Efficacy of DNA Vaccines in Protecting Rainbow Trout against VHS and IHN under Intensive Farming Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. VHS and IHN Plasmids Expression in EPC Cell Line
2.2. Fish
2.3. Viruses and Phylogenetic Analysis
2.4. Controlled Conditions Trial
2.5. Histological Analysis
2.6. Field Trial
2.7. Plasmid Detection
2.8. Statistical Analysis
3. Results
3.1. VHS and IHN Plasmids Expression in EPC Cell Line
3.2. Viruses and Phylogenetic Analysis
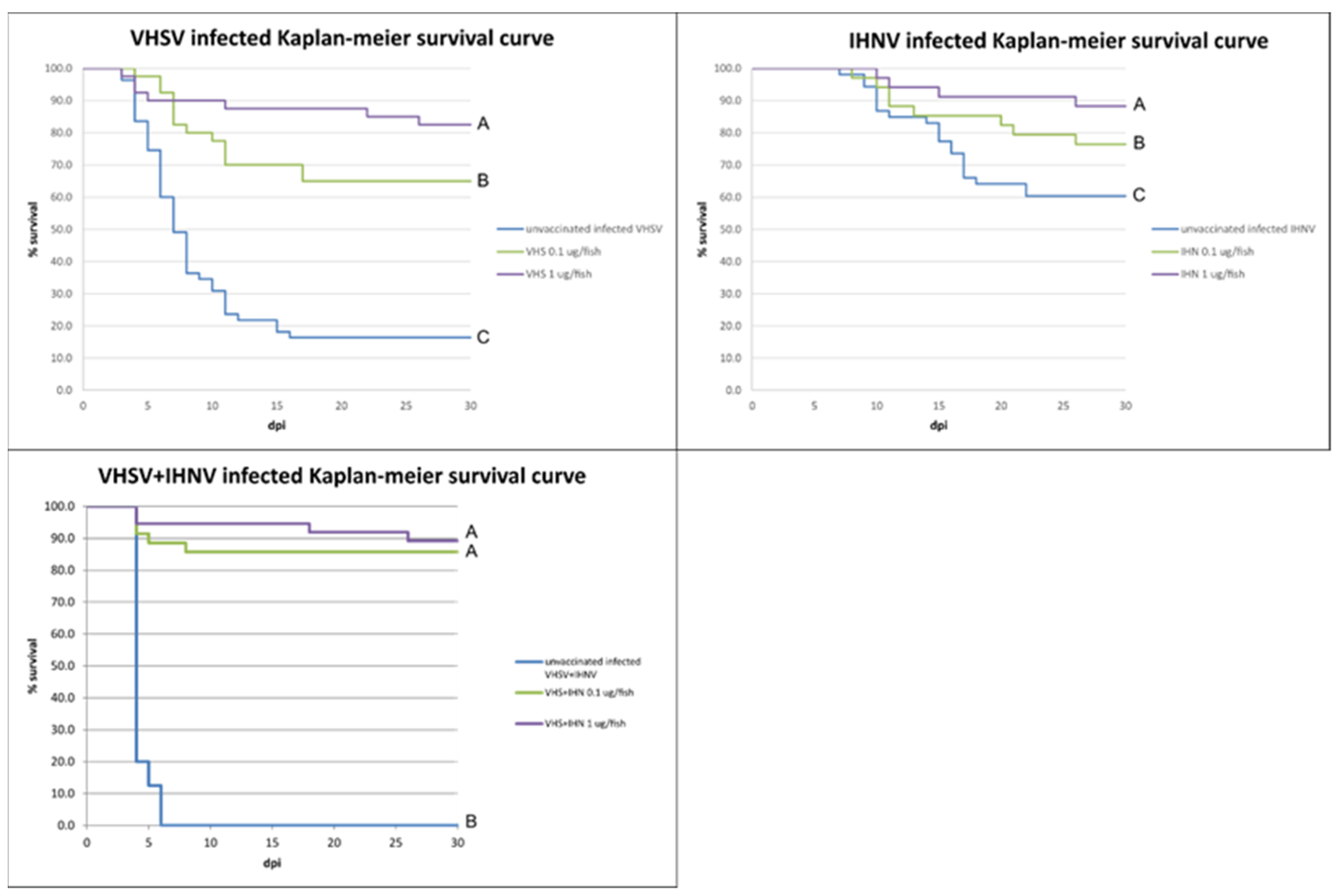
3.3. Controlled Conditions Trial
3.4. Histology
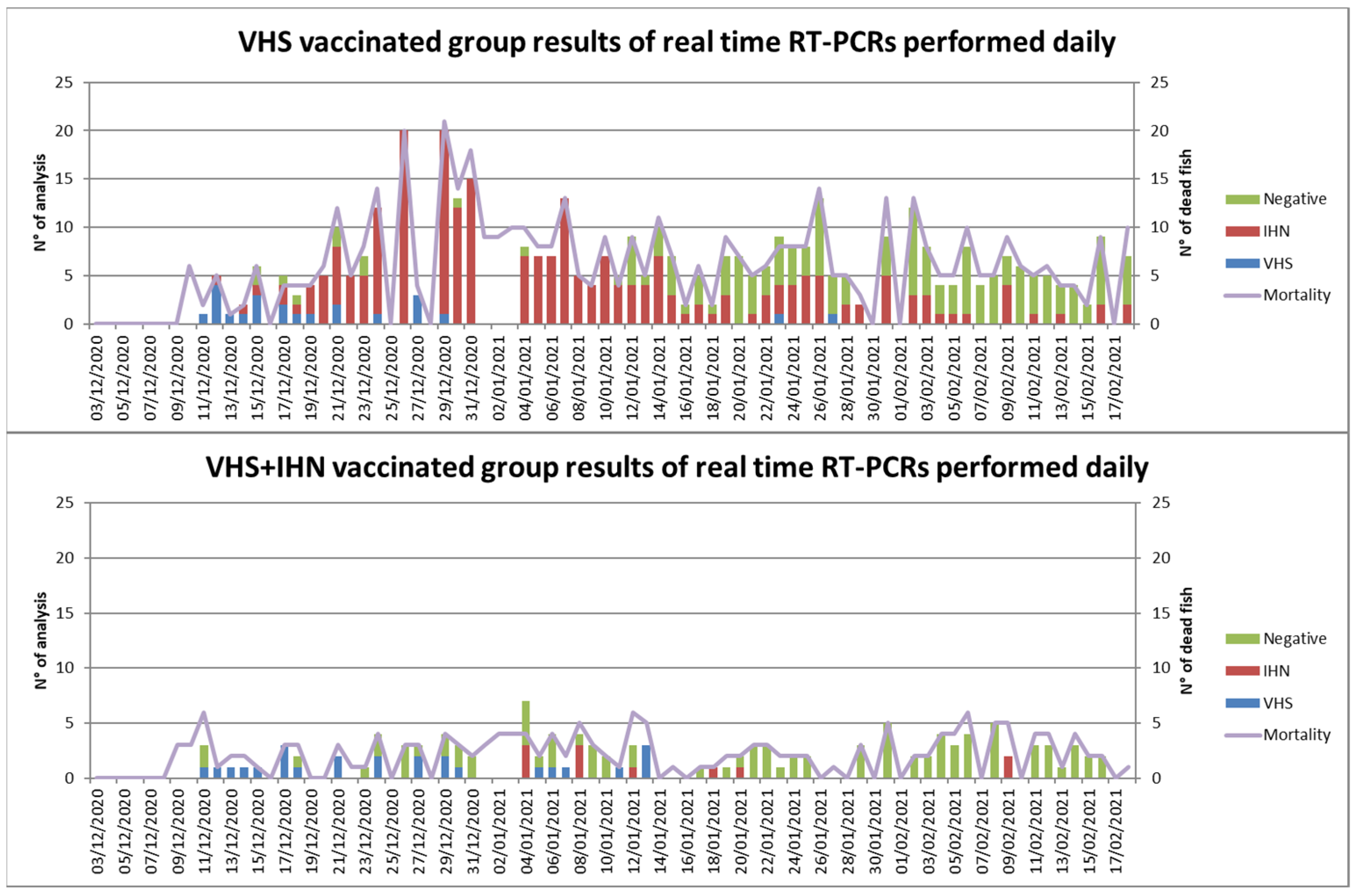
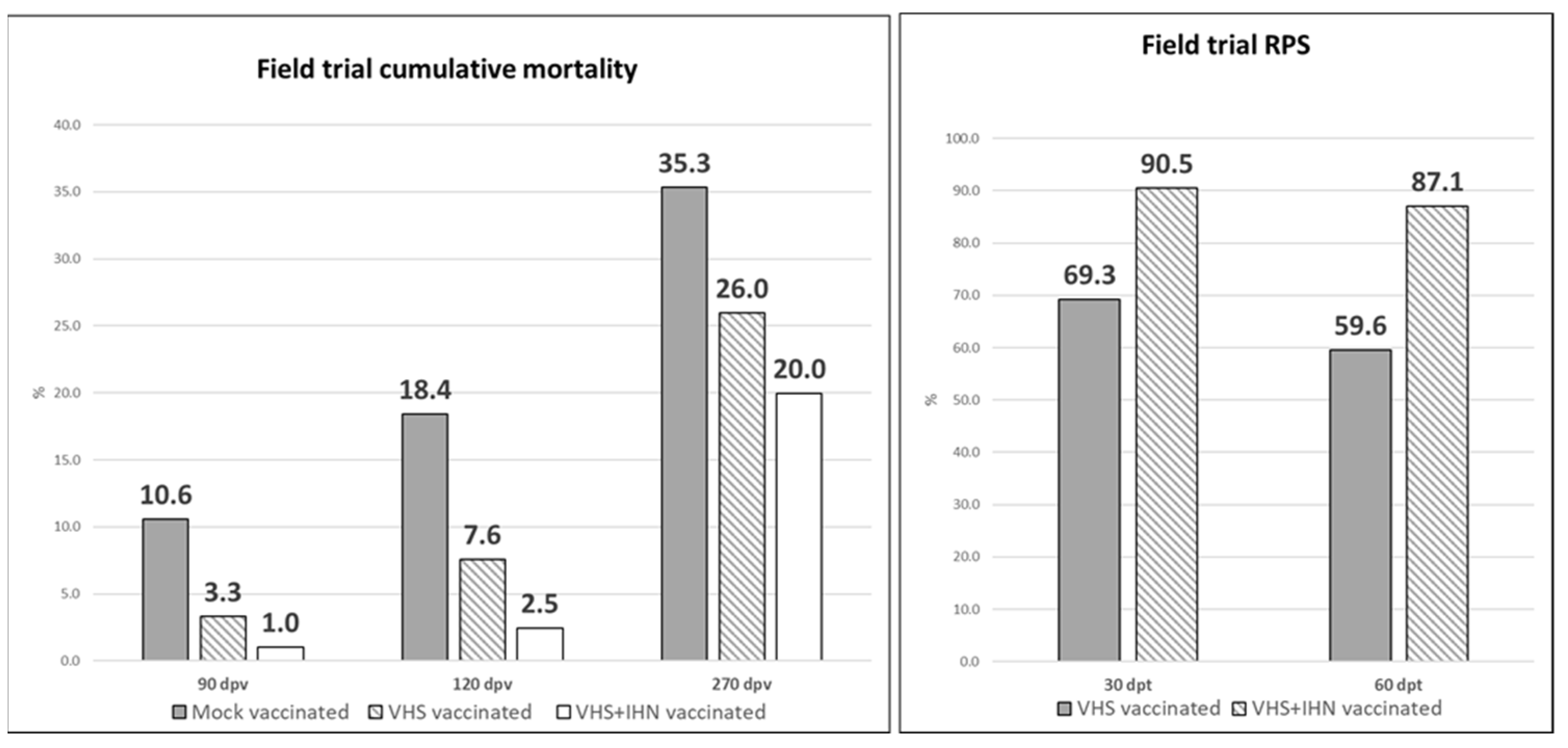
3.5. Field Trial
3.6. Plasmid Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Povinelli, M.; Toffan, A.; Piacini, A.; Gatti, F.; Ballestrazzi, R. Incidenza economica di Setticemia Emorragica Virale (SEV) e Necrosi Ematopoietica Infettiva (NEI) nelle troticolture italiane: Il caso studio del Trentino Economic impact of Viral Haemorrhagic Septicaemia (VHS) and Infectious Haematopoietic Necrosi. Ittiopat 2020, 17, 53–66. [Google Scholar]
- Abbadi, M.; Gastaldelli, M.; Pascoli, F.; Zamperin, G.; Buratin, A.; Bedendo, G.; Toffan, A.; Panzarin, V. Increased virulence of Italian infectious hematopoietic necrosis virus (IHNV) associated with the emergence of new strains. Virus Evol. 2021, 7, veab056. [Google Scholar] [CrossRef]
- Kantala, T. Update on Control and Management of IHN Outbreak in Finland Cases of IHN in Finland 2017–2018; 24thAW of the National Reference Laboratories for Fish Diseases: Lyngby, Denmark, 2020. [Google Scholar]
- Olesen, N.J.; Cuenca, A.; Vendramin, N.; Iburg, T.M. First Outbreak of IHN in Denmark IHN in Denmark for the First Time Ever; 25thAW of the National Reference Laboratories for Fish Diseases: Lyngby, Denmark, 2021. [Google Scholar]
- Lorenzen, N.; Olesen, N.J. Immunization with viral antigens: Viral haemorrhagic septicaemia. Dev. Biol. Stand. 1997, 90, 201–209. [Google Scholar]
- Biacchesi, S.; Brémont, M. Vaccination against Viral Hemorrhagic Septicemia and Infectious Hematopoietic Necrosis. Fish Vaccin. 2014, 12, 289–302. [Google Scholar] [CrossRef]
- Lapatra, S.E.; Corbeil, S.; Jones, G.R.; Shewmaker, W.D.; Kurath, G. Feature the dose-dependent effect on protection and humoral response to a dna vaccine against infectious hematopoietic necrosis (Ihn) virus in sub yearling rainbow trout. J. Aquat. Anim. Health 2000, 12, 167–180. [Google Scholar] [CrossRef]
- Lorenzen, E.; Einer-Jensen, K.; Martinussen, T.; Lapatra, S.E.; Lorenzen, N. Feature dna vaccination of rainbow trout against viral hemorrhagic septicemia virus: A dose–response and time–course study. J. Aquat. Anim. Health 2000, 12, 167–180. [Google Scholar] [CrossRef]
- Collins, C.; Lorenzen, N.; Collet, B. DNA vaccination for finfish aquaculture. Fish Shellfish Immunol. 2018, 85, 106–125. [Google Scholar] [CrossRef]
- Tonheim, T.C.; Bøgwald, J.; Dalmo, R.A. What happens to the DNA vaccine in fish? A review of current knowledge. Fish Shellfish Immunol. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Ballesteros, N.A.; Saint-Jean, S.R.; Perez-Prieto, S.I. Food pellets as an effective delivery method for a DNA vaccine against infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss, Walbaum). Fish Shellfish Immunol. 2014, 37, 220–228. [Google Scholar] [CrossRef]
- Valero, Y.; Awad, E.; Buonocore, F.; Arizcun, M.; Esteban, M.; Meseguer, J.; Chaves-Pozo, E.; Cuesta, A. An oral chitosan DNA vaccine against nodavirus improves transcription of cell-mediated cytotoxicity and interferon genes in the European sea bass juveniles gut and survival upon infection. Dev. Comp. Immunol. 2016, 65, 64–72. [Google Scholar] [CrossRef]
- Reyes, M.; Ramírez, C.; Ñancucheo, I.; Villegas, R.; Schaffeld, G.; Kriman, L.; Gonzalez, J.; Oyarzun, P. A novel “in-feed” delivery platform applied for oral DNA vaccination against IPNV enables high protection in Atlantic salmon (Salmon salar). Vaccine 2017, 35, 626–632. [Google Scholar] [CrossRef]
- Schalk, J.A.; Mooi, F.R.; Berbers, G.A.; Van Aerts, L.A.; Ovelgönne, H.; Kimman, T.G. Preclinical and Clinical Safety Studies on DNA Vaccines. Hum. Vaccines 2006, 2, 45–53. [Google Scholar] [CrossRef]
- Kurath, G.; Garver, K.A.; Corbeil, S.; Elliott, D.G.; Anderson, E.D.; LaPatra, S.E. Protective immunity and lack of histopathological damage two years after DNA vaccination against infectious hematopoietic necrosis virus in trout. Vaccine 2006, 24, 345–354. [Google Scholar] [CrossRef]
- Fijan, N.; Sulimanović, D.; Bearzotti, M.; Muzinić, D.; Zwillenberg, L.; Chilmonczyk, S.; Vautherot, J.; de Kinkelin, P. Some properties of the Epithelioma papulosum cyprini (EPC) cell line from carp cyprinus carpio. Ann. de l’Institut Pasteur/Virol. 1983, 134, 207–220. [Google Scholar] [CrossRef]
- Pascoli, F.; Bilò, F.; Marzano, F.N.; Borghesan, F.; Mancin, M.; Manfrin, A.; Toffan, A. Susceptibility of genotyped marble trout Salmo marmoratus (Cuvier, 1829) strains to experimental challenge with European viral hemorrhagic septicemia virus (VHSV) and infectious hematopoietic necrosis virus (IHNV). Aquaculture 2015, 435, 152–156. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Abbadi, M.; Fusaro, A.; Ceolin, C.; Casarotto, C.; Quartesan, R.; Pozza, M.D.; Cattoli, G.; Toffan, A.; Holmes, E.C.; Panzarin, V. Molecular Evolution and Phylogeography of Co-circulating IHNV and VHSV in Italy. Front. Microbiol. 2016, 7, 1306. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Stamatakis, A. Phylogenetic models of rate heterogeneity: A high performance computing perspective. In Proceedings of the 20th IEEE International Parallel & Distributed Processing Symposium, Rhodes, Greece, 25–29 April 2006; Volume 8, p. 253. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Overturf, K.; LaPatra, S.; Powell, M. Real-time PCR for the detection and quantitative analysis of IHNV in salmonids. J. Fish Dis. 2001, 24, 325–333. [Google Scholar] [CrossRef]
- Purcell, M.K.; Hart, S.A.; Kurath, G.; Winton, J.R. Strand-specific, real-time RT-PCR assays for quantification of genomic and positive-sense RNAs of the fish rhabdovirus, Infectious hematopoietic necrosis virus. J. Virol. Methods 2006, 132, 18–24. [Google Scholar] [CrossRef]
- Jonstrup, S.P.; Kahns, S.; Skall, H.F.; Boutrup, T.S.; Olesen, N.J. Development and validation of a novel Taqman-based real-time RT-PCR assay suitable for demonstrating freedom from viral haemorrhagic septicaemia virus. J. Fish Dis. 2012, 36, 9–23. [Google Scholar] [CrossRef]
- Kahns, S.; Skall, H.F.; Kaas, R.S.; Korsholm, H.; Jensen, B.B.; Jonstrup, S.P.; Dodge, M.J.; Einer-Jensen, K.; Stone, D.; Olesen, J. European freshwater VHSV genotype Ia isolates divide into two distinct subpopulations. Dis. Aquat. Org. 2012, 99, 23–35. [Google Scholar] [CrossRef]
- Enzmann, P.; Kurath, G.; Fichtner, D.; Bergmann, S. Infectious hematopoietic necrosis virus: Monophyletic origin of European isolates from North American Genogroup M. Dis. Aquat. Org. 2005, 66, 187–195. [Google Scholar] [CrossRef]
- Einer-Jensen, K.; Delgado, L.; Lorenzen, E.; Bovo, G.; Evensen, Ø.; LaPatra, S.; Lorenzen, N. Dual DNA vaccination of rainbow trout (Oncorhynchus mykiss) against two different rhabdoviruses, VHSV and IHNV, induces specific divalent protection. Vaccine 2009, 27, 1248–1253. [Google Scholar] [CrossRef]
- Lorenzen, E.; Lorenzen, N.; Einer-Jensen, K.; Brudeseth, B.; Evensen, O. Time course study of in situ expression of antigens following DNA-vaccination against VHS in rainbow trout (Oncorhynchus mykiss Walbaum) fry. Fish Shellfish Immunol. 2005, 19, 27–41. [Google Scholar] [CrossRef]
- Lorenzen, N.; Jensena, K.; Lapatra, S. DNA vaccines as a tool for analysing the protective immune response against rhabdoviruses in rainbow trout. Fish Shellfish Immunol. 2002, 12, 439–453. [Google Scholar] [CrossRef]
- Sommerset, I.; Lorenzen, E.; Lorenzen, N.; Bleie, H.; Nerland, A.H. A DNA vaccine directed against a rainbow trout rhabdovirus induces early protection against a nodavirus challenge in turbot. Vaccine 2003, 21, 4661–4667. [Google Scholar] [CrossRef]
- Lorenzen, N. Immunity induced shortly after DNA vaccination of rainbow trout against rhabdoviruses protects against heterologous virus but not against bacterial pathogens. Dev. Comp. Immunol. 2002, 26, 173–179. [Google Scholar] [CrossRef]
- Lorenzen, E.; Einer-Jensen, K.; Rasmussen, J.; Kjær, T.; Collet, B.; Secombes, C. The protective mechanisms induced by a fish rhabdovirus DNA vaccine depend on temperature. Vaccine 2009, 27, 3870–3880. [Google Scholar] [CrossRef]
- Sepúlveda, D.; Lorenzen, N. Can VHS Virus Bypass the Protective Immunity Induced by DNA Vaccination in Rainbow Trout? PLoS ONE 2016, 11, e0153306. [Google Scholar] [CrossRef]
- Long, A.; Richard, J.; Hawley, L.; LaPatra, S.E.; Garver, K.A. Transmission potential of infectious hematopoietic necrosis virus in APEX-IHN®-vaccinated Atlantic salmon. Dis. Aquat. Org. 2017, 122, 213–221. [Google Scholar] [CrossRef]
- Boudinot, P.; Blanco, M.D.M.; de Kinkelin, P.; Benmansour, A. Combined DNA Immunization with the Glycoprotein Gene of Viral Hemorrhagic Septicemia Virus and Infectious Hematopoietic Necrosis Virus Induces Double-Specific Protective Immunity and Nonspecific Response in Rainbow Trout. Virology 1998, 249, 297–306. [Google Scholar] [CrossRef]
- Tonheim, T.C.; Leirvik, J.; Løvoll, M.; Myhr, A.I.; Bøgwald, J.; Dalmo, R.A. Detection of supercoiled plasmid DNA and luciferase expression in Atlantic salmon (Salmo salar L.) 535days after injection. Fish Shellfish Immunol. 2007, 23, 867–876. [Google Scholar] [CrossRef]

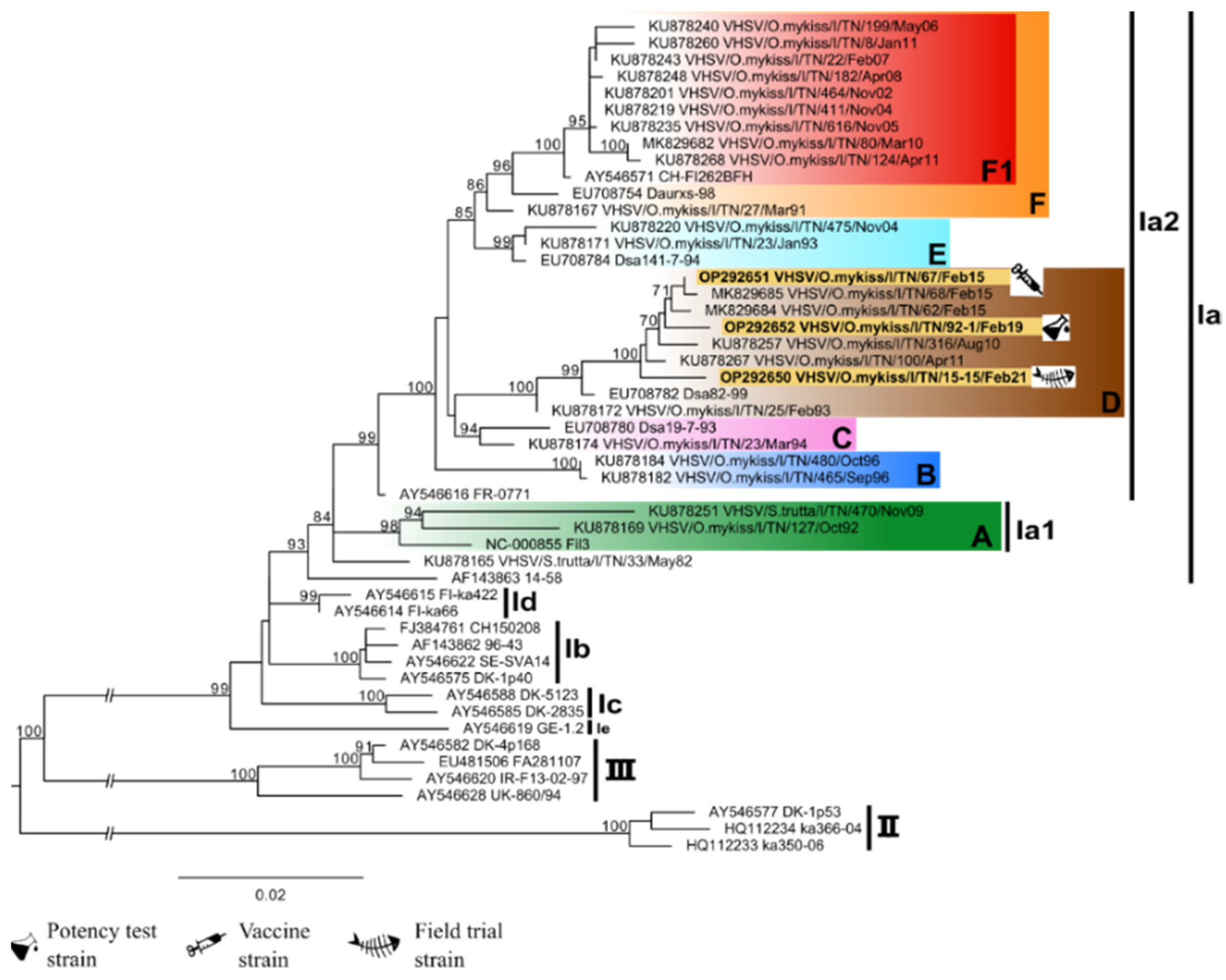
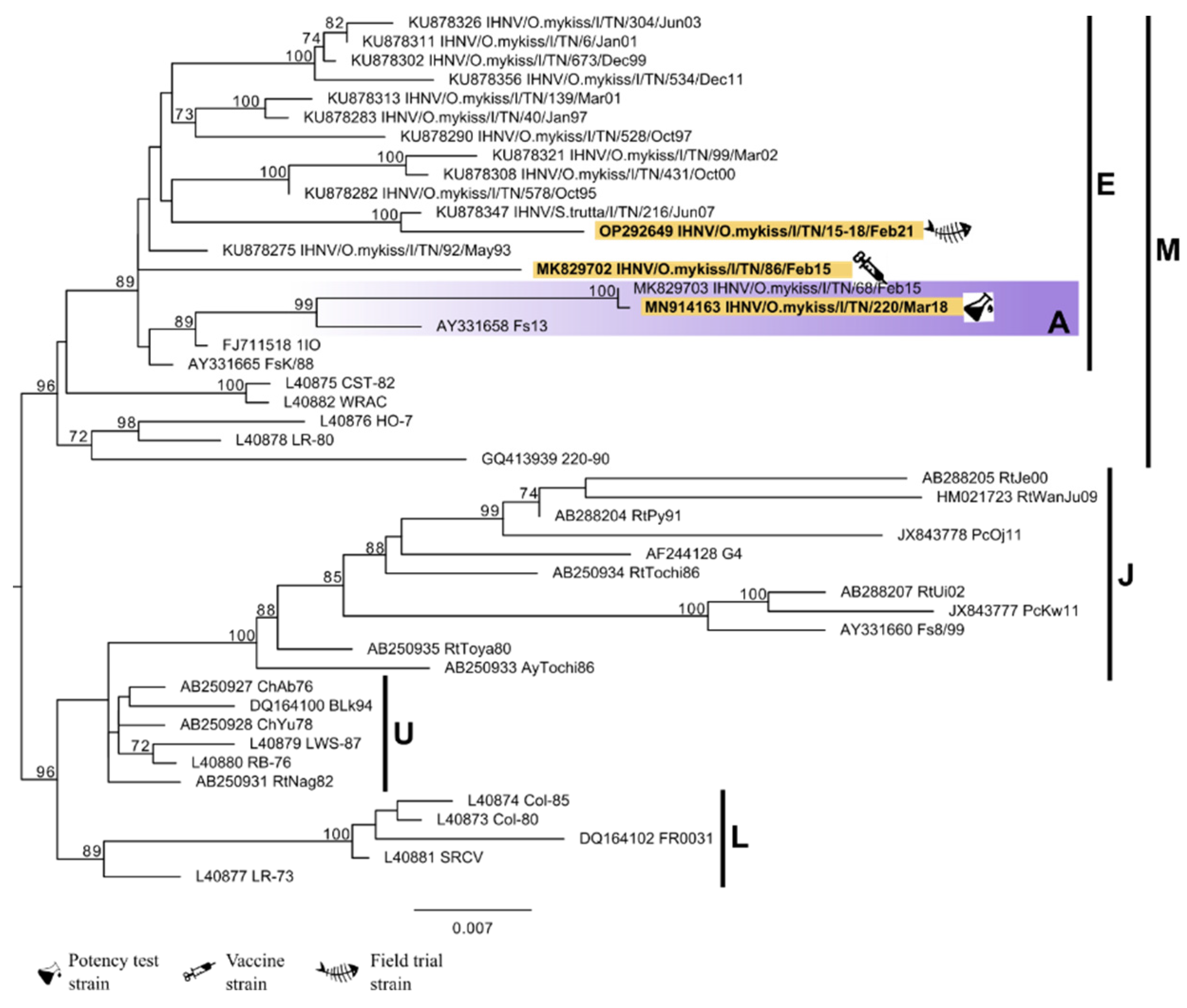
| Use within the Study | Viral Strain | Year of Isolation | Host | Accession Number |
|---|---|---|---|---|
| VHS vaccine strain | VHSV/O.mykiss /I/TN/67/Feb15 | 2015 | O. mykiss | OP292651 |
| VHS potency strain | VHSV/O.mykiss/I/TN/92-1/Feb19 | 2019 | O. mykiss | OP292652 |
| VHS field trial strain | VHSV/O.mykiss/I/TN/15-15/Feb21 | 2021 | O. mykiss | OP292650 |
| IHN vaccine strain | IHNV/O.mykiss/I/TN/86/Feb15 | 2015 | O. mykiss | MK829702 |
| IHN potency strain | IHNV/O. mykiss/I/TN/220-2/Mar18 | 2018 | O. mykiss | MN914163 |
| IHN field trial strain | IHNV/O.mykiss/I/TN/15-18/Feb21 | 2021 | O. mykiss | OP292649 |
| Tank | Group | Treatment | Challenge |
|---|---|---|---|
| 1 | Safety VHS | 2 μg/fish VHS plasmid | Unchallenged |
| 2 | Safety IHN | 2 μg/fish IHN plasmid | Unchallenged |
| 3 | Negative control | Unvaccinated | Unchallenged |
| 4 | VHS/IHN mock | Mock vaccinated | VHSV + IHNV bath challenged |
| 5 | VHS mock | Mock vaccinated | VHSV bath challenged |
| 6 | IHN mock | Mock vaccinated | IHNV bath challenged |
| 7 | VHS 0.1 | 0.1 μg/fish VHS plasmid | VHSV bath challenged |
| 8 | VHS 1 | 1 μg/fish VHS plasmid | VHSV bath challenged |
| 9 | IHN 0.1 | 0.1 μg/fish IHN plasmid | IHNV bath challenged |
| 10 | IHN 1 | 1 μg/fish IHN plasmid | IHNV bath challenged |
| 11 | VHS/IHN 0.1 | 0.1 μg/fish VHS plasmid + 0.1 μg/fish IHN plasmid | VHSV + IHNV bath challenged |
| 12 | VHS/IHN 1 | 1 μg/fish VHS plasmid + 1 μg/fish IHN plasmid | VHSV + IHNV bath challenged |
| PCR | Primer | Sequence 5’→3’ | Amplicon Size | T Annealing (°C) |
|---|---|---|---|---|
| pVax1-vhsG | F1_(pVAX1)_VHSV R1_pVAX1_(VHSV) | CAATGGGAGTTTGTTTTGGCACCAA TGCAGTTGGAGGGATGAGTGTATAG | 370 | 62 |
| pVax1-ihnG | F1_(pVAX1)_IHNV R1_pVAX1_(IHNV) | GGCTAACTAGAGAACCCACTGCTTA GGATAGGCAATTAGTCCCCTGTTCT | 327 | 58 |
| Endogenous gene | B-Glob_FOR B-Glob_REV | TCCATGGACTCAGAGACACTTC GACACAACGACAGCCAGGAA | 443 | 57 |
| Group ID | Tank | Treatment | Challenge Virus and Dose | Mortality (%) | RPS |
|---|---|---|---|---|---|
| Safety test VHS | 1 | 2 μg VHS plasmid | - | 0 | - |
| Safety test IHN | 2 | 2 μg IHN plasmid | - | 0 | - |
| Negative control | 3 | 100 μL PBS | - | 0 | - |
| Positive control VHS IHN | 4 | 100 μL PBS | 106.30 TCID50/mL × (VHSV 92-1 + IHNV 220-2) | 100 | - |
| Positive control VHS | 5 | 100 μL PBS | 104.55 TCID50/mL × VHSV 92-1 | 83.6 | - |
| Positive control IHN | 6 | 100 μL PBS | 104.55 TCID50/mL × IHNV 220-2 | 40.4 | - |
| VHS 0,1 | 7 | 0.1 μg VHS plasmid | 104.55 TCID50/mL × VHSV 92-1 | 42.5 | 49.2 |
| VHS 1 | 8 | 1 μg VHS plasmid | 104.55 TCID50/mL × VHSV 92-1 | 17.9 | 78.5 |
| IHN 0,1 | 9 | 0.1 μg IHN plasmid | 104.30 TCID50/mL × IHNV 220-2 | 23.5 | 44.6 |
| IHN 1 | 10 | 1 μg IHN plasmid | 104.55/mL × IHNV 220-2 | 11.1 | 72.5 |
| VHS IHN 0,1 | 11 | 0.1 μg/fish VHS plasmid + 0.1 μg/fish IHN plasmid | 106.05 TCID50/mL × (VHSV 92-1+ IHNV 220-2) | 14.3 | 87.5 |
| VHS IHN 1 | 12 | 1 μg/fish VHS plasmid + 1 μg/fish IHN plasmid | 105.80 TCID50/mL × (VHS 92-1+ IHN 220-2) | 10.8 | 90 |
| Tank | ID | Organ | VHSV | IHNV |
|---|---|---|---|---|
| 11 | 1 | Spleen | Pos (26.98) | Pos (30.55) |
| Kidney | Pos (20.48) | Pos (27.31) | ||
| 2 | Spleen | Pos (27.74) | Pos (32.69) | |
| Kidney | Pos (22.53) | Pos (28.92) | ||
| 3 | Spleen | Pos (24.89) | Pos (32.54) | |
| Kidney | Pos (19.71) | Pos (28.82) | ||
| 4 | Spleen | Pos (30.58) | Pos (29.03) | |
| Kidney | Pos (30.14) | Pos (33.55) | ||
| 5 | Spleen | Pos (22.39) | Neg | |
| Kidney | Pos (22.39) | Neg | ||
| 12 | 1 | Spleen | Pos (29.67) | Neg |
| Kidney | Pos (24.64) | Neg | ||
| 2 | Spleen | Pos (30.06) | Neg | |
| Kidney | Pos (20.07) | Neg | ||
| 3 | Spleen | Doubt (36.00) | Neg | |
| Kidney | Pos (33.65) | Neg | ||
| 4 | Spleen | Neg | Neg | |
| Kidney | Neg | Neg |
| Timepoint | VHSV | IHNV | Bacteriological Examinations |
|---|---|---|---|
| 64 dpv | Pos (18.79 ct) | Neg | Neg |
| 69 dpv | Pos (19 ct) | Neg | Neg |
| 76 dpv | Pos (22.54 ct) | Neg | Neg |
| 84 dpv | Pos (22.94 ct) | Neg | Positive to A.papoffii, A.sobria, A. bestiarum |
| 90 dpv | Pos (23.00 ct) | Pos (18.50 ct) | Neg |
| 98 dpv | Pos * | Pos * | Positive to A. sobria and P. shigelloides |
| 105 dpv | Pos (21.35 ct) | Positive to A. sobria | |
| 111 dpv | Pos (27.24 ct) | Pos (26.33 ct) | Neg |
| 118 dpv | Neg | Pos (28.2 ct) | Positive to A. dhakensis |
| 125 dpv | Neg | Pos (20.96 ct) | Positive to Y. Ruckeri biotype II |
| 134 dpv | Pos (32.33 ct) | Neg | Neg |
| Timepoint | Group | Real-Time RT-PCR VHSV | Real-Time RT-PCR IHNV | Bacteriological Examinations |
|---|---|---|---|---|
| 182 dpv | Mock | Pos (35.28 ct) | Pos (20.86 ct) | Neg |
| VHS | Pos (35.14 ct) | Pos (19.75 ct) | Neg | |
| VHS+IHN | Pos (31.22 ct) | Pos (18.67 ct) | Neg | |
| 210 dpv | Mock | Neg | Pos (24.85 ct) | Positive to Y. Ruckeri biotype II |
| VHS | Pos (26.30 ct) | Neg | Positive to Y. Ruckeri biotype II | |
| VHS+IHN | Neg | Pos (31.69 ct) | Positive to Y. Ruckeri biotype II | |
| 232 dpv | Mock | Neg | Pos (27.90 ct) | Neg |
| VHS | Neg | Pos (33.86 ct) | Neg | |
| VHS+IHN | Neg | Pos (27.40 ct) | Neg | |
| 280 dpv | Mock | Neg | Pos (31.77 ct) | Neg |
| VHS | Neg | Pos (34.66 ct) | Neg | |
| VHS+IHN | Neg | Neg | Neg |
| Trial | Plasmid Detection | pVax1-vhsG-Positive | pVax1-ihnG-Positive | |
|---|---|---|---|---|
| Time Point (dpv) | ||||
| Potency test | 90 | 5 /5 | 5/5 | |
| Field trial | 120 | 1/5 | 1/5 | |
| 160 | 3/5 | 3/5 | ||
| 180 | 3/5 | 2/5 | ||
| 210 | 2/5 | 2/5 | ||
| 230 | 3/5 | 3/5 | ||
| 260 | 4/5 | 0/5 | ||
| 280 | 0/5 | 0/5 | ||
| 320 | 6/15 | 6/15 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsella, A.; Pascoli, F.; Pretto, T.; Buratin, A.; Biasini, L.; Abbadi, M.; Cortinovis, L.; Berto, P.; Manfrin, A.; Vanelli, M.; et al. Efficacy of DNA Vaccines in Protecting Rainbow Trout against VHS and IHN under Intensive Farming Conditions. Vaccines 2022, 10, 2062. https://doi.org/10.3390/vaccines10122062
Marsella A, Pascoli F, Pretto T, Buratin A, Biasini L, Abbadi M, Cortinovis L, Berto P, Manfrin A, Vanelli M, et al. Efficacy of DNA Vaccines in Protecting Rainbow Trout against VHS and IHN under Intensive Farming Conditions. Vaccines. 2022; 10(12):2062. https://doi.org/10.3390/vaccines10122062
Chicago/Turabian StyleMarsella, Andrea, Francesco Pascoli, Tobia Pretto, Alessandra Buratin, Lorena Biasini, Miriam Abbadi, Luana Cortinovis, Paola Berto, Amedeo Manfrin, Marco Vanelli, and et al. 2022. "Efficacy of DNA Vaccines in Protecting Rainbow Trout against VHS and IHN under Intensive Farming Conditions" Vaccines 10, no. 12: 2062. https://doi.org/10.3390/vaccines10122062
APA StyleMarsella, A., Pascoli, F., Pretto, T., Buratin, A., Biasini, L., Abbadi, M., Cortinovis, L., Berto, P., Manfrin, A., Vanelli, M., Perulli, S., Rasmussen, J. S., Sepúlveda, D., Vendramin, N., Lorenzen, N., & Toffan, A. (2022). Efficacy of DNA Vaccines in Protecting Rainbow Trout against VHS and IHN under Intensive Farming Conditions. Vaccines, 10(12), 2062. https://doi.org/10.3390/vaccines10122062






