Abstract
This prospective cohort survey evaluated the concordance of clinicians’ perceptions of parental intentions and parents’ actual intentions to vaccinate their infants against influenza. During a routine healthy baby visit, clinicians provided parents with information about influenza, children’s vulnerability to influenza, and nonadjuvanted and adjuvanted trivalent influenza vaccines (TIV and aTIV, respectively). Before and after the clinician–parent interaction, parents were surveyed about their attitudes, their perceptions of support from significant others, and the intention to vaccinate their infant with aTIV. Clinicians were asked about their perception of parents’ intentions to choose aTIV for their children. These assessments included 24 clinicians at 15 community practices and nine public health clinics, and 207 parents. The correlation coefficients of the clinicians’ assessment of parents’ intention to vaccinate were 0.483 (p < 0.001) if the vaccine was presented as free of cost, 0.266 (p < 0.001) if the cost was $25, and 0.146 (p = 0.036) if the cost was $50, accounting for 23%, 7%, and 2% of the variance in parental intentions, respectively. The clinicians were poor at predicting parental intentions to immunize, particularly when cost was involved. Information on vaccine options and influenza infection should be provided for every eligible patient to allow parents to determine if the vaccine is appropriate for their child.
1. Introduction
Children represent approximately 13% of the population infected with influenza globally and are at elevated risk of influenza-related complications [1,2]. Worldwide, influenza-related mortality ranges from 2.1 to 23.8 per 100,000 population among children younger than 5 years, and the highest percentages of hospitalizations and death are among children younger than 2 years [3].
Seasonal vaccination against influenza is recommended for certain subpopulations, including children aged 6 through 23 months, that account for a large proportion of influenza-related hospitalizations and deaths [4,5]. In Canada, three types of influenza vaccines are currently licensed for use in children of 6 through to 23 months of age: trivalent inactivated influenza vaccines (TIV), quadrivalent inactivated influenza vaccines (QIV), and MF59-adjuvanted trivalent inactivated influenza vaccines (aTIV; Fluad®, Seqirus UK Limited). aTIV has been shown to elicit an earlier, stronger, broader, and more persistent immune response in children when compared to nonadjuvanted TIV [6,7], particularly when evaluated across pediatric-relevant correlates of protection [8].
Despite the significant morbidity and mortality caused by influenza each year, as well as the availability of safe vaccines, low influenza vaccine uptakes within high-risk groups remain a global challenge and contribute to the burden of disease [9]. While parental vaccine concerns and vaccine misinformation circulating in social media and other outlets play an important role in parents’ immunization intentions [10], studies have shown that physician recommendations are the single most important facilitator of vaccine uptake in children of 6 through to 23 months of age [11,12,13].
At least anecdotally, clinicians often report that they do not offer vaccines with the same intensity of recommendation or even at all if they perceive that patients (or their parents) may be uninterested or unwilling to accept the vaccination in question. The ability of clinicians to correctly infer their patients’ readiness to accept seasonal influenza vaccination, however, remains largely unstudied. The objective of this research was to evaluate the concordance, or lack thereof, of clinicians’ perceptions of parents’ intentions to vaccinate their infants and the actual parental intentions to do so with a novel seasonal influenza vaccine. An additional aim of this study was to determine whether the accuracy of clinician perceptions of parental intentions to vaccinate may deteriorate when cost is involved, as in the case of a novel, approved, but not yet publicly funded vaccines. Accordingly, we assessed the accuracy of clinician perceptions of parental intentions to vaccinate infants with publicly funded influenza vaccines, as well as with vaccines that cost increasing amounts at parental expense.
2. Methods
2.1. Study Design and Population
This was a prospective cohort survey design conducted during the 2015–2016 influenza season. A complete description of the study design can be found in the PIVOT-I study report by Fisher et al. Briefly, the study population consisted of the parents of infants aged 6 to 23 months of age who were presented for a scheduled healthy baby visit. During the consultation with clinicians (physicians in community settings or nurses in a public health setting), information about influenza and aTIV was provided to parents (Figure 1). Parents were administered surveys by research nurses before and after the clinician interaction.
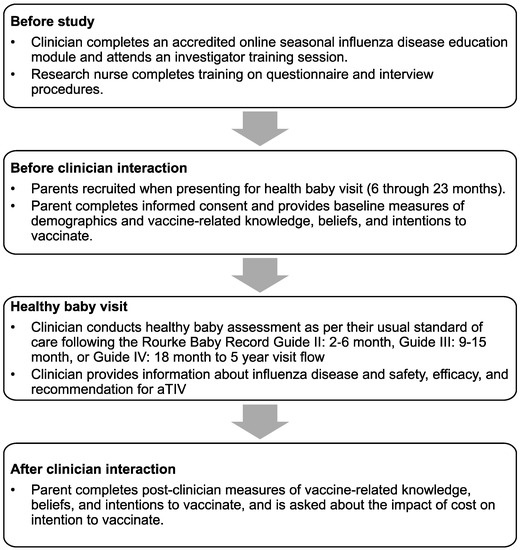
Figure 1.
Study recruitment procedures and protocol flow.
2.2. Study Procedures
After the clinician interaction, parents were asked about their intentions to vaccinate their infant with aTIV if it were provided free of charge, if it cost $25 and if it cost $50 and were asked to report their perceptions of the strength of the clinicians’ recommendation of vaccination with aTIV. Clinicians were asked, separately, about their perception of the parent’s intention to have their infants vaccinated with aTIV if free of charge, $25, and $50.
Parents’ intentions to vaccinate their infants with aTIV were assessed with a 7-point Likert scale where 1 = “Strongly agree” and 7 = “Strongly disagree” that the parent intended to vaccinate their infant with aTIV. The clinicians’ assessments of the parental intention to vaccinate with aTIV were assessed similarly with 7-point Likert scales where 1 = “Strongly agree that the parent intends to vaccinate their baby with the adjuvanted seasonal flu vaccine” and 7 = “Strongly disagree that the parent intends to vaccinate their baby with the adjuvanted seasonal flu vaccine.” Parental perceptions of the strength of the clinician’s recommendation to vaccinate with aTIV were assessed with a 7-point Likert scale where 1 = “Strongly recommended” and 7 = “Strongly discouraged.” This study was conducted prior to the availability of aTIV and focused on the parental intentions and clinicians’ assessment of parental intentions to opt for the vaccine when it became available. The research protocol received ethics approval from the Western University Health Sciences Research Ethics Board, the Fraser Health Research Ethics Board, and IRB Services.
2.3. Statistical Analysis
Correlation coefficients and corresponding 2-tailed measures of significance were calculated to assess the association of the clinicians’ perceptions of parental intentions to vaccinate their infants with aTIV and parental intentions to vaccinate per se. Correlation coefficients also assessed the relationship between the clinicians’ assessments of parental intentions to vaccinate their infants with aTIV and parents’ perceptions of the strength of the clinicians’ recommendations to do so.
3. Results
3.1. Sample Characteristics
A total of 18 community practice and public health clinics across Canada participated; 15 community practice clinics enrolled 136 parents, while three public health clinics enrolled 71 parents (N = 207). The baseline demographics and characteristics are presented in Table 1.

Table 1.
Sociodemographic characteristics.
3.2. Clinician Perceptions of Parental Intentions
Healthcare providers were generally poor at predicting parental intentions to vaccinate their children with aTIV (Table 2). Clinician perceptions were most concordant with parental intentions in relation to parental intentions to accept the vaccine “when it becomes available” (r = 0.60, p < 0.001) and when the vaccine was presented as free (r = 0.483, p < 0.001). Even in the context of these statistically significant correlations, we noted that the clinician’s perceptions only accounted for 23% to 36% of the variance in parental intentions. When adding the cost of the vaccine, as is the case with approved but unfunded vaccines, the clinician’s concordance with parental intentions deteriorated. The clinician’s perceptions of the parental intentions to vaccinate accounted for only 7% of the variance if the vaccine cost $25 (r = 0.266, p < 0.001) and for only 2% of the variance if the vaccine cost $50 (r = 0.146, p < 0.02).

Table 2.
Correlation between clinicians’ assessment of parents’ intentions to vaccinate with the adjuvanted seasonal flu vaccine and parents’ actual intentions to vaccinate.
In a related analysis, it was determined that the clinicians’ perceptions of parents’ intentions to vaccinate their infants were associated with the parents’ perceptions of the strength of the clinicians’ recommendations to do so (r = 0.324, p < 0.001).
4. Discussion
These results shed light on the accuracy of clinicians’ perceptions of parents’ interests in vaccinating their children against influenza. Clinicians’ perceptions of parental interest in vaccinating infants with aTIV accounted for substantially less than half of the variance in the parents’ actual intentions to do so. When cost was involved, as is the case with approved but not publicly funded vaccines, the clinicians’ assessments of parental intentions to vaccinate their infants accounted for a very small amount (2% to 7%) of the variance of the parent’s actual intentions to vaccinate. Moreover, the clinicians’ assessments of the parent’s intentions to vaccinate their infants were associated with the parent’s perceptions of the strength of clinicians’ recommendations to vaccinate their infants. While this finding is correlational, and causality cannot be determined, it is possible that the clinicians who perceived parents’ interest in vaccinating their infants to be weaker gave correspondingly weaker recommendations to vaccinate. It is also possible that the clinicians who gave weaker recommendations to vaccinate influenced the parents to form weaker intentions to vaccinate their infant or that parents with weaker intentions to vaccinate were inclined to perceive clinician recommendations as relatively weak. All of these possibilities are worthy of further investigation.
Clinician recommendations are widely regarded as an important influence on vaccine acceptance. Our findings indicate that clinicians cannot accurately predict the parental acceptance of infant vaccination against seasonal influenza with a particular vaccine, especially if cost is involved. Our findings also suggest that parents who perceive weaker clinician recommendations of vaccination express weaker intentions to vaccinate their infants. While further research is needed—among the limitations of this research, we focused on intentions to use a vaccine that was not yet available, not on vaccination per se—and it seems clear that clinicians are not on particularly solid ground when attempting to gauge parental interest in vaccinating their infants. Accordingly, it would seem most reasonable to provide vaccine-related and preventable disease–related information to all parents of eligible infants so as to permit them to make vaccine decisions that are independent of clinician assumptions about parental acceptance. This seems to be especially important in the case of approved but unfunded vaccines because the clinician assessment of parental interest was weakest in this setting. It would also seem important to untangle the association we observed between the clinician perception of weaker parental intentions to vaccinate and the parental perception of weaker clinician recommendations of vaccination, given the importance of clinician recommendation influence on vaccine uptake.
5. Conclusions
Clinicians were poor at predicting the parental intention to immunize with aTIV. Information on the vaccine and the disease should be provided to every eligible patient to allow parents the option to determine if the vaccine is appropriate for their child.
Author Contributions
All authors participated in the design of the study, the acquisition of data, or analysis and interpretation of data. All authors made substantial contributions to the drafting of the article or provided important and critical revisions for intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Novartis Vaccines and Diagnostics, Inc. and Seqirus Inc. Of note, Novartis’ influenza vaccine business was acquired by the CSL-group on 31 July 2015, and currently operates as Seqirus, Inc. The sponsor had primary responsibility for study design and study vaccines, protocol development, study monitoring, data management, and statistical analyses.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Western University Health Sciences Research Ethics Board, the Fraser Health Research Ethics Board, and IRB Services (protocol 106417, 21 October 2015).
Informed Consent Statement
All participants provided written and informed consent.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank the team at MD Briefcase for CME development and collaboration for project management. The authors additionally wish to thank C. Gordon Beck and Amanda Justice (Princeton Biomedical Communications, LLC, Princeton, NJ), for editorial assistance in the preparation of the manuscript. Funding for this support was provided by Seqirus, Inc.
Conflicts of Interest
Constantina Boikos is an employee of Seqirus. William A. Fisher has been a consultant to Novartis and Seqirus. James A. Mansi was an employee of Seqirus at the time of this study and is currently employed by Moderna, Inc.
References
- Nair, H.; Brooks, W.A.; Katz, M.; Roca, A.; Berkley, J.A.; Madhi, S.A.; Simmerman, J.M.; Gordon, A.; Sato, M.; Howie, S.; et al. Global burden of respiratory infections due to seasonal influenza in young children: A systematic review and meta-analysis. Lancet 2011, 378, 1917–1930. [Google Scholar] [CrossRef]
- Schanzer, D.L.; Langley, J.M.; Tam, T.W.S. Hospitalization Attributable to Influenza and Other Viral Respiratory Illnesses in Canadian Children. Pediatr. Infect. Dis. J. 2006, 25, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Poehling, K.A.; Edwards, K.M.; Griffin, M.R.; Szilagyi, P.G.; Staat, M.A.; Iwane, M.K.; Snively, B.M.; Suerken, C.K.; Hall, C.B.; Weinberg, G.A.; et al. The burden of influenza in young children, 2004–2009. Pediatrics 2013, 131, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Iwane, M.K.; Edwards, K.M.; Szilagyi, P.G.; Walker, F.J.; Griffin, M.R.; Weinberg, G.A.; Coulen, C.; Poehling, K.A.; Shone, L.P.; Balter, S.; et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics 2004, 113, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Coffin, S.E.; Zaoutis, T.E.; Rosenquist, A.B.; Heydon, K.; Herrera, G.; Bridges, C.B.; Watson, B.; Localio, R.; Hodinka, R.L.; Keren, R. Incidence, complications, and risk factors for prolonged stay in children hospitalized with community-acquired influenza. Pediatrics 2007, 119, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Bravo, L.; Ceballos, A.; Mitha, E.; Gray, G.; Quiambao, B.; Patel, S.S.; Bizjajeva, S.; Bock, H.; Nazaire-Bermal, N.; et al. Enhanced and persistent antibody response against homologous and heterologous strains elicited by a MF59-adjuvanted influenza vaccine in infants and young children. Vaccine 2014, 32, 6146–6156. [Google Scholar] [CrossRef]
- Vesikari, T.; Knuf, M.; Wutzler, P.; Karvonen, A.; Kieninger-Baum, D.; Schmitt, H.J.; Baehner, F.; Borkowski, A.; Tsai, T.F.; Clemens, R. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N. Engl. J. Med. 2011, 365, 1406–1416. [Google Scholar] [CrossRef]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; Del Giudice, G.; Della Cioppa, G.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr. Infect. Dis. J. 2011, 30, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Rudan, I.; Boschi-Pinto, C.; Biloglav, Z.; Mulholland, K.; Campbell, H. Epidemiology and etiology of childhood pneumonia. Bull. World Health Organ. 2008, 86, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.; Buttenheim, A.M. Parental vaccine concerns, information source, and choice of alternative immunization schedules. Hum. Vaccines Immunother. 2013, 9, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Nowalk, M.P.; Zimmerman, R.K.; Lin, C.J.; Ko, F.S.; Raymund, M.; Hoberman, A.; Kearney, D.H.; Greenberg, D.P. Parental perspectives on influenza immunization of children aged 6 to 23 months. Am. J. Prev. Med. 2005, 29, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Nowalk, M.P.; Lin, C.J.; Zimmerman, R.K.; Ko, F.S.; Hoberman, A.; Zoffel, L.; Kearney, D.H. Changes in parents’ perceptions of infant influenza vaccination over two years. J. Natl. Med. Assoc. 2007, 99, 636–641. [Google Scholar] [PubMed]
- Ma, K.K.; Schaffner, W.; Colmenares, C.; Howser, J.; Jones, J.; Poehling, K.A. Influenza vaccinations of young children increased with media coverage in 2003. Pediatrics 2006, 117, e157–e163. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).