Effectiveness of the Booster of SARS-CoV-2 Vaccine among Japanese Adolescents: A Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedures and Ethical Considerations
2.2. Study Population and Mass Vaccination
2.3. Diagnosis of SARS-CoV-2 Infecion
2.4. Statistical Analysis
3. Results
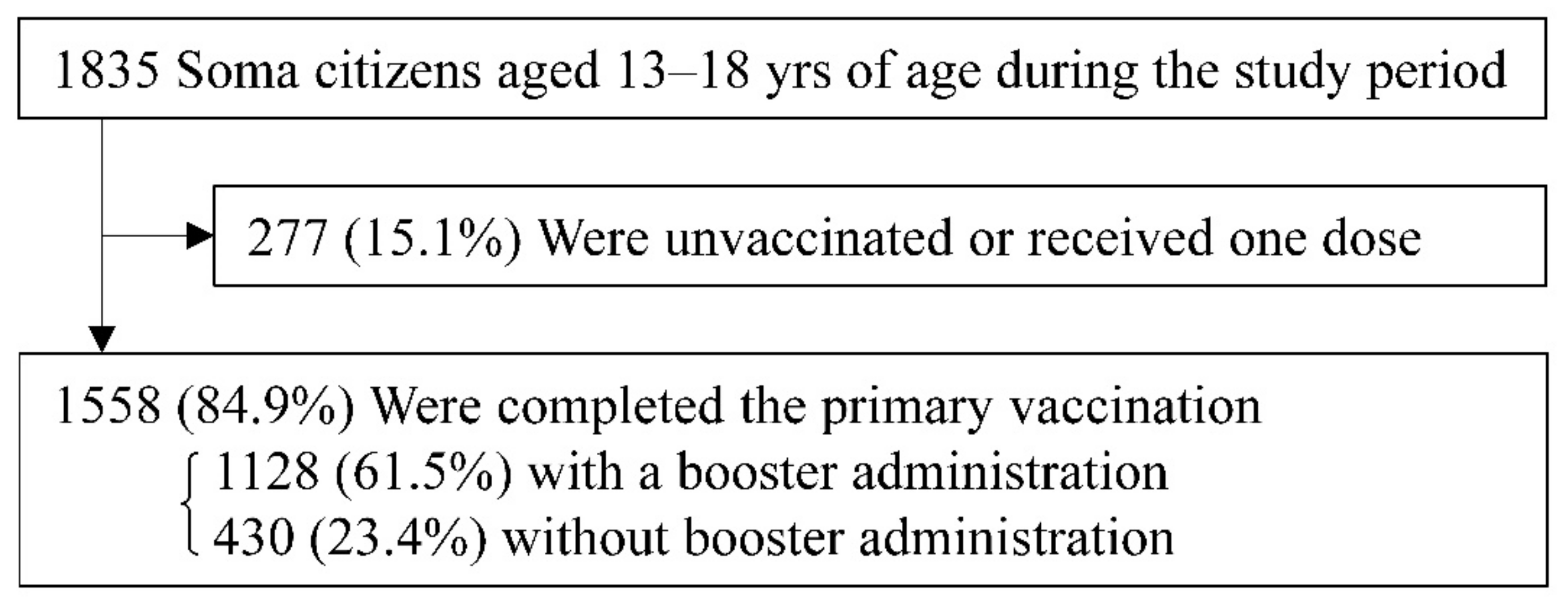
3.1. Study Population
3.2. Cohorts with SARS-CoV-2 Infection
3.3. Crude Effectiveness of a Booster Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preston, L.E.; Chevinsky, J.R.; Kompaniyets, L.; Lavery, A.M.; Kimball, A.; Boehmer, T.K.; Goodman, A.B. Characteristics and Disease Severity of US Children and Adolescents Diagnosed With COVID-19. JAMA Net. Open 2021, 4, e215298. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, K.E.C.; Backer, J.A.; de Boer, P.T.; van Hoek, A.J.; Klinkenberg, D.; Korthals Altes, H.; Leung, K.Y.; de Melker, H.; Miura, F.; Wallinga, J. A scenario modelling analysis to anticipate the impact of COVID-19 vaccination in adolescents and children on disease outcomes in the Netherlands, summer 2021. Eurosurveillance 2022, 27, 2101090. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Pereira, S.M.P.; Shafran, R.; de Stavola, B.L.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’Mahoney, L.; Chalder, T.; et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): A national matched cohort study. Lancet Child Adolesc. Health 2022, 6, 230–239. [Google Scholar] [CrossRef]
- Berg, S.K.; Nielsen, S.D.; Nygaard, U.; Bundgaard, H.; Palm, P.; Rotvig, C.; Christensen, A.V. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child Adolesc. Health 2022, 6, 240–248. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo Del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Harrison, P.J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immun. 2022, 103, 154–162. [Google Scholar] [CrossRef]
- Price, A.M.; Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Boom, J.A.; Sahni, L.C.; Pannaraj, P.S.; Irby, K.; Bline, K.E.; Maddux, A.B.; et al. BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N. Engl. J. Med. 2022, 386, 1899–1909. [Google Scholar] [CrossRef]
- Sawano, T.; Ito, N.; Ozaki, A.; Nishikawa, Y.; Nonaka, S.; Kobashi, Y.; Higuchi, A.; Tsubokura, M. Evacuation of residents in a natural disaster during the COVID-19 era. QJM 2021, 114, 445–446. [Google Scholar] [CrossRef]
- Yamamoto, N.; Takahashi, Y.; Hayashi, S. Legal and regulatory processes for Japan’s COVID-19 immunization program. Vaccine 2021, 39, 6449–6450. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare of Japan. Clinical Guidance on Diagnosis and Treatment of COVID-19. Available online: https://www.mhlw.go.jp/content/000967699.pdf (accessed on 10 September 2022).
- King, C.; Beard, J.; Crampin, A.C.; Costello, A.; Mwansambo, C.; Cunliffe, N.A.; Heyderman, R.S.; French, N.; Bar-Zeev, N.; VacSurv, C. Methodological challenges in measuring vaccine effectiveness using population cohorts in low resource settings. Vaccine 2015, 33, 4748–4755. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Austin, P.C.; Laupacis, A. A tutorial on methods to estimating clinically and policy-meaningful measures of treatment effects in prospective observational studies: A review. Int. J. Biostat. 2011, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- WHO. Evaluation of COVID-19 Vaccine Effectiveness: Interim Guidance, 17 March 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Nextstrain Team. Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused Globally over the Past 6 Months. Available online: https://nextstrain.org/ncov/gisaid/global/6m?dmax=2022-06-15&dmin=2022-05-14&f_country=Japan (accessed on 10 September 2022).
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.D., Jr.; Kitchin, N.; Xu, X.; Dychter, S.S.; Lockhart, S.; Gurtman, A.; Perez, J.L.; Zerbini, C.; Dever, M.E.; Jennings, T.W.; et al. Safety and Efficacy of a Third Dose of BNT162b2 COVID-19 Vaccine. N. Engl. J. Med. 2022, 386, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Chiew, C.J.; Premikha, M.; Chong, C.Y.; Wei, W.E.; Ong, B.; Lye, D.C.; Heng, D.; Lee, V.J.; Tan, K.B. Effectiveness of primary series and booster vaccination against SARS-CoV-2 infection and hospitalisation among adolescents aged 12-17 years in Singapore: A national cohort study. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. JAMA 2022, 327, 2210–2219. [Google Scholar] [CrossRef]
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use (accessed on 10 September 2022).
- Kohane, I.; Omenn, G.S. Understanding COVID Vaccine Efficacy over Time—Bridging a Gap between Public Health and Health Care. N. Engl. J. Med. 2022, 387, 483–485. [Google Scholar] [CrossRef]
- Akaishi, T.; Kushimoto, S.; Katori, Y.; Sugawara, N.; Egusa, H.; Igarashi, K.; Fujita, M.; Kure, S.; Takayama, S.; Abe, M.; et al. Effectiveness of third vaccine dose for coronavirus disease 2019 during the Omicron variant pandemic: A prospective observational study in Japan. Sci. Rep. 2022, 12, 13589. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Gram, M.A.; Emborg, H.D.; Schelde, A.B.; Friis, N.U.; Nielsen, K.F.; Moustsen-Helms, I.R.; Legarth, R.; Lam, J.U.H.; Chaine, M.; Malik, A.Z.; et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: A nationwide Danish cohort study. PLoS Med. 2022, 19, e1003992. [Google Scholar] [CrossRef]
- Tan, S.H.X.; Cook, A.R.; Heng, D.; Ong, B.; Lye, D.C.; Tan, K.B. Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 387, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; AlMukdad, S.; Ayoub, H.H.; Altarawneh, H.N.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; et al. COVID-19 Vaccine Protection among Children and Adolescents in Qatar. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Soma City. COVID-19 Information in Soma City. Available online: https://www.city.soma.fukushima.jp/kenko_fukushi/COVID19/index.html (accessed on 10 September 2022).
- Mevorach, D.; Anis, E.; Cedar, N.; Hasin, T.; Bromberg, M.; Goldberg, L.; Parnasa, E.; Dichtiar, R.; Hershkovitz, Y.; Ash, N.; et al. Myocarditis after BNT162b2 Vaccination in Israeli Adolescents. N. Engl. J. Med. 2022, 386, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Baggs, J.; Marquez, P.; Abara, W.E.; Olubajo, B.; Myers, T.R.; Su, J.R.; Thompson, D.; Gee, J.; Shimabukuro, T.T.; et al. Safety Monitoring of COVID-19 Vaccine Booster Doses Among Persons Aged 12-17 Years—United States, December 9, 2021–February 20, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Lim, S.R.; Yau, C.E.; Liew, T.M. Examining the Prevailing Negative Sentiments Related to COVID-19 Vaccination: Unsupervised Deep Learning of Twitter Posts over a 16 Month Period. Vaccines 2022, 10, 1457. [Google Scholar] [CrossRef]
- Soma City. Results of Safety Survey of COVID Vaccination in Adolescents. Available online: https://www.city.soma.fukushima.jp/kenko_fukushi/COVID19/medical/10168.html (accessed on 10 September 2022).
- Shimabukuro, T.T.; Nguyen, M.; Martin, D.; DeStefano, F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015, 33, 4398–4405. [Google Scholar] [CrossRef]
- Swift, R. City in Fukushima uses lessons learned from tsunami to boost COVID-19 vaccine rollout. The Japan Times, 11 June 2021. [Google Scholar]
- Dib, F.; Mayaud, P.; Chauvin, P.; Launay, O. Online mis/disinformation and vaccine hesitancy in the era of COVID-19: Why we need an eHealth literacy revolution. Hum. Vaccin. Immunother. 2021, 18, 1–3. [Google Scholar] [CrossRef]
- Remschmidt, C.; Wichmann, O.; Harder, T. Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: A systematic review. BMC Infect. Dis. 2015, 15, 429. [Google Scholar] [CrossRef]
- Patel, M.M.; Jackson, M.L.; Ferdinands, J. Postlicensure Evaluation of COVID-19 Vaccines. JAMA 2020, 324, 1939–1940. [Google Scholar] [CrossRef]
- De Smedt, T.; Merrall, E.; Macina, D.; Perez-Vilar, S.; Andrews, N.; Bollaerts, K. Bias due to differential and non-differential disease- and exposure misclassification in studies of vaccine effectiveness. PLoS ONE 2018, 13, e0199180. [Google Scholar] [CrossRef]
- Lipsitch, M.; Kahn, R.; Mina, M.J. Antibody testing will enhance the power and accuracy of COVID-19-prevention trials. Nat. Med. 2020, 26, 818–819. [Google Scholar] [CrossRef] [PubMed]
- Fukushima Prefecture. The COVID-19 Infection Status in Fukushima Prefecure. Available online: https://www.pref.fukushima.lg.jp/sec/21045c/fukushima-hasseijyoukyou.html (accessed on 10 September 2022).
| Vaccination Status | |||
|---|---|---|---|
| Unvaccinated or Partially Vaccinated for Primary Dose | Primary Vaccination Alone | A Booster Vaccination Received | |
| (n = 277, 15.1%) | (n = 430, 23.4%) | (n = 1128, 61.5%) | |
| SARS-CoV-2 infection | |||
| Asymptomatic | 0 | 0 | 1 |
| Mild symptom | 3 | 11 | 3 |
| Vaccination Status | Number of Adolescents | Number of COVID-19 Cases | Crude Incidence Rate (95% CI) | Crude Vaccine Effectiveness (95% CI) |
|---|---|---|---|---|
| Primary vaccination alone | 430 | 11 | 2.6 (1.4–4.6) | Ref |
| A booster administration received | 1128 | 4 | 0.4 (0.1–0.9) | 86.4 (57.2–95.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, Y.; Yamamoto, K.; Takita, M.; Kami, M.; Tsubokura, M.; Shibuya, K. Effectiveness of the Booster of SARS-CoV-2 Vaccine among Japanese Adolescents: A Cohort Study. Vaccines 2022, 10, 1914. https://doi.org/10.3390/vaccines10111914
Saito Y, Yamamoto K, Takita M, Kami M, Tsubokura M, Shibuya K. Effectiveness of the Booster of SARS-CoV-2 Vaccine among Japanese Adolescents: A Cohort Study. Vaccines. 2022; 10(11):1914. https://doi.org/10.3390/vaccines10111914
Chicago/Turabian StyleSaito, Yoshika, Kana Yamamoto, Morihito Takita, Masahiro Kami, Masaharu Tsubokura, and Kenji Shibuya. 2022. "Effectiveness of the Booster of SARS-CoV-2 Vaccine among Japanese Adolescents: A Cohort Study" Vaccines 10, no. 11: 1914. https://doi.org/10.3390/vaccines10111914
APA StyleSaito, Y., Yamamoto, K., Takita, M., Kami, M., Tsubokura, M., & Shibuya, K. (2022). Effectiveness of the Booster of SARS-CoV-2 Vaccine among Japanese Adolescents: A Cohort Study. Vaccines, 10(11), 1914. https://doi.org/10.3390/vaccines10111914






