Long-Term CD4+ T-Cell and Immunoglobulin G Immune Responses in Oncology Workers following COVID-19 Vaccination: An Interim Analysis of a Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Blood-Sample Collection
2.2. Isolation of Peripheral Blood Mononuclear Cells and Serum from Whole Blood
2.3. Interferon Gamma Enzyme-Linked Immunospot Assay
2.4. Immunoglobulin G Antibody Enzyme-Linked Immunosorbent Assay
2.5. Antibody Response to SARS-CoV-2 Variants
2.6. HLA Antigen Class I– and II–Blocking
2.7. Endpoints and Statistical Methods
3. Results
3.1. Baseline Participant Characteristics, Risk of COVID-19, Vaccination Status, and Complications during Study
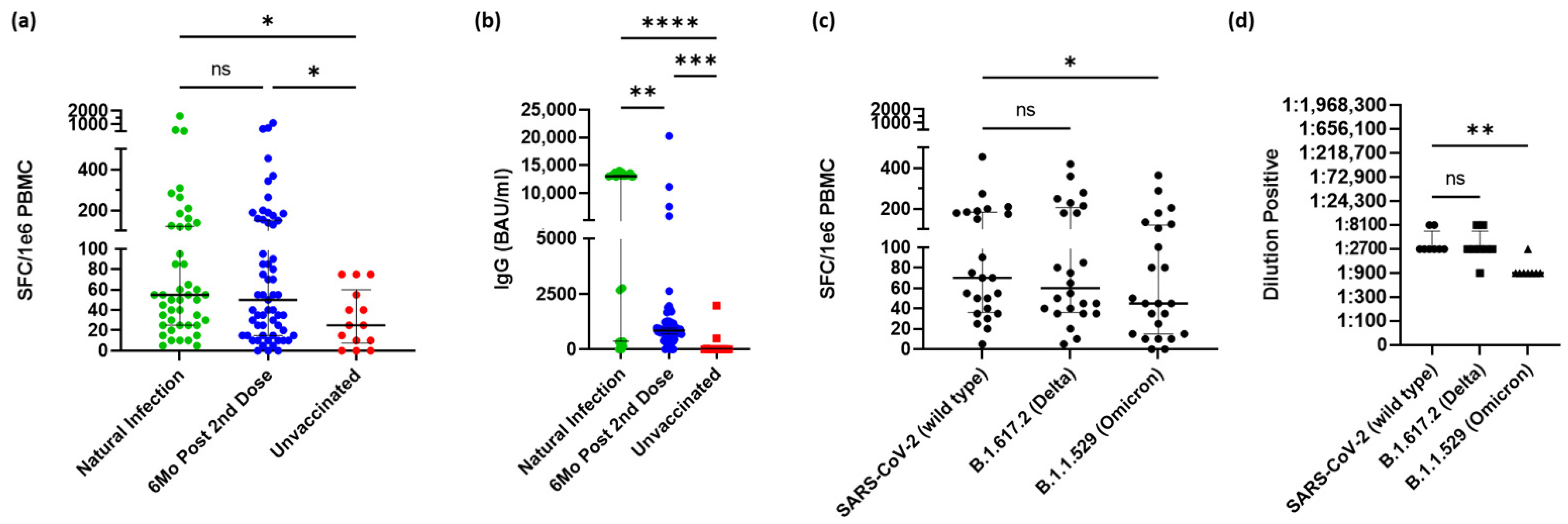
3.2. SARS-CoV-2 Immune Response 6 Months after Vaccination
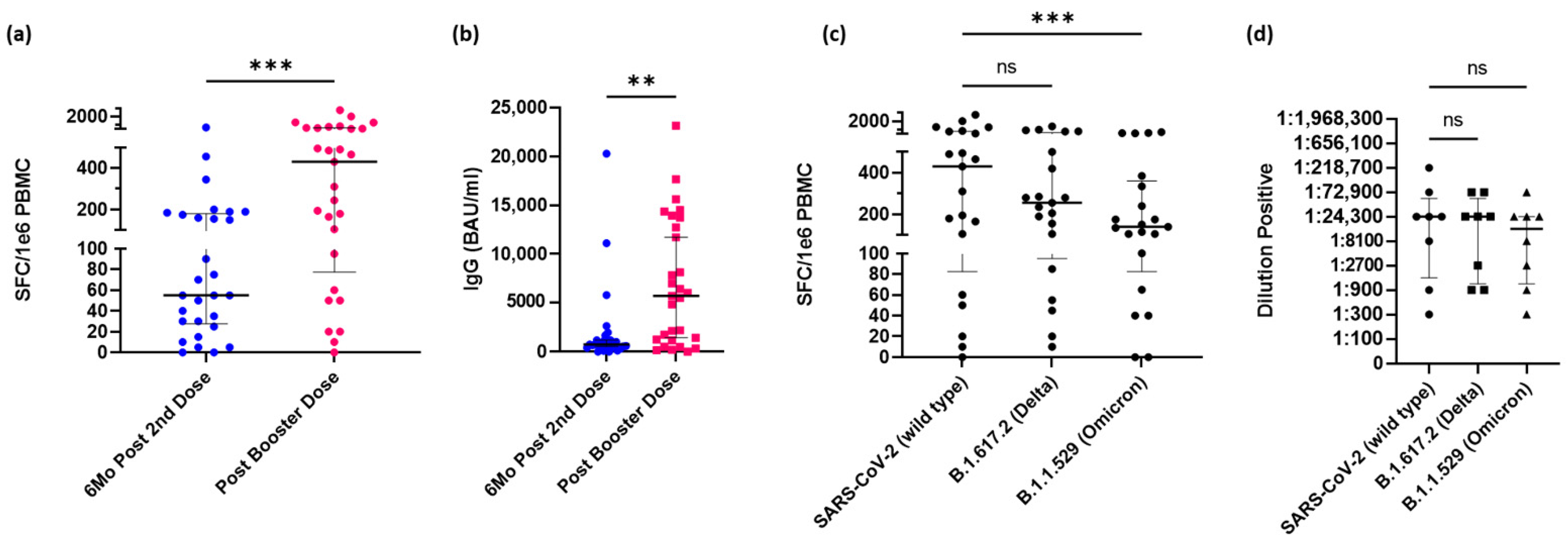
3.3. Booster Dose of mRNA Vaccine Increases Immune Response
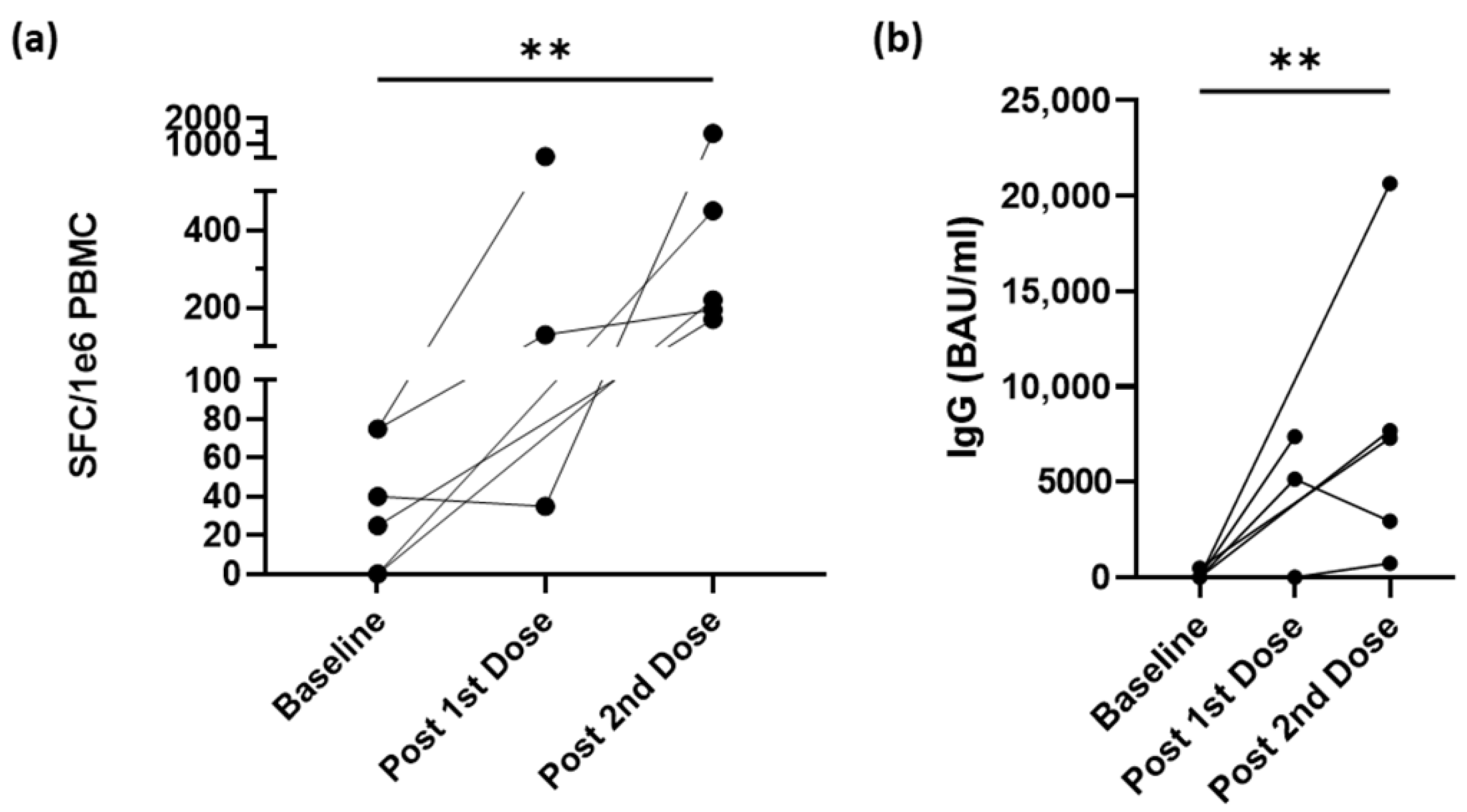
3.4. Vaccination Increases IFN-γ–Secreting CD4+ T-cells and IgG Antibodies
3.5. Spike-Reactive CD4+ T-cells Wane following Booster but Not below Pre-Boosted Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baric, R.S. Emergence of a Highly Fit SARS-CoV-2 Variant. N. Engl. J. Med. 2020, 383, 2684–2686. [Google Scholar] [CrossRef] [PubMed]
- Diken, M.; Kreiter, S.; Vascotto, F.; Selmi, A.; Attig, S.; Diekmann, J.; Huber, C.; Tureci, O.; Sahin, U. mTOR inhibition improves antitumor effects of vaccination with antigen-encoding RNA. Cancer Immunol. Res. 2013, 1, 386–392. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef]
- Primorac, D.; Vrdoljak, K.; Brlek, P.; Pavelic, E.; Molnar, V.; Matisic, V.; Erceg Ivkosic, I.; Parcina, M. Adaptive Immune Responses and Immunity to SARS-CoV-2. Front. Immunol. 2022, 13, 848582. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.; Lewis, M.W.; Hansen, S.G.; Strelow, L.I.; Nelson, J.A.; Sexton, G.J.; Hanifin, J.M.; Slifka, M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003, 9, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Barrat Hernández, A.Q.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.K.; Moore, Z.; Zeng, D. Effectiveness of COVID-19 Vaccines over a 9-Month Period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Pilon-Thomas, S.; Schell, M.J.; Abrahamsen, M.; Islam, J.Y.; Isaacs-Soriano, K.; Kennedy, K.; Dukes, C.W.; Whiting, J.; Rathwell, J.; et al. SARS-CoV-2 Period Seroprevalence and Related Factors, Hillsborough County, Florida, USA, October 2020–March 2021. Emerg. Infect. Dis. 2022, 28, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Lancet, J.E.; Pilon-Thomas, S.; Dong, N.; Jain, A.G.; Tan, E.; Ball, S.; Tworoger, S.S.; Siegel, E.M.; Whiting, J.; et al. Evaluation of Antibody Response to SARS-CoV-2 mRNA-1273 Vaccination in Patients with Cancer in Florida. JAMA Oncol. 2022, 8, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, D.; Amanat, F.; Chromikova, V.; Jiang, K.; Strohmeier, S.; Arunkumar, G.A.; Tan, J.; Bhavsar, D.; Capuano, C.; Kirkpatrick, E.; et al. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr. Protoc. Microbiol. 2020, 57, e100. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Diaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverria, L.E.; Glisic, M.; Muka, T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]


| Vaccinated Participant N = 60 (81%) | Unvaccinated Participant N = 14 (19%) | Total N = 74 (100%) | |
|---|---|---|---|
| Age (median and range) | 42.5 (24–70) | 37 (25–55) | 42 (24–70) |
| Gender | |||
| Male | 12 (20%) | 0 (0%) | 12 (16%) |
| Female | 48 (80%) | 14 (100%) | 62 (83%) |
| Race | |||
| White | 52 (87%) | 12 (86%) | 64 (86%) |
| Black or African American | 1 (2%) | 2 (14%) | 3 (4%) |
| Asian | 5 (8%) | 0 | 5 (7%) |
| Some other race | 2 (3%) | 0 | 2 (3%) |
| Ethnicity | |||
| NonHispanic or Latino | 50 (83%) | 10 (71%) | 60 (81%) |
| Hispanic or Latino | 6 (10%) | 4 (29%) | 10 (14%) |
| Other | 1 (2%) | 0 | 1 (1%) |
| Unknown | 3 (5%) | 0 | 3 (4%) |
| Occupation | |||
| Patient-facing | 52 (87%) | 13 (93%) | 65 (88%) |
| Nonpatient-facing | 8 (13%) | 1 (7%) | 9 (12%) |
| Vaccine manufacturer | |||
| Moderna | 36 (60%) | 4 (29%) | 40 (54%) |
| Pfizer | 22 (37%) | 1 (7%) | 23 (31%) |
| Other | 2 (3%) | NA | 2 (3%) |
| Third dose/booster | 29 (48%) | NA | 29 (39%) |
| Reported positive COVID-19 test * | 19 (32%) | 6 (43%) | 25 (34%) |
| Before enrollment | 8 (13%) | 5 (36%) | 13 (18%) |
| During study | 11 (18%) | 1 (7%) | 12 (12%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallen, C.; Dukes, C.W.; Aldrich, A.; Macaisa, L.; Mo, Q.; Cubitt, C.L.; Pilon-Thomas, S.; Giuliano, A.R.; Czerniecki, B.J.; Costa, R.L.B. Long-Term CD4+ T-Cell and Immunoglobulin G Immune Responses in Oncology Workers following COVID-19 Vaccination: An Interim Analysis of a Prospective Cohort Study. Vaccines 2022, 10, 1931. https://doi.org/10.3390/vaccines10111931
Gallen C, Dukes CW, Aldrich A, Macaisa L, Mo Q, Cubitt CL, Pilon-Thomas S, Giuliano AR, Czerniecki BJ, Costa RLB. Long-Term CD4+ T-Cell and Immunoglobulin G Immune Responses in Oncology Workers following COVID-19 Vaccination: An Interim Analysis of a Prospective Cohort Study. Vaccines. 2022; 10(11):1931. https://doi.org/10.3390/vaccines10111931
Chicago/Turabian StyleGallen, Corey, Christopher W. Dukes, Amy Aldrich, Lauren Macaisa, Qianxing Mo, Christopher L. Cubitt, Shari Pilon-Thomas, Anna R. Giuliano, Brian J. Czerniecki, and Ricardo L. B. Costa. 2022. "Long-Term CD4+ T-Cell and Immunoglobulin G Immune Responses in Oncology Workers following COVID-19 Vaccination: An Interim Analysis of a Prospective Cohort Study" Vaccines 10, no. 11: 1931. https://doi.org/10.3390/vaccines10111931
APA StyleGallen, C., Dukes, C. W., Aldrich, A., Macaisa, L., Mo, Q., Cubitt, C. L., Pilon-Thomas, S., Giuliano, A. R., Czerniecki, B. J., & Costa, R. L. B. (2022). Long-Term CD4+ T-Cell and Immunoglobulin G Immune Responses in Oncology Workers following COVID-19 Vaccination: An Interim Analysis of a Prospective Cohort Study. Vaccines, 10(11), 1931. https://doi.org/10.3390/vaccines10111931





