Mycobacterium tuberculosis: Implications of Ageing on Infection and Maintaining Protection in the Elderly
Abstract
1. Introduction
2. Inflammaging
3. T-Cell Immune System
4. M. tb and Granuloma Formation
5. Immune Response of Mycobacterium tuberculosis in Young Healthy Individuals
5.1. Initial Immune Responses
5.2. Latent M. tb
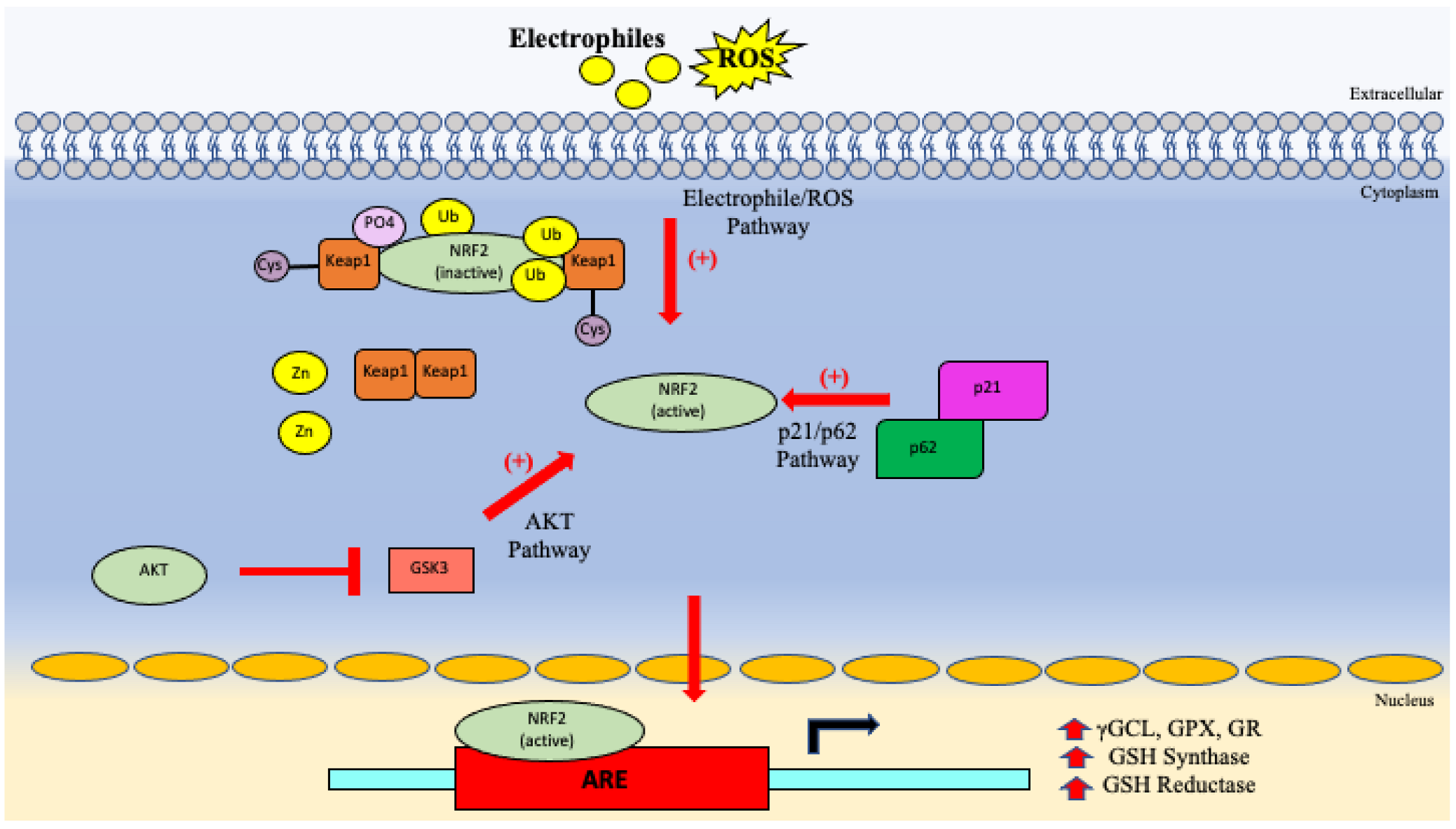
5.3. GSH System
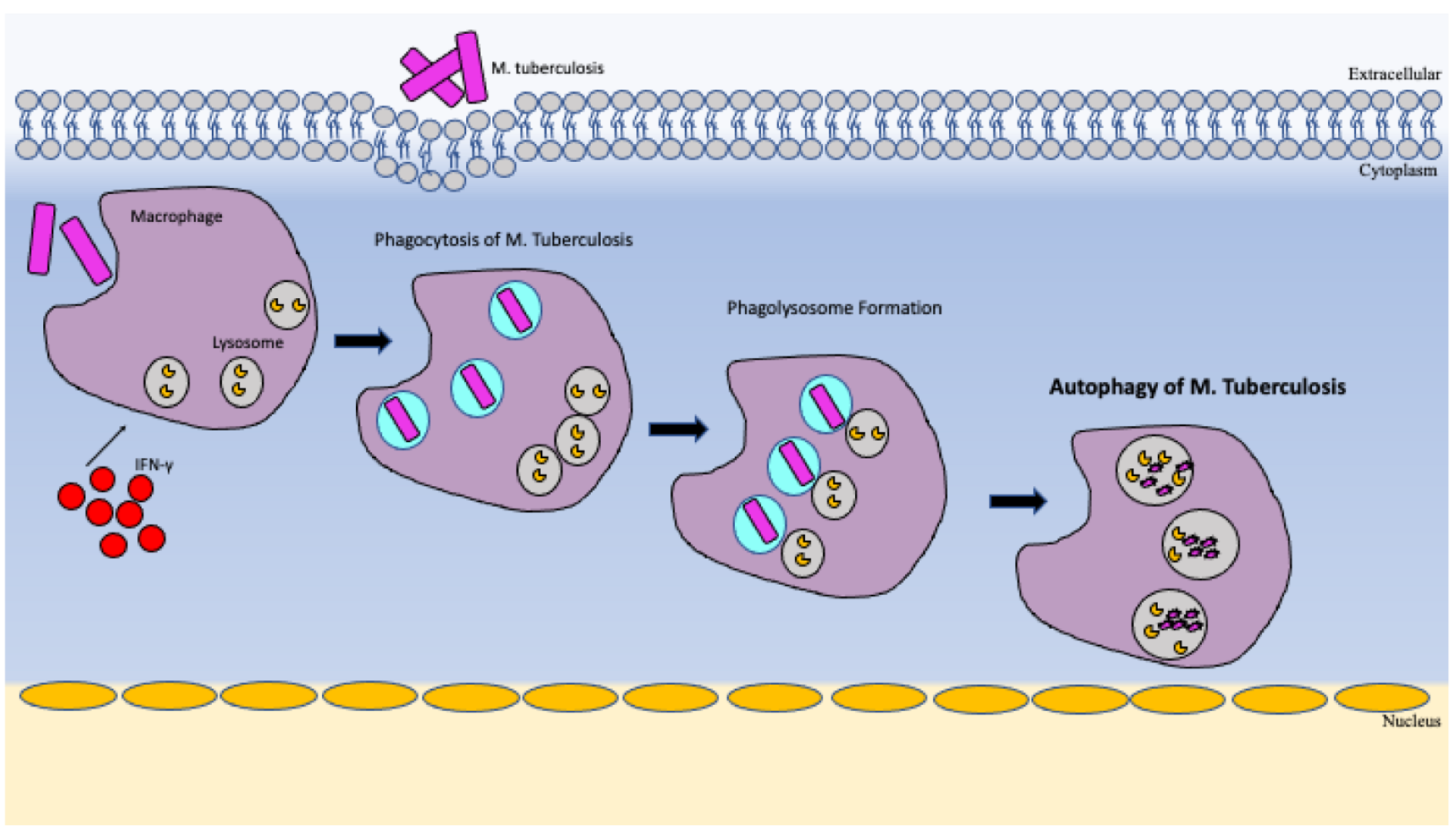
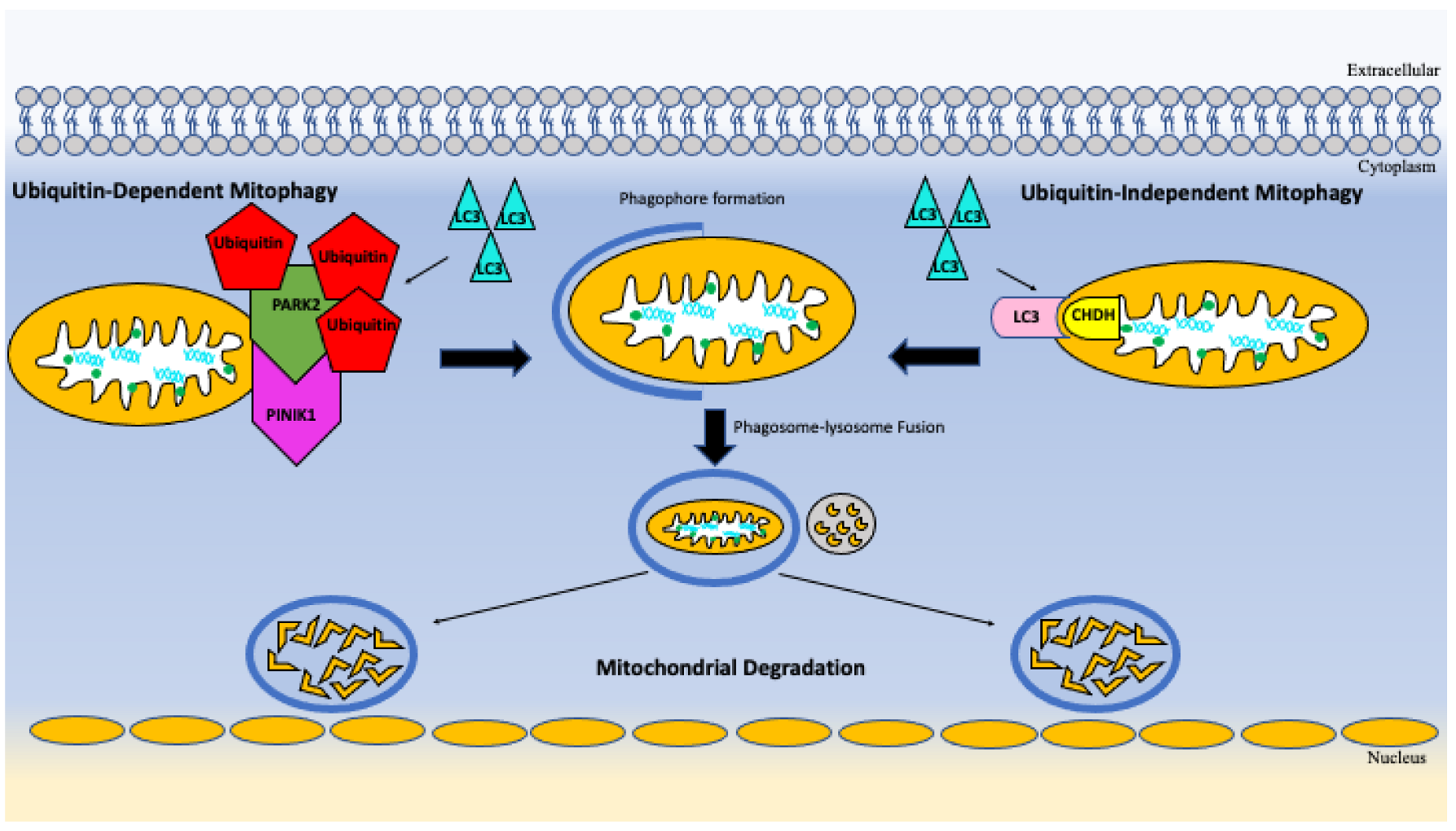
5.4. Autophagy and Mitophagy
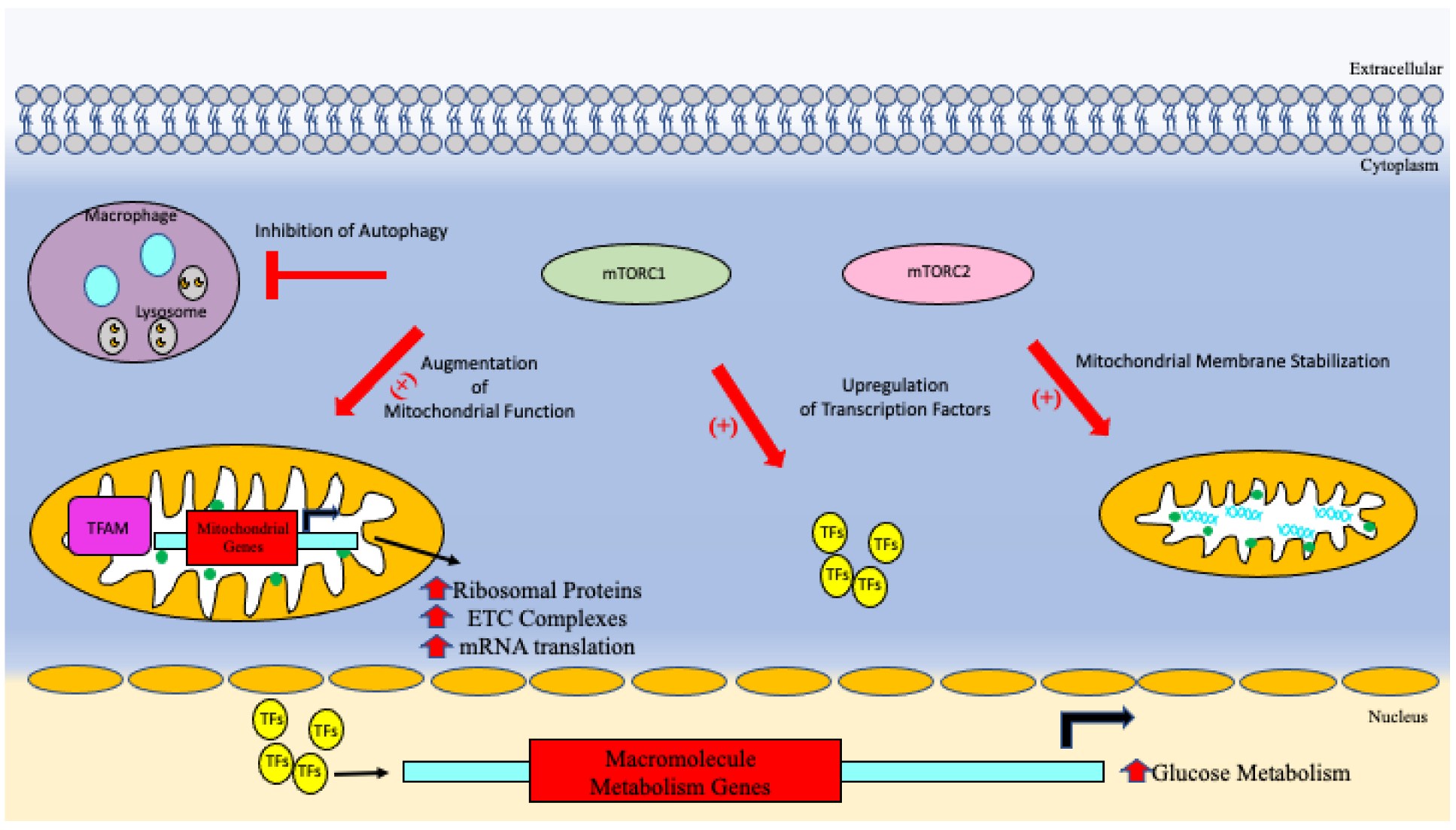
5.5. mTOR Signaling
6. Detecting M. tb Infection
7. Ageing and Tuberculosis
7.1. Ageing Effect on Mitochondrial Function
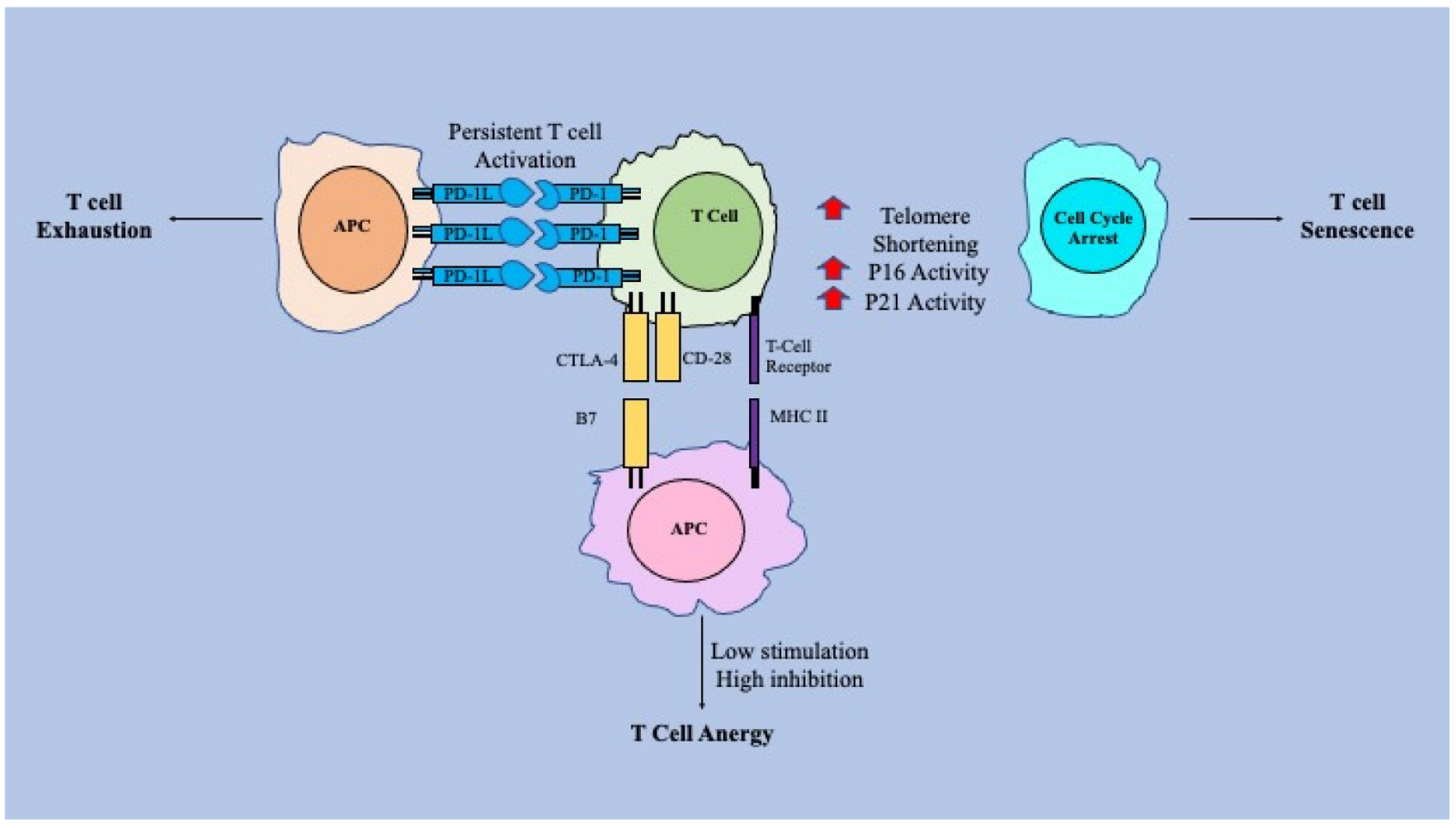
7.2. Ageing within the T-Cell System
7.3. How Ageing Affects mTOR Signaling, Autophagy & Mitophagy
7.4. Ageing Effect on GSH System
7.5. Genes/Transcription Factors That Play a Role in Ageing
8. Ageing Effects on Susceptibility to M. tb Infection and Possible Preventative Strategies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanasi, E.; Ayilavarapu, S.; Jones, J. The aging population: Demographics and the biology of aging. Periodontology 2000 2016, 72, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Behar, S.; Gottlieb, S. Aging and Diseases of the Heart. Circulation 2003, 108, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S. Connecting obesity, aging and diabetes. Nat. Med. 2009, 15, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Scordo, J.M.; Aguillón-Durán, G.P.; Ayala, D.; Quirino-Cerrillo, A.P.; Rodríguez-Reyna, E.; Mora-Guzmán, F.; Caso, J.A.; Ledezma-Campos, E.; Schlesinger, L.S.; Torrelles, J.B.; et al. A prospective cross-sectional study of tuberculosis in elderly Hispanics reveals that BCG vaccination at birth is protective whereas diabetes is not a risk factor. PLoS ONE 2021, 16, e0255194. [Google Scholar] [CrossRef] [PubMed]
- Suastika, K.; Dwipayana, P.; Siswadi, M.; Tuty, R.A. Age is an Important Risk Factor for Type 2 Diabetes Mellitus and Cardiovascular Diseases. In Glucose Tolerance; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative Stress, Mitochondrial Dysfunction, and Aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- DiLoreto, R.; Murphy, C.T. The cell biology of aging. Mol. Biol. Cell 2015, 26, 4524–4531. [Google Scholar] [CrossRef]
- Ravera, S.; Podestà, M.; Sabatini, F.; Dagnino, M.; Cilloni, D.; Fiorini, S.; Barla, A.; Frassoni, F. Discrete Changes in Glucose Metabolism Define Aging. Sci. Rep. 2019, 9, 10347. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Lactic Acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Recent Advances in Nutritional Sciences Glutathione Metabolism and Its Implications for Health 1. 2004. Available Online: https://academic.oup.com/jn/article/134/3/489/4688681 (accessed on 29 June 2022).
- Saeidi, A.; Zandi, K.; Cheok, Y.Y.; Saeidi, H.; Wong, W.F.; Lee, C.Y.Q.; Cheong, H.C.; Yong, Y.K.; Larsson, M.; Shankar, E.M. T-Cell Exhaustion in Chronic Infections: Reversing the State of Exhaustion and Reinvigorating Optimal Protective Immune Responses. Front. Immunol. 2018, 9, 2569. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef]
- Palmer, B.E.; Neff, C.P.; LeCureux, J.; Ehler, A.; Dsouza, M.; Remling-Mulder, L.; Korman, A.J.; Fontenot, A.P.; Akkina, R. In Vivo Blockade of the PD-1 Receptor Suppresses HIV-1 Viral Loads and Improves CD4+ T Cell Levels in Humanized Mice. J. Immunol. 2012, 190, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Sun, H.; Welling, T.H.; Tian, Z.; Zou, W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013, 25, 214–221. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2016, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Canan, C.H.; Gokhale, N.S.; Carruthers, B.; Lafuse, W.P.; Schlesinger, L.S.; Torrelles, J.B.; Turner, J. Characterization of lung inflammation and its impact on macrophage function in aging. J. Leukoc. Biol. 2014, 96, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Ault, R.; Dwivedi, V.; Koivisto, E.; Nagy, J.; Miller, K.; Nagendran, K.; Chalana, I.; Pan, X.; Wang, S.-H.; Turner, J. Altered monocyte phenotypes but not impaired peripheral T cell immunity may explain susceptibility of the elderly to develop tuberculosis. Exp. Gerontol. 2018, 111, 35–44. [Google Scholar] [CrossRef]
- Desdín-Micó, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabandé-Rodríguez, E.; Blanco, E.M.; Alfranca, A.; Cussó, L.; Desco, M.; et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Adibzadeh, M.; Solana, R.; Beckman, I. The T cell in the ageing individual1This article is based on a presentation to the First International Conference on Aging and Immunology, Bethesda, MD, 16–19 June, 1996.1. Mech. Ageing Dev. 1997, 93, 35–45. [Google Scholar] [CrossRef]
- Minato, N.; Hattori, M.; Hamazaki, Y. Physiology and pathology of T-cell aging. Int. Immunol. 2020, 32, 223–231. [Google Scholar] [CrossRef]
- Bobak, C.A.; Abhimanyu; Natarajan, H.; Gandhi, T.; Grimm, S.L.; Nishiguchi, T.; Koster, K.; Longlax, S.C.; Dlamini, Q.; Kahari, J.; et al. Increased DNA methylation, cellular senescence and premature epigenetic aging in guinea pigs and humans with tuberculosis. Aging 2022, 14, 2174–2193. Available Online: www.aging-us.comwww.aging-us.com (accessed on 2 November 2022). [CrossRef]
- Wahl, S.M. Transforming Growth Factor Beta (TGF-β) in Inflammation: A Cause and a Cure. J. Clin. Immunol. 1992, 12, 61–74. [Google Scholar] [CrossRef]
- Rozot, V.; Vigano, S.; Mazza-Stalder, J.; Idrizi, E.; Day, C.L.; Perreau, M.; Lazor-Blanchet, C.; Petruccioli, E.; Hanekom, W.; Goletti, D.; et al. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur. J. Immunol. 2013, 43, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Pagán, A.J.; Ramakrishnan, L. Immunity and Immunopathology in the Tuberculous Granuloma. Cold Spring Harb. Perspect. Med. 2014, 5, a018499. [Google Scholar] [CrossRef]
- Ehlers, S.; Schaible, U.E. The Granuloma in Tuberculosis: Dynamics of a Host–Pathogen Collusion. Front. Immunol. 2013, 3, 411. [Google Scholar] [CrossRef] [PubMed]
- Sia, J.K.; Rengarajan, J. Immunology of Mycobacterium tuberculosis Infections. Microbiol. Spectr. 2019, 7, 1–19. [Google Scholar] [CrossRef]
- Faridgohar, M.; Nikoueinejad, H. New findings of Toll-like receptors involved in Mycobacterium tuberculosis infection. Pathog. Glob. Health 2017, 111, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Joosten, L.A.B.; van de Veerdonk, F.L.; Savage, N.; van Crevel, R.; Kullberg, B.J.; van der Ven, A.; Ottenhoff, T.H.M.; Dinarello, C.A.; van der Meer, J.W.M.; et al. Transcriptional and inflammasome-mediated pathways for the induction of IL-1β production by Mycobacterium tuberculosis. Eur. J. Immunol. 2009, 39, 1914–1922. [Google Scholar] [CrossRef]
- Ravesloot-Chávez, M.M.; Van Dis, E.; Stanley, S.A. The Innate Immune Response to Mycobacterium tuberculosis Infection. Annu. Rev. Immunol. 2021, 39, 611–637. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Barber, D.L. Innate and Adaptive Cellular Immune Responses to Mycobacterium tuberculosis Infection. Cold Spring Harb. Perspect. Med. 2015, 5, a018424. [Google Scholar] [CrossRef]
- Boom, W.H.; Schaible, U.E.; Achkar, J.M. The knowns and unknowns of latent Mycobacterium tuberculosis infection. J. Clin. Investig. 2021, 131, 1–8. [Google Scholar] [CrossRef]
- Morris, D.; Khurasany, M.; Nguyen, T.; Kim, J.; Guilford, F.; Mehta, R.; Gray, D.; Saviola, B.; Venketaraman, V. Glutathione and infection. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3329–3349. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lv, J.; Carlson, C.; Liu, H.; Wang, H.; Xu, T.; Wu, F.; Song, C.; Wang, X.; Wang, T.; et al. Trained immunity contributes to the prevention of Mycobacterium tuberculosis infection, a novel role of autophagy. Emerg. Microbes Infect. 2021, 10, 578–588. [Google Scholar] [CrossRef]
- Lam, A.; Prabhu, R.; Gross, C.M.; Riesenberg, L.A.; Singh, V.; Aggarwal, S. Role of apoptosis and autophagy in tuberculosis. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, 218–229. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Chung, C.; Jo, E.-K. Autophagy: A new strategy for host-directed therapy of tuberculosis. Virulence 2019, 10, 448–459. [Google Scholar] [CrossRef]
- Yoo, S.-M.; Jung, Y.-K. A Molecular Approach to Mitophagy and Mitochondrial Dynamics. Mol. Cells 2018, 41, 18–26. [Google Scholar] [CrossRef]
- Patrick, K.L.; Watson, R.O. Mitochondria: Powering the Innate Immune Response to Mycobacterium tuberculosis Infection. Infect. Immun. 2021, 89, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Mahla, R.S.; Kumar, A.; Tutill, H.J.; Krishnaji, S.T.; Sathyamoorthy, B.; Noursadeghi, M.; Breuer, J.; Pandey, A.K.; Kumar, H. NIX-mediated mitophagy regulate metabolic reprogramming in phagocytic cells during mycobacterial infection. Tuberculosis 2020, 126, 102046. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Zhang, W. Role of mTOR in Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2018, 19, 2043. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.-L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.-P.; Hulea, L.; Larsson, O.; Pollak, M.; St-Pierre, J.; Topisirovic, I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 2015, 14, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Subbian, S. Harnessing the mTOR Pathway for Tuberculosis Treatment. Front. Microbiol. 2018, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Cerni, S.; Shafer, D.; To, K.; Venketaraman, V. Investigating the Role of Everolimus in mTOR Inhibition and Autophagy Promotion as a Potential Host-Directed Therapeutic Target in Mycobacterium tuberculosis Infection. J. Clin. Med. 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Lachmandas, E.; Beigier-Bompadre, M.; Cheng, S.-C.; Kumar, V.; van Laarhoven, A.; Wang, X.; Ammerdorffer, A.; Boutens, L.; de Jong, D.; Kanneganti, T.-D.; et al. Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur. J. Immunol. 2016, 46, 2574–2586. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M. Latent Mycobacterium tuberculosis Infection and Interferon-Gamma Release Assays. Microbiol. Spectr. 2016, 4(5), 2–8. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines on the Management of Latent Tuberculosis Infection. In Guidelines on the Management of Latent Tuberculosis Infection; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Pahal, P.; Sharma, S. PPD Skin Test. In Treasure Island (FL); StatPearls Publishing: Bethesda, ML, USA, 2022; Available Online: https://www-ncbi-nlm-nih-gov.proxy.westernu.edu/books/NBK556037/ (accessed on 2 November 2022).
- Stavri, H.R.; Murgoci, G.; Ulea, I.; Popa, L.G.; Popa, M.I.; Stavri, H. Prospective Comparison of Two Brands of Tuberculin Skin Tests and Quantiferon-TB Gold in-tube Assay Performances for Tuberculosis Infection in Hospitalized Children. Maedica 2010, 5, 271–276. [Google Scholar]
- Hasløv, K.; Ponce-De-, S.; Rosales, L.; Rangel-Frausto, S.; Larsen, S.O.; Hasløv, K. Tuberculin PPD RT23: Still going strong Counterpoint. Int. J. Tuberc. Lung Dis. 1998, 2, 793–796. [Google Scholar]
- Gualano, G.; Mencarini, P.; Lauria, F.N.; Palmieri, F.; Mfinanga, S.; Mwaba, P.; Chakaya, J.; Zumla, A.; Ippolito, G. Tuberculin skin test—Outdated or still useful for Latent TB infection screening? Int. J. Infect. Dis. 2019, 80, S20–S22. [Google Scholar] [CrossRef]
- Jurčev-Savičević, A.; Katalinić-Janković, V.; Mise, K.; Gudelj, I. The Role of Interferon-gamma Release Assay in Tuberculosis Control. Arch. Ind. Hyg. Toxicol. 2012, 63, 49–59. [Google Scholar] [CrossRef][Green Version]
- Belson, A.; Schmidt, T.; Fernando, D.; Hardes, K.; Scott, N.; Brett, S.; Clark, D.; Oliveira, J.; Davis, B.; McHugh, S.; et al. Characterisation of the clinical and activated T cell response to repeat delayed-type hypersensitivity skin challenges in human subjects, with KLH and PPD, as a potential model to test T cell-targeted therapies. Inflamm. Res. 2016, 65, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; King, M.L. Tuberculosis and Aging: A Global Health Problem. Aging Infect. Dis. 2001, 33, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Falzon, D.; Floyd, K.; Raviglione, M.; Glaziou, P. Global Epidemiology of Tuberculosis. Semin. Respir. Crit. Care Med. 2013, 34, 003–016. [Google Scholar] [CrossRef][Green Version]
- Sakhno, L.V.; Shevela, E.Y.; Tikhonova, M.A.; Nikonov, S.D.; Ostanin, A.A.; Chernykh, E.R. Impairments of Antigen-Presenting Cells in Pulmonary Tuberculosis. J. Immunol. Res. 2015, 2015, 793292. [Google Scholar] [CrossRef]
- McDermott, W.; Rogers, D.E.; Med, J.H.; Bloom, B.R.; Murray, C.J.L. 15. National MDR-TB Task Force, National Action Plan to Combat Multidrug-Resistant Tuberculosis (Centers for Disease Control); American Society for Microbiology: Washington, DC, USA, 1982. [Google Scholar]
- Akha, A.A.S. Aging and the immune system: An overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Perkins, G. Dynamics of mitochondrial structure during apoptosis and the enigma of Opa1. Biochim. Biophys. Acta 2009, 1787, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-J.; Sohal, R.S. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc. Natl. Acad. Sci. USA 1998, 95, 12896–12901. Available Online: www.pnas.org (accessed on 26 June 2022). [CrossRef] [PubMed]
- Dunn, J.; Grider, M.H. Physiology, Adenosine Triphosphate. StatPearls 2022. Available Online: https://pubmed-ncbi-nlm-nih-gov.proxy.westernu.edu/31985968/ (accessed on 25 June 2022).
- Leist, M.; Single, B.; Castoldi, A.F.; Kühnle, S.; Nicotera, P. Intracellular Adenosine Triphosphate (ATP) Concentration: A Switch in the Decision Between Apoptosis and Necrosis. J. Exp. Med. 1997, 185, 1481–1486. Available Online: http://rupress.org/jem/article-pdf/185/8/1481/1111225/5497.pdf (accessed on 27 June 2022). [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Yan, J.; Greer, J.M.; Hull, R.; O’Sullivan, J.D.; Henderson, R.D.; Read, S.J.; A McCombe, P. The effect of ageing on human lymphocyte subsets: Comparison of males and females. Immun. Ageing 2010, 7, 4. Available Online: http://www.immunityageing.com/content/7/1/4 (accessed on 21 August 2022). [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Successful and Maladaptive T Cell Aging. Immunity 2017, 46, 364–378. [Google Scholar] [CrossRef]
- Moskowitz, D.M.; Zhang, D.W.; Hu, B.; Le Saux, S.; Yanes, R.E.; Ye, Z.; Buenrostro, J.D.; Weyand, C.M.; Greenleaf, W.J.; Goronzy, J.J. Epigenomics of human CD8 T cell differentiation and aging. Sci. Immunol. 2017, 2, 2–12. [Google Scholar] [CrossRef]
- Bengsch, B.; Johnson, A.L.; Kurachi, M.; Odorizzi, P.M.; Pauken, K.E.; Attanasio, J.; Stelekati, E.; McLane, L.M.; Paley, M.A.; Delgoffe, G.M.; et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8 + T Cell Exhaustion. Immunity 2016, 45, 358–373. [Google Scholar] [CrossRef]
- Zhang, J.; He, T.; Xue, L.; Guo, H. Senescent T cells: A potential biomarker and target for cancer therapy. eBioMedicine 2021, 68, 103409. [Google Scholar] [CrossRef]
- Shammas, M.A. Telomeres, lifestyle, cancer, and aging. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 28–34. [Google Scholar] [CrossRef]
- Victorelli, S.; Passos, J.F. Telomeres and Cell Senescence-Size Matters Not. eBioMedicine 2017, 21, 14–20. [Google Scholar] [CrossRef]
- Chou, J.P.; Effros, R.B. T Cell Replicative Senescence in Human Aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar]
- Byng-Maddick, R.; Noursadeghi, M. Does tuberculosis threaten our ageing populations? BMC Infect. Dis. 2016, 16, 119. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.A.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8, 998. [Google Scholar] [CrossRef]
- Guillén, C.; Benito, M. mTORC1 Overactivation as a Key Aging Factor in the Progression to Type 2 Diabetes Mellitus. Front. Endocrinol. 2018, 9, 621. [Google Scholar] [CrossRef]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An Emerging Role in Aging and Age-Associated Diseases. Front. Cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef]
- Markofski, M.; Dickinson, J.M.; Drummond, M.J.; Fry, C.S.; Fujita, S.; Gundermann, D.M.; Glynn, E.L.; Jennings, K.; Paddon-Jones, D.; Reidy, P.T.; et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp. Gerontol. 2015, 65, 1–7. [Google Scholar] [CrossRef]
- Erden-Inal, M.; Sunal, E.; Kanbak, G. Age-related changes in the glutathione redox system. Cell Biochem. Funct. 2002, 20, 61–66. [Google Scholar] [CrossRef]
- Zhu, Y.; Carvey, P.M.; Ling, Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006, 1090, 35–44. [Google Scholar] [CrossRef]
- Allen, M.; Bailey, C.; Cahatol, I.; Dodge, L.; Yim, J.; Kassissa, C.; Luong, J.; Kasko, S.; Pandya, S.; Venketaraman, V. Mechanisms of Control of Mycobacterium tuberculosis by NK Cells: Role of Glutathione. Front. Immunol. 2015, 6, 508. [Google Scholar] [CrossRef]
- Mustikaningtyas, D.; Widyarti, S.; Rifa’I, M.; Widodo, N. Proposed Mechanism of Antibacterial Activity of Glutathione by Inhibition of the d-Alanyl-d-alanine Carboxypeptidase Enzyme. Int. J. Pept. Res. Ther. 2020, 27, 843–849. [Google Scholar] [CrossRef]
- Shaw, P.; Chattopadhyay, A. Nrf2–ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Matsuyama, M.; Nonaka, M.; Nakajima, M.; Morishima, Y.; Ishii, Y.; Hizawa, N. The Role of NRF2 in Mycobacterial Infection. Antioxidants 2021, 10, 1861. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 2019, 19, 573–583. [Google Scholar] [CrossRef]
- Ly, J.; Lagman, M.; Saing, T.; Singh, M.K.; Tudela, E.V.; Morris, D.; Anderson, J.; Daliva, J.; Ochoa, C.; Patel, N.; et al. Liposomal Glutathione Supplementation Restores TH1 Cytokine Response to Mycobacterium tuberculosis Infection in HIV-Infected Individuals. J. Interf. Cytokine Res. 2015, 35, 875–887. [Google Scholar] [CrossRef]
- Fatima, S.; Kumari, A.; Das, G.; Dwivedi, V.P. Tuberculosis vaccine: A journey from BCG to present. Life Sci. 2020, 252, 117594. [Google Scholar] [CrossRef]
- Satcher, D.; Gayle, H.D.; Castro, K.G.; Thacker, S.B.; Goodman, R.A.; Hewitt, S.M.; Wolcott, L.B.; Higgins, M.M.; Jenkins, P.M. The Role of BCG Vaccine in the Prevention and Control of Tuberculosis in the United States. 1996. Available Online: https://www.cdc.gov/mmwr/pdf/rr/rr4504.pdf (accessed on 27 August 2022).
| Mediators | Mechanism of Immune Response |
|---|---|
| Macrophages | Macrophages are the first responders to infection. Recognition of M. Tb occurs through TLR and PAMP interaction. This leads to activation of NF-κB to upregulate mediators that promote inflammation and the immune response [21,22]. |
| T-Cells | Both CD4+ and CD8+ play a role in preventing the spread of M. tb throughout the body. CD4+ cells promote antibody production and increase the recruitment of CD8+ cells. Those found to have lower counts of CD4+ are associated with an increased risk for infection [26]. |
| Cytokines | Important cytokine mediators include IFN-γ which accentuates macrophage recruitment, while TNF-α, IL-1α and Il-1β promote the host inflammatory response. [22,23,24]. |
| Reactive Oxygen Species | The host defense with reactive oxygen species begins with the production of superoxide radical O2- via the NADPH oxidase pathway which promotes control and elimination of M. Tb [24]. |
| Autophagy | Mediates intracellular degradation to decrease bacterial load. Upon infection, there is an upregulation of ubiquitin-dependent autophagy of M. tb [21,24]. |
| Factors of Immunity | Mechanism of Immune Dysfunction |
|---|---|
| ATP Production | ROS accumulation leads to mitochondria dysfunction, depleting ATP generation within cells that are imperative to the immune response [6,47,49]. |
| T-cell responses to infection | CD8 T-cell exhaustion and senescence exacerbated in ageing leads to a suboptimal response to infection [11,13,53,60]. |
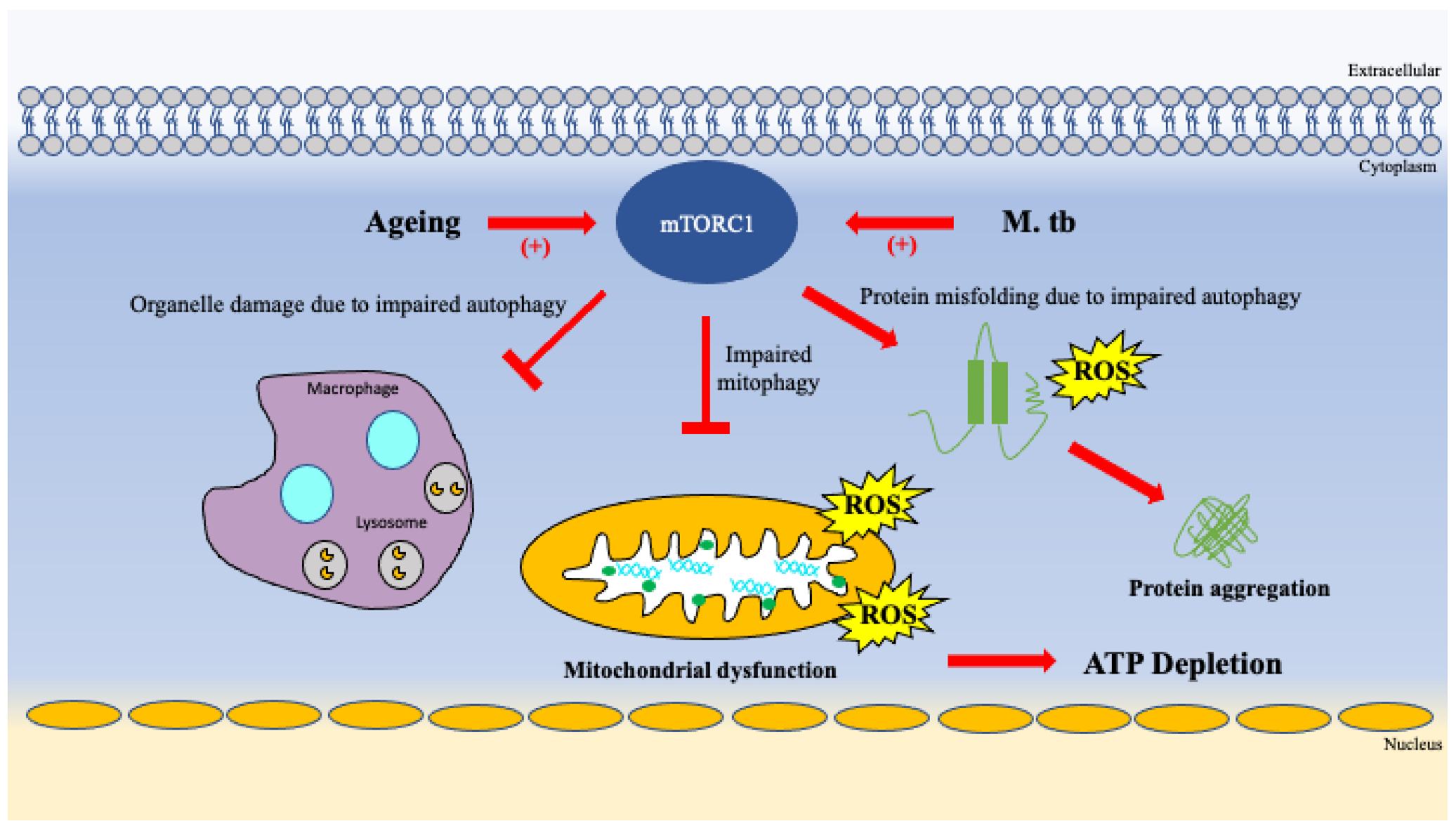
| Cell clearance and turnover | Overactivation of mTORC1 signaling impairs autophagy and mitophagy resulting toxic build up cellular waste products, misfolded proteins, and damage organelles [63,64]. |
| Defense against oxidative stress | Diminished levels of GSH through reduced GSH synthesis attenuates the immune response in aging populations, increasing susceptibility to infection [47,67]. |
| Genes promoting cell protection | Decreased gene expression of NRF2 reduces GSH synthase and reductase levels which promotes immunosuppression and the inability to neutralize oxidative stress [67,71]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonavida, V.; Frame, M.; Nguyen, K.H.; Rajurkar, S.; Venketaraman, V. Mycobacterium tuberculosis: Implications of Ageing on Infection and Maintaining Protection in the Elderly. Vaccines 2022, 10, 1892. https://doi.org/10.3390/vaccines10111892
Bonavida V, Frame M, Nguyen KH, Rajurkar S, Venketaraman V. Mycobacterium tuberculosis: Implications of Ageing on Infection and Maintaining Protection in the Elderly. Vaccines. 2022; 10(11):1892. https://doi.org/10.3390/vaccines10111892
Chicago/Turabian StyleBonavida, Victor, Mitchell Frame, Kevin H. Nguyen, Shlok Rajurkar, and Vishwanath Venketaraman. 2022. "Mycobacterium tuberculosis: Implications of Ageing on Infection and Maintaining Protection in the Elderly" Vaccines 10, no. 11: 1892. https://doi.org/10.3390/vaccines10111892
APA StyleBonavida, V., Frame, M., Nguyen, K. H., Rajurkar, S., & Venketaraman, V. (2022). Mycobacterium tuberculosis: Implications of Ageing on Infection and Maintaining Protection in the Elderly. Vaccines, 10(11), 1892. https://doi.org/10.3390/vaccines10111892









