Early Real-World Data to Assess Benefits and Risks of COVID-19 Vaccines: A Systematic Review of Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Source
2.2. Eligibility Criteria
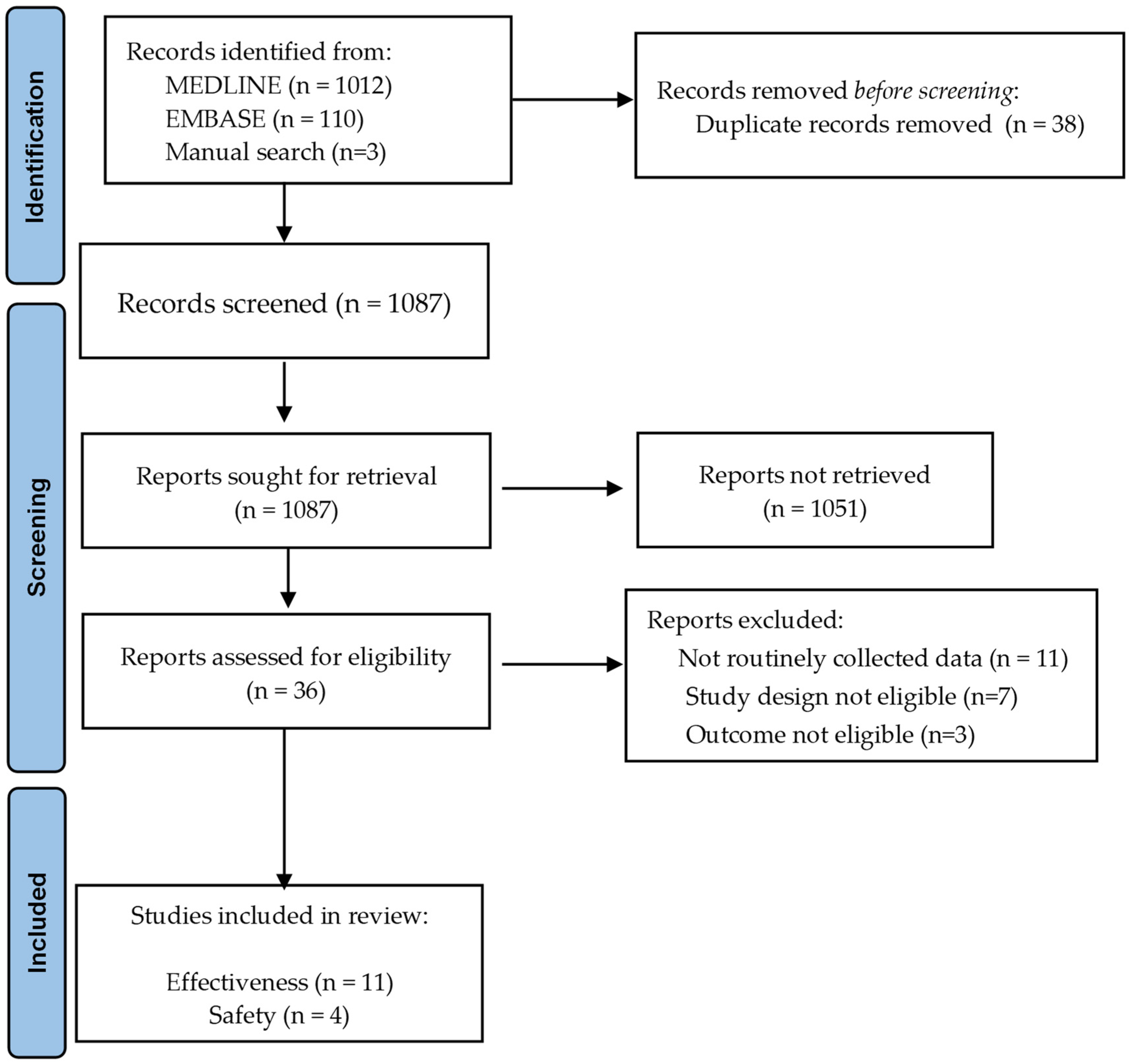
2.3. Study Selection
2.4. Data Collection
2.5. Risk of Bias of Included Studies
2.6. Data Synthesis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucinotta, D.V.M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Considerations for the Use of Real-World Data and Real-World Evidence to Support Regulatory Decision-Making for Drug and Biological Products-Draft Guidance for Industry. Available online: https://www.fda.gov/media/154714/download (accessed on 1 November 2021).
- Kim, H.S.; Lee, S.; Kim, J.H. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J. Korean Med. Sci. 2018, 33, e213. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Lythgoe, M.P.; Middleton, P. Comparison of COVID-19 Vaccine Approvals at the U.S. Food and Drug Administration, European Medicines Agency, and Health Canada. JAMA Netw. Open. 2021, 4, e2114531. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; el Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Rosen, B.; Waitzberg, R. Israel’s rapid rollout of vaccinations for COVID-19. Isr. J. Health Policy Res. 2021, 10, 114. [Google Scholar] [CrossRef]
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giatino, C.; Ortiz-Ospina, E.; Hasel, J.; McDonalds, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). 2022. Our World in Data. Available online: https://ourworldindata.org/coronavirus (accessed on 1 November 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Cohort Studies; University of Ottawa: Ottawa, ON, Canada, 2014; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 1 November 2021).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Newcastle-Ottawa Quality Assessment Scale Case Control Studies; University of Ottawa: Ottawa, ON, Canada; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 1 November 2021).
- Ho, P.J.; Gernaat, S.A.; Hartman, M.; Verkooijen, H.M. Health-related quality of life in Asian patients with breast cancer: A systematic review. BMJ Open 2018, 8, e020512. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in healthcare workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Hartronft, S.; McConeghy, K.; Kennedy, M.; Intrator, O.; Minor, L.; Hubert, T.L.; Goldstein, M.K. Proportion of SARS-CoV-2 positive tests and vaccination in Veterans Affairs Community Living Centers. J. Am. Geriatr. Soc. 2021, 69, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Swift, M.D.; Breker, L.E.; Tande, A.J.; Tommaso, C.P.; Hainy, C.M.; Chu, H.; Murad, M.H.; Berbari, E.F. Effectiveness of Messenger RNA Coronavirus Disease 2019 (COVID-19) Vaccines Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in a Cohort of Healthcare Personnel. Clin. Infect. Dis. 2021, 73, e1376–e1379. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Pollock, B.D.; Shah, N.D.; Farrugia, G.; Virk, A.; Swift, M.; Breeher, L.; Binnicker, M.; Berbari, E.F. Impact of the Coronavirus Disease 2019 (COVID-19) Vaccine on Asymptomatic Infection Among Patients Undergoing Preprocedural COVID-19 Molecular Screening. Clin. Infect. Dis. 2022, 74, 59–65. [Google Scholar] [CrossRef]
- Gras-Valentí, P.; Chico-Sánchez, P.; Algado-Sellés, N.; Jiménez-Sepúlveda, N.J.; Gómez-Sotero, I.L.; Fuster-Pérez, M.; Cartagena-Llopis, L.; Sánchez-Valero, M.; Cerezo-Milán, P.; Martínez-Tornero, I.; et al. Efectividad de la primera dosis de vacuna BNT162b2 para prevenir la COVID-19 en personal sanitario [Effectiveness of the first dose of BNT162b2 vaccine to preventing COVID-19 in healthcare personnel.]. Rev. Esp. Salud. Publica. 2021, 95, e202104070. (In Spanish) [Google Scholar]
- Teran, R.A.; Walblay, K.A.; Shane, E.L.; Xydis, S.; Gretsch, S.; Gagner, A.; Samala, U.; Choi, H.; Zelinski, C.; Black, S.R. Postvaccination SARS-CoV-2 infections among skilled nursing facility residents and staff members-Chicago, Illinois, December 2020–March 2021. Am. J. Transplant. 2021, 21, 2290–2297. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers-Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar]
- Vasileiou, E.; Simpson, C.R.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; Chuter, A.; et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet 2021, 397, 1646–1657. [Google Scholar] [CrossRef]
- Angel, Y.; Spitzer, A.; Henig, O.; Saiag, E.; Sprecher, E.; Padova, H.; Ben-Ami, R. Association Between Vaccination with BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers. JAMA 2021, 325, 2457–2465. [Google Scholar] [CrossRef]
- Domi, M.; Leitson, M.; Gifford, D.; Nicolaou, A.; Sreenivas, K.; Bishnoi, C. The BNT162b2 vaccine is associated with lower new COVID-19 cases in nursing home residents and staff. J. Am. Geriatr. Soc. 2022, 69, 2079–2089. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.T.; Boyarsky, B.J.; Motter, J.D.; Greenberg, R.S.; Teles, A.T.; Ruddy, J.A.; Krach, M.R.; Jain, V.S.; Werbel, W.A.; Avery, R.K.; et al. Safety and Reactogenicity of 2 Doses of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation 2021, 105, 2170–2174. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Dolev, M.; Menascu, S.; Zohar, D.-N.; Dreyer-Alster, S.; Miron, S.; Shirbint, E.; Magalashvili, D.; Flechter, S.; Givon, U.; et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult. Scler. 2021, 27, 864–870. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Robinson, L.B.; Camargo, C.A., Jr.; Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA 2021, 325, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.M.; Lin, K.J.; Gatto, N.M.; Rassen, J.A.; Glynn, R.J.; Schneeweiss, S. Real-World Evidence for Assessing Pharmaceutical Treatments in the Context of COVID-19. Clin. Pharmacol. Ther. 2021, 109, 816–828. [Google Scholar] [CrossRef]
- Rho, Y.; Cho, D.Y.; Son, Y.; Lee, Y.J.; Kim, J.W.; Lee, H.J.; You, S.C.; Park, R.W.; Lee, J.Y. COVID-19 International Collaborative Research by the Health Insurance Review and Assessment Service Using Its Nationwide Real-world Data: Database, Outcomes, and Implications. J. Prev. Med. Public Health Yebang Uihakhoe Chi 2021, 54, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Modin, D.; Jørgensen, M.E.; Gislason, G.; Jensen, J.S.; Køber, L.; Claggett, B.; Hegde, S.M.; Solomon, S.D.; Torp-Pedersen, C.; Biering-Sørensen, T. Influenza Vaccine in Heart Failure. Circulation 2019, 139, 575–586. [Google Scholar] [CrossRef]
- Nohynek, H.; Baum, U.; Syrjänen, R.; Ikonen, N.; Sundman, J.; Jokinen, J. Effectiveness of the live attenuated and the inactivated influenza vaccine in two-year-olds—A nationwide cohort study Finland, influenza season 2015/16. Eurosurveillance 2016, 21, 30346. [Google Scholar] [CrossRef]
- Pacheco, R.L.; Martimbianco, A.; Riera, R. Let’s end “real-world evidence” terminology usage: A study should be identified by its design. J. Clin. Epidemiol. 2022, 142, 249–251. [Google Scholar] [CrossRef]
- Langan, S.M.; Schmidt, S.A.; Wing, K.; Ehrenstein, V.; Nicholls, S.G.; Filion, K.B.; Klungel, O.; Petersen, I.; Sorensen, H.T.; Dixon, W.G.; et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ Clin. Res. Ed. 2018, 363, k3532. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Simera, I.; Hoey, J.; Moher, D.; Schulz, K. EQUATOR: Reporting guidelines for health research. Open Med. 2008, 2, e49–e50. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. Available online: https://www.bmj.com/content/355/bmj.i4919 (accessed on 5 November 2021). [CrossRef] [PubMed]

| Characteristics | Number | Proportion from the Total of Studies |
|---|---|---|
| Population (n = 15) | ||
| Healthcare professionals | 7 of 15 | 47% |
| Nursing home residents | 3 of 15 | 20% |
| Patient in hospital setting or Other setting | 3 of 15 | 20% |
| Population-based | 2 of 15 | 13% |
| Country (n = 15) | ||
| USA | 8 of 15 | 53% |
| Israel | 3 of 15 | 20% |
| UK | 2 of 15 | 13% |
| Spain | 1 of 15 | 7% |
| Czech Republic | 1 of 15 | 7% |
| Study type (n = 15) | ||
| Cohort | 12 of 15 | 80% |
| Cross-sectional ¹ | 2 of 15 | 13% |
| Case-control | 1 of 15 | 7% |
| Vaccines (study might include >1 vaccine) | ||
| BNT162b2 | 14 of 15 | 93% |
| mRNA-1273 | 6 of 15 | 40% |
| ChAdOx1 | 1 of 15 | 7% |
| Non-specified | 1 of 15 | 7% |
| Data source (study might include >1 database) | ||
| Specific database ² | 5 of 15 | 33% |
| Institutional database | 5 of 15 | 33% |
| Eletronic-Health records | 4 of 15 | 27% |
| Patient-generated data | 4 of 15 | 27% |
| Effectiveness primary outcome (study might include >1 outcome) | ||
| SARS-CoV-2 infection | 10 of 11 | 91% |
| Hospitalization | 2 of 11 | 18% |
| Mortality | 2 of 11 | 18% |
| Safety Outcomes (study might include >1 outcome) | ||
| Adverse events general | 3 of 4 | 75% |
| Acute allergic reaction | 1 of 4 | 25% |
| Reactogenicity | 1 of 4 | 25% |
| Study | Population N (Sample Size) | Country | COVID-19 Vaccine | Study Design | Database | Main Outcomes | Result (Relative Measure Compared to Unvaccinated) Effectiveness (%), If Available |
|---|---|---|---|---|---|---|---|
| Hall et al., 2021 [15] | Healthcare workers, and staff from hospital n = 23,324 | UK | BNT162b2 | Cohort | SIREN database (Staff from publicly funded hospitals in the UK) | SARS-CoV-2 infection confirmed by a PCR test | OR 0.59 [CI 95% 0.54–0.64) 85% (CI 95% 74–96) |
| Rudolph et al., 2021 [16] | Community living center residents N = 130 clinics (>6000 residents) | USA and Puerto Rico | Not specified | Cohort | COVID nursing home data website and electronic health records | Positive SARS-CoV-2 tests as reported | RR 0.37 (CI 95% 0.20–0.68) |
| Swift et al., 2021 [17] | Healthcare workers n = 71,152 | USA | BNT162b2 or mRNA-1273 | Cohort | Occupational Health Services database | SARS-CoV-2 infection confirmed by a PCR test | BNT162b2r % effectiveness 2 doses = 0.968 (0.953, 0.978); mRNA-1273a % effectiveness 2 doses = 0.986 (0.901, 0.998) BNT162b2 % effectiveness 1 dose = 0.781 (0.711, 0.820) mRNA-1273 % effectiveness 1 dose = 0.912 (0.806, 0.961) |
| Tande et al., 2021 [18] | Patients screened (preprocedural and presurgical) in clinical/hospital n = 48,333 | USA | BNT162b2 or mRNA-1273 | Cohort | Eletronic Health records and institutionally curated COVID-19 database | SARS-CoV-2 infection confirmed by a PCR test | RR 0.35 (CI 95% 0.26–0.47) |
| Gras-Valentí et al., 2021 [19] | Healthcare workers, and staff from hospitals and clinics n = 268 | Spanish | BNT162b2 | Case-Control | Hospital workforce database | SARS-CoV-2 infection confirmed by a PCR test | OR 0.47 (0.23–0.99) |
| Teran et al., 2021 [20] | Nursing Facility Residents and Staff Members n = 627 | USA | BNT162b2 or mRNA-1273 | Cohort | Chicago Department of Public Health (CDPH) database | SARS-CoV-2 infection (NE detection) | 22 of 627 SARS-CoV-2 infections occurred among vaccinated |
| Thompson et al., 2021 [21] | Healthcare workers, and staff from hospital n = 3950 | USA | BNT162b2 or mRNA-1273 | Cohort | HERO database (USA eight locations), hospital setting | SARS-CoV-2 infection confirmed by a PCR test | Fully immunized 90% (68–97) Partially immunized 80% (59–90) |
| Vasileiou et al., 2021 [22] | Scotland population-based n = 1,331,993 (vaccinated) | Scotland | BNT162b2 or ChAdOx1 nCoV-19 | Cohort | Early Pandemic Evaluation and Enhanced Surveillance of COVID-19—EAVE II Electronic Communication of Surveillance in Scotland (ECOSS) Turas Vaccination Management Tool (TVMT) | Hospital admissions with COVID-19 as the main cause of admission | ChAdOx1 vaccine 88% (95% CI 75–94) BNT162b2 mRNA 91% (95% CI 85–94) |
| Angel et al., 2021 [23] | Health care workers from hospital n = 6710 | Israel | BNT162b2 | Cohort | Hospital workforce database | Symptomatic SARS-CoV-2 infection confirmed by a PCR test | Adjusted IRR 0.14 [95% CI, 0.07–0.31) |
| Domi et al., 2021 [24] | Nursing Facility Residents and staffs n = 2501 | USA | BNT162b2 | Cohort | CMS National Health Safety Network (NHSN) Public File data | New COVID-19 resident cases per resident-week and Resident deaths | Resident cases (6w) IRR: 0.64 (95% CI 0.48–0.86) Resident deaths (6w) IRR: 0.45 (95%CI 0.31–0.65) |
| Dagan et al., 2021 [25] | Israel population-based n = 596,618 (vaccinated) | Israel | BNT162b2 | Cohort | Electronic medical records of Clalit Health Services (CHS) | SARS-CoV-2 infection confirmed by a PCR test Hospital admission for COVID-19 Death from COVID-19 | 7 or more after 2nd dose Prevent Infection: 92% (95% CI, 88 to 95) Prevent hospitalization: 87% (95% CI, 55 to 100) Prevent Death: 72% (95% CI, 19 to 100) |
| Study | Population | Country | COVID-19 Vaccine | Study Design | Database | Main Outcome | Result |
|---|---|---|---|---|---|---|---|
| Ou et al., 2021 [26] | Solid organ transplant recipients | USA | BNT162b2 or mRNA-1273 | Cohort | Patient-generated data by questionnaires from Social media or transplant centers (Johns Hopkins) | Reactogenicity and most frequent adverse events | The most common were pain, fatigue (Dose1–36%; Dose2–56%), and headache (D1–28%; D2–42%) |
| Riad et al., 2021 [27] | Health care workers | Czech Republic | BNT162b2 | Cross-Sectional | Patient-generated data by questionnaire, from hospital setting | Prevalence of adverse effects | Injection site pain (89.8%), fatigue (62.2%), headache (45.6%), muscle pain (37.1%), and chills (33.9%) |
| Achiron et al., 2021 [28] | Multiple sclerosis patients | Israel | BNT162b2 | Cohort | Patient-generated data from Multiple Sclerosis center | Adverse event proportion | Safety profile of COVID-19 vaccine was characterized by pain at the injection site (14.2%), fatigue (15.9%), and headache (7.3%) |
| Blumenthal et al., 2021 [29] | Health care workers | USA | BNT162b2 or mRNA-1273 | Cross-Sectional | Eletronic health records and patient-generated data “self-reported” from hospitals | Acute Allergic Reactions | Acute allergic reactions were reported by 1365 employees overall (2.10% [95% CI, 1.99–2.22%]), more frequently with the Moderna vaccine compared with Pfizer-BioNTech (2.20% [95% CI, 2.06–2.35%] vs. 1.95% [95% CI, 1.79–2.13%]; P = 0.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.B.; Roque, F.; Ida, F.; Plácido, A.I.; Vu, M.; Hernández-Muñoz, J.J.; Herdeiro, M.T. Early Real-World Data to Assess Benefits and Risks of COVID-19 Vaccines: A Systematic Review of Methods. Vaccines 2022, 10, 1896. https://doi.org/10.3390/vaccines10111896
Ribeiro TB, Roque F, Ida F, Plácido AI, Vu M, Hernández-Muñoz JJ, Herdeiro MT. Early Real-World Data to Assess Benefits and Risks of COVID-19 Vaccines: A Systematic Review of Methods. Vaccines. 2022; 10(11):1896. https://doi.org/10.3390/vaccines10111896
Chicago/Turabian StyleRibeiro, Tatiane B., Fátima Roque, Fidelia Ida, Ana I. Plácido, Mai Vu, Jose J. Hernández-Muñoz, and Maria Teresa Herdeiro. 2022. "Early Real-World Data to Assess Benefits and Risks of COVID-19 Vaccines: A Systematic Review of Methods" Vaccines 10, no. 11: 1896. https://doi.org/10.3390/vaccines10111896
APA StyleRibeiro, T. B., Roque, F., Ida, F., Plácido, A. I., Vu, M., Hernández-Muñoz, J. J., & Herdeiro, M. T. (2022). Early Real-World Data to Assess Benefits and Risks of COVID-19 Vaccines: A Systematic Review of Methods. Vaccines, 10(11), 1896. https://doi.org/10.3390/vaccines10111896







