Levels and Adverse Effects Following Vaccination with the BNT162b2 Vaccine among Japanese Healthcare Workers. Vaccines 2021, 9, 1149. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef]
- Zhong, D.; Xiao, S.; Debes, A.K.; Egbert, E.R.; Caturegli, P.; Colantuoni, E.; Milstone, A.M. Durability of antibody levels after vaccination with mRNA SARS-CoV-2 vaccine in individuals with or without prior infection. JAMA 2021, 326, 2524–2526. [Google Scholar] [CrossRef]
- Buonfrate, D.; Piubelli, C.; Gobbi, F.; Martini, D.; Bertoli, G.; Ursini, T.; Moro, L.; Ronzoni, N.; Angheben, A.; Rodari, P.; et al. Antibody response induced by the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers, with or without prior SARS-CoV-2 infection: A prospective study. Clin. Microbiol. Infect. 2021, 27, 1845–1850. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Ciliberto, G. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef]
- Campo, F.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Noia, V.; Di Domenico, E.G.; et al. Antibody Persistence 6 Months Post-Vaccination with BNT162b2 among Health Care Workers. Vaccines 2021, 9, 1125. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Moon, J.-Y.; Lee, S.-K.; Lee, H.; Moon, S.; Chung, S.J.; Yeo, Y.; Park, T.S.; Park, D.W.; Kim, T.-H.; et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 nCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association With Age, Sex, Obesity, and Adverse Reactions. Front. Immunol. 2021, 12, 779212. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Dall’Olmo, L.; della Rocca, F.; Barbaro, F.; Cosma, C.; Basso, D.; Cattelan, A.; Cianci, V.; Plebani, M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta 2021, 519, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, Y.R.; Heo, S.T.; Oh, H.; Kim, M.; Lee, H.R.; Yoo, J.R. Healthcare Workers in South Korea Maintain a SARS-CoV-2 Antibody Response Six Months After Receiving a Second Dose of the BNT162b2 mRNA Vaccine. Front. Immunol. 2022, 13, 827306. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, J.A.; Englund, J.A.; Wang, X.; Brown, J.C.; Zerr, D.M.; Strelitz, B.; Klein, E.J. Higher Antibody Concentrations in U.S. Health Care Workers Associated with Greater Reactogenicity Post-Vaccination. Vaccines 2022, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Takahashi, S.; Hakim, J.; Kelly, J.D.; Torres, L.; Iyer, N.S.; Greenhouse, B. SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci. Adv. 2021, 7, eabh3409. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Terpos, E.; Zirou, C.; Sklirou, A.D.; Apostolakou, F.; Gumeni, S.; Dimopoulos, M.A. Comparative kinetics of SARS-CoV-2 anti-spike protein RBD IgGs and neutralizing antibodies in convalescent and naïve recipients of the BNT162b2 mRNA vaccine versus COVID-19 patients. BMC Med. 2021, 19, 208. [Google Scholar] [CrossRef]
| Variable | All (n = 847) | Non-COVID-19 (n = 601) | Prior-COVID-19 (n = 246) | p |
|---|---|---|---|---|
| Age (years) | 45 (34–53) | 44 (32–53) | 46 (37–53) | 0.057 |
| Gender [n (%)] | Female 677 (79.9%) | Female 485 (80.7%) | Female 192(78.4%) | 0.001 |
| Male 170 (20.1%) | Male 116 (19.3%) | Male 54 (21.6%) | ||
| BMI (kg/m2) | 24.5 (21.9–28.1) | 24.4 (21.8–27.5) | 24.7 (22.4–29.0) | 0.034 |
| Overweight [n (%)] | 265 (31.3%) | 189 (31.4%) | 76 (30.9%) | 0.874 |
| Obese [n (%)] | 127 (15.0%) | 83 (13.8%) | 44 (17.9%) | 0.131 |
| Smoking [n (%)] | 108 (12.7%) | 77 (12.8%) | 31 (12.4%) | 0.933 |
| Allergy [n (%)] | 166 (19.6%) | 127 (21.2%) | 39 (12.8%) | 0.079 |
| Diabetes [n (%)] | 55 (6.5%) | 40 (6.7%) | 12 (4.8%) | 0.328 |
| Hypertension [n (%)] | 156 (18.4%) | 96 (16.0%) | 60 (24.4%) | 0.004 |
| Hyperlipidemia [n (%)] | 146 (17.2%) | 97 (16.2%) | 49 (20.0%) | 0.237 |
| Cardiac diseases [n (%)] | 61 (7.2%) | 36 (6.0%) | 25 (10%) | 0.042 |
| Thromboembolic diseases [n (%)] | 26 (3.0%) | 19 (3.1%) | 7 (2.9%) | 0.808 |
| Autoimmune diseases [n (%)] | 122 (14.4%) | 81 (13.4%) | 41 (16.7%) | 0.230 |
| CKD G4, G5 [n (%)] | 9 (1.1%) | 7 (1.1%) | 2 (1.0%) | 0.650 |
| Pulmonary diseases [n (%)] | 43 (5.1%) | 31 (5.2%) | 12 (4.8%) | 0.866 |
| Cancer [n (%)] | 58 (6.8%) | 43 (7.1%) | 15 (6.2%) | 0.580 |
| SARS-CoV-2 IgG (BAU) after 3 months | 1145 (543–2095) | 829 (452–1640) | 2042 (1267–2812) | 0.0001 |
| SARS-CoV-2 IgG (BAU) after 6 months | 225 (100.0–510.3) | 163.7 (86.5–329.4) | 517.0 (251.2–1049.3) | 0.0001 |
| Adverse Reactions | After First Dose of Vaccine [n (%)] | After Second Dose of Vaccine [n (%)] | p |
|---|---|---|---|
| None | 142 (16.7%) | 150 (17.7%) | 0.265 |
| Malaise | 149 (17.6%) | 215 (25.4%) | 0.0001 |
| Loss of smell | 0 | 1 (0.1%) | NA |
| Loss of taste | 2 (0.2%) | 2 (0.2%) | 1.00 |
| Feverish state (<38 °C) | 101 (11.9%) | 159 (18.8%) | 0.0001 |
| Fever (>38 °C) | 38 (4.5%) | 118 (13.9%) | 0.0001 |
| Runny nose | 5 (0.6%) | 7 (0.8%) | 0.772 |
| Cough | 6 (0.7%) | 6 (0.7%) | NA |
| Sore throat | 12 (1.4%) | 15 (1.8%) | 0.560 |
| Dyspnea | 4 (0.5%) | 12 (1.4%) | 0.079 |
| Respiratory failure requiring oxygen therapy | 1 (0.1%) | 0 | NA |
| Muscle pain | 142 (16.8%) | 237 (28.0%) | 0.0001 |
| Gastrointestinal complaints | 16 (1.9%) | 33 (3.9%) | 0.014 |
| Headache | 107 (12.6%) | 200 (23.6%) | 0.0001 |
| Injection site soreness | 651 (76.9%) | 548 (64.7%) | 0.0001 |
| Others | 68 (8%) | 102 (12%) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krintus, M.; Piasecki, M.; Lackowski, P.; Buszko, K.; Kubica, A.; Kosobucka-Ozdoba, A.; Michalski, P.; Pietrzykowski, L.; Stolarek, W.; Wojcik, A.; et al. Determinants of the Level of Anti-SARS-CoV-2 IgG ANTibodiEs after Vaccination (DANTE-SIRIO 7) Study in a Large Cohort of Healthcare Workers. Vaccines 2022, 10, 2125. https://doi.org/10.3390/vaccines10122125
Krintus M, Piasecki M, Lackowski P, Buszko K, Kubica A, Kosobucka-Ozdoba A, Michalski P, Pietrzykowski L, Stolarek W, Wojcik A, et al. Determinants of the Level of Anti-SARS-CoV-2 IgG ANTibodiEs after Vaccination (DANTE-SIRIO 7) Study in a Large Cohort of Healthcare Workers. Vaccines. 2022; 10(12):2125. https://doi.org/10.3390/vaccines10122125
Chicago/Turabian StyleKrintus, Magdalena, Maciej Piasecki, Piotr Lackowski, Katarzyna Buszko, Aldona Kubica, Agata Kosobucka-Ozdoba, Piotr Michalski, Lukasz Pietrzykowski, Wioleta Stolarek, Agata Wojcik, and et al. 2022. "Determinants of the Level of Anti-SARS-CoV-2 IgG ANTibodiEs after Vaccination (DANTE-SIRIO 7) Study in a Large Cohort of Healthcare Workers" Vaccines 10, no. 12: 2125. https://doi.org/10.3390/vaccines10122125
APA StyleKrintus, M., Piasecki, M., Lackowski, P., Buszko, K., Kubica, A., Kosobucka-Ozdoba, A., Michalski, P., Pietrzykowski, L., Stolarek, W., Wojcik, A., Tomczak, M., Wojtal, E., Krys, J., Wlodarczyk, Z., & Kubica, J. (2022). Determinants of the Level of Anti-SARS-CoV-2 IgG ANTibodiEs after Vaccination (DANTE-SIRIO 7) Study in a Large Cohort of Healthcare Workers. Vaccines, 10(12), 2125. https://doi.org/10.3390/vaccines10122125







