Neutrophils in Mycobacterium tuberculosis
Abstract
1. Introduction
2. Pathogenesis of TB
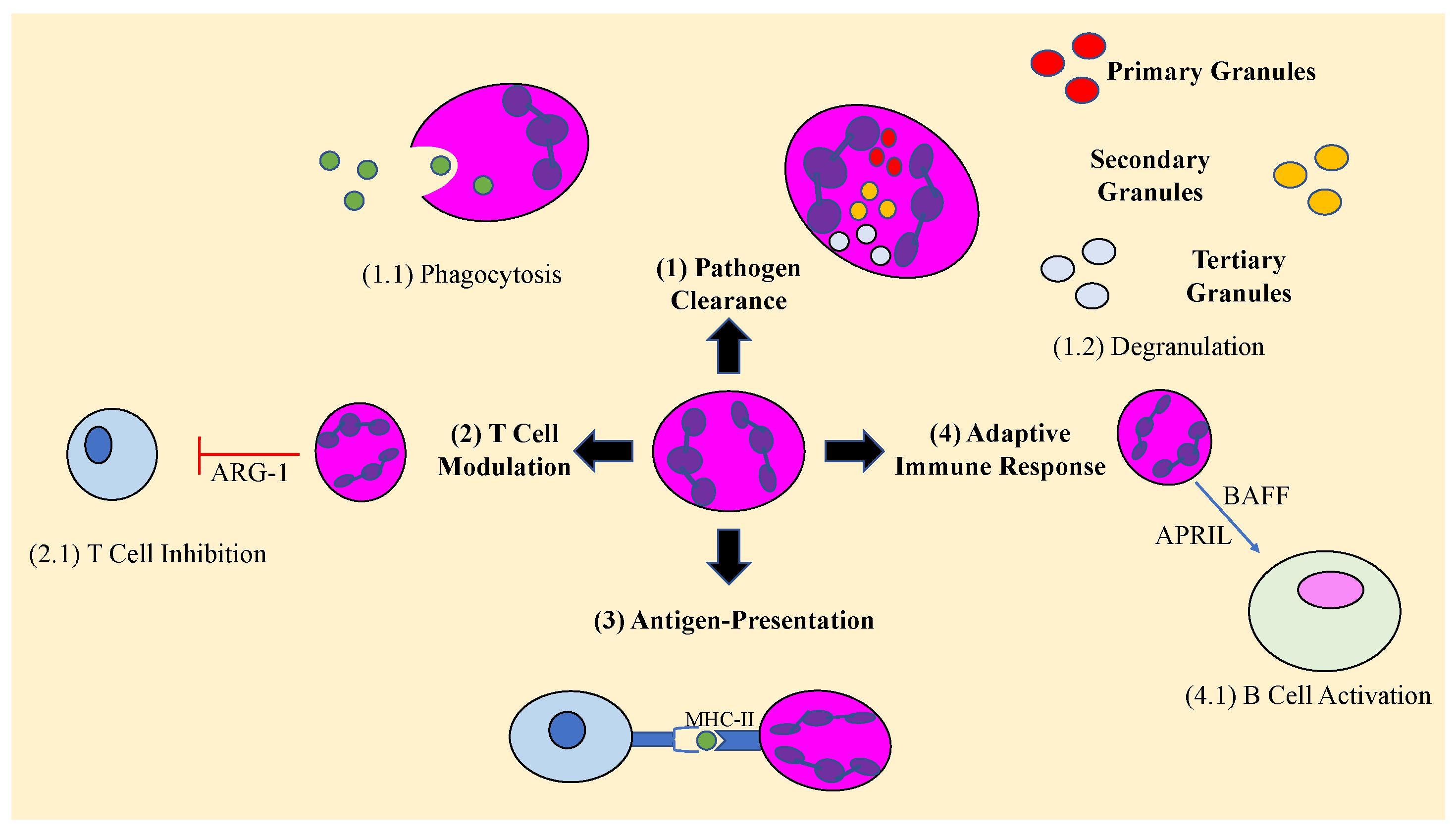
3. Role of Neutrophils in the Immune Response
4. Neutrophils and Extracellular Traps
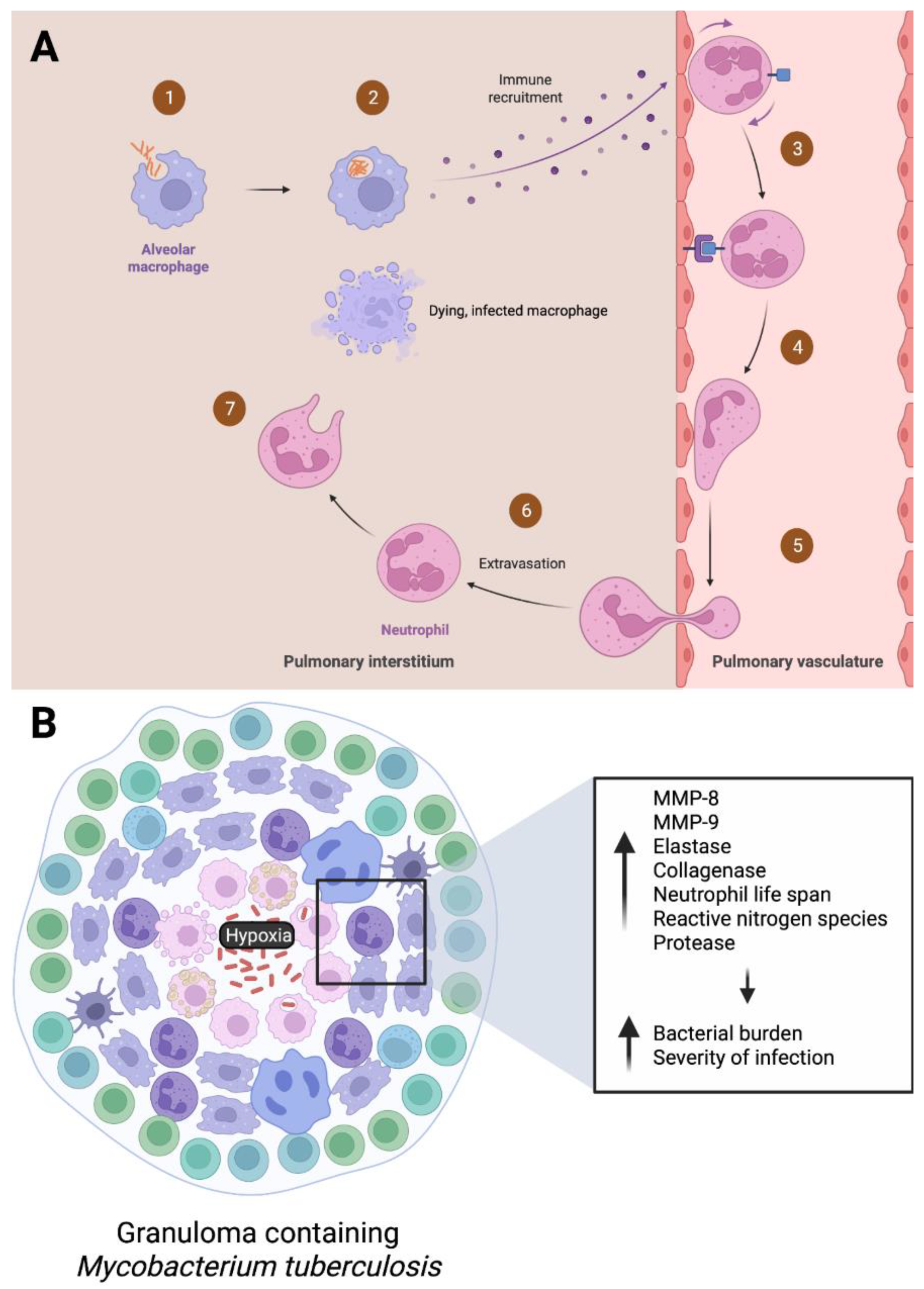
5. Neutrophils in the Acute Stage of M. tb
6. Neutrophils in Latent Phases of M. tb Infection
7. Using Neutrophils for Good: Vaccine Development
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Tuberculosis. 27 October 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 2 January 2023).
- Formad, H.F. The Etiology of Tuberculosis. J. Am. Med. Assoc. 1882, IV, 312–316. [Google Scholar] [CrossRef]
- CDC. Tuberculosis General Information Fact Sheet; CDC: Atlanta, GA, USA, 2011. [Google Scholar]
- Long, R.; Divangahi, M.; Schwartzman, K. Chapter 2: Transmission and pathogenesis of tuberculosis. Can. J. Respir. Crit. Care Sleep Med. 2022, 6, 22–32. [Google Scholar] [CrossRef]
- Dutta, N.K.; Karakousis, C. Latent Tuberculosis Infection: Myths, Models, and Molecular Mechanisms. Microbiol. Mol. Biol. Rev. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Eguirado, E.; Schlesinger, L.S. Modeling the Mycobacterium tuberculosis Granuloma—The Critical Battlefield in Host Immunity and Disease. Front. Immunol. 2013, 4, 98. [Google Scholar] [CrossRef]
- Sia, J.K.; Rengarajan, J. Immunology of Mycobacterium Tuberculosis Infections. In Microbiology Spectrum; American Society for Microbiology: Washingtong, DC, USA, 2019; Volume 7. [Google Scholar]
- Narasimhan, P.; Wood, J.; MacIntyre, C.R.; Mathai, D. Risk Factors for Tuberculosis. Pulm. Med. 2013, 2013, 828939. [Google Scholar] [CrossRef] [PubMed]
- Madzime, M.; Rossouw, T.M.; Theron, A.J.; Anderson, R.; Steel, H.C. Interactions of HIV and Antiretroviral Therapy with Neutrophils and Platelets. Front. Immunol. 2021, 12, 634386. [Google Scholar] [CrossRef]
- Dowey, R.; Iqbal, A.; Heller, S.R.; Sabroe, I.; Prince, L.R. A Bittersweet Response to Infection in Diabetes; Targeting Neutrophils to Modify Inflammation and Improve Host Immunity. Front. Immunol. 2021, 12, 678771. [Google Scholar] [CrossRef] [PubMed]
- Hilda, J.N.; Das, S.; Tripathy, S.P.; Hanna, L.E. Role of neutrophils in tuberculosis: A bird’s eye view. J. Endotoxin Res. 2019, 26, 240–247. [Google Scholar] [CrossRef]
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J.; et al. Requirement of Interleukin 17 Receptor Signaling for Lung Cxc Chemokine and Granulocyte Colony-Stimulating Factor Expression, Neutrophil Recruitment, and Host Defense. J. Exp. Med. 2001, 194, 519–528. [Google Scholar] [CrossRef]
- de Oliveira, F.M.; Trentini, M.M.; Junqueira-Kipnis, A.P.; Kipnis, A. The mc2-CMX vaccine induces an enhanced immune response against Mycobacterium tuberculosis compared to Bacillus Calmette-Guérin but with similar lung inflammatory effects. Mem. Inst. Oswaldo Cruz 2016, 111, 223–231. [Google Scholar] [CrossRef]
- Heemskerk, D.; Caws, M.; Marais, B.; Farrar, J. Chapter 2: Pathogenesis. In Tuberculosis in Adults and Children; Springer: Cham, Switzerland, 2015; pp. 9–15. [Google Scholar]
- Loudon, R.G.; Roberts, R.M. Singing and the dissemination of tuberculosis. Am. Rev. Respir. Dis. 1968, 98, 297–300. [Google Scholar] [CrossRef]
- Lin, P.L.; Flynn, J.L. The End of the Binary Era: Revisiting the Spectrum of Tuberculosis. J. Immunol. 2018, 201, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Lillebaek, T.; Dirksen, A.; Baess, I.; Strunge, B.; Thomsen, V.; Andersen, Å.B. Molecular Evidence of Endogenous Reactivation of Mycobacterium tuberculosis after 33 Years of Latent Infection. J. Infect. Dis. 2002, 185, 401–404. [Google Scholar] [CrossRef]
- Zhai, W.; Wu, F.; Zhang, Y.; Fu, Y.; Liu, Z. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Corleis, B.; Korbel, D.; Wilson, R.; Bylund, J.; Chee, R.; Schaible, U.E. Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell. Microbiol. 2012, 14, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Simeone, R.; Bobard, A.; Lippmann, J.; Bitter, W.; Majlessi, L.; Brosch, R.; Enninga, J. Phagosomal Rupture by Mycobacterium tuberculosis Results in Toxicity and Host Cell Death. PLoS Pathog. 2012, 8, e1002507. [Google Scholar] [CrossRef] [PubMed]
- Hilda, J.N.; Das, S. Neutrophil CD64, TLR2 and TLR4 expression increases but phagocytic potential decreases during tuberculosis. Tuberculosis 2018, 111, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Cheng, A.-C.; Yang, K.-Y.; Chen, N.-J.; Hsu, T.-L.; Jou, R.; Hsieh, S.-L.; Tseng, P.-H. CLEC9A modulates macrophage-mediated neutrophil recruitment in response to heat-killed Mycobacterium tuberculosis H37Ra. PLoS ONE 2017, 12, e0186780. [Google Scholar] [CrossRef]
- Pokkali, S.; Das, S.D. Augmented chemokine levels and chemokine receptor expression on immune cells during pulmonary tuberculosis. Hum. Immunol. 2009, 70, 110–115. [Google Scholar] [CrossRef]
- Remot, A.; Doz, E.; Winter, N. Neutrophils and Close Relatives in the Hypoxic Environment of the Tuberculous Granuloma: New Avenues for Host-Directed Therapies? Front. Immunol. 2019, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Cacciotto, C.; Alberti, A. Eating the Enemy: Mycoplasma Strategies to Evade Neutrophil Extracellular Traps (NETs) Promoting Bacterial Nucleotides Uptake and Inflammatory Damage. Int. J. Mol. Sci. 2022, 23, 15030. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Cipak, A.; Schaur, R.J.; Zarkovic, N. Pathophysiology of neutrophil-mediated extracellular redox reactions. Front. Biosci. 2016, 21, 839–855. [Google Scholar] [CrossRef]
- Fu, L.M. The potential of human neutrophil peptides in tuberculosis therapy. Int. J. Tuberc. Lung Dis. 2003, 7, 1027–1032. [Google Scholar] [PubMed]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Abdallah, D.S.A.; Egan, C.E.; Butcher, B.A.; Denkers, E.Y. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int. Immunol. 2011, 23, 317–326. [Google Scholar] [CrossRef]
- Tan, B.H.; Meinken, C.; Bastian, M.; Bruns, H.; Legaspi, A.; Ochoa, M.T.; Krutzik, S.R.; Bloom, B.R.; Ganz, T.; Modlin, R.L.; et al. Macrophages Acquire Neutrophil Granules for Antimicrobial Activity against Intracellular Pathogens. J. Immunol. 2006, 177, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Lyadova, I.V.; Panteleev, A.V. Th1 and Th17 Cells in Tuberculosis: Protection, Pathology, and Biomarkers. Mediat. Inflamm. 2015, 2015, 854507. [Google Scholar] [CrossRef]
- Ong, C.W.M.; Fox, K.; Ettorre, A.; Elkington, P.T.; Friedland, J.S. Hypoxia increases neutrophil-driven matrix destruction after exposure to Mycobacterium tuberculosis. Sci. Rep. 2018, 8, 11475. [Google Scholar] [CrossRef]
- Ramos-Kichik, V.; Mondragón-Flores, R.; Mondragón-Castelán, M.; Gonzalez-Pozos, S.; Muñiz-Hernandez, S.; Rojas-Espinosa, O.; Chacón-Salinas, R.; Estrada-Parra, S.; Estrada-García, I. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis 2009, 89, 29–37. [Google Scholar] [CrossRef]
- Meier, S.; Seddon, J.A.; Maasdorp, E.; Kleynhans, L.; du Plessis, N.; Loxton, A.G.; Malherbe, S.T.; Zak, D.E.; Thompson, E.; Duffy, F.J.; et al. Neutrophil degranulation, NETosis and platelet degranulation pathway genes are co-induced in whole blood up to six months before tuberculosis diagnosis. PLoS ONE 2022, 17, e0278295. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.; Vermeren, S. Anti-Inflammatory Neutrophil Functions in the Resolution of Inflammation and Tissue Repair. Cells 2022, 11, 4076. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.J.; Butler, E.R.; Stewart, G.R. Mycobacterium tuberculosis ESAT-6 is a leukocidin causing Ca2+ influx, necrosis and neutrophil extracellular trap formation. Cell Death Dis. 2014, 5, e1474. [Google Scholar] [CrossRef]
- Dang, G.; Cui, Y.; Wang, L.; Li, T.; Cui, Z.; Song, N.; Chen, L.; Pang, H.; Liu, S. Extracellular Sphingomyelinase Rv0888 of Mycobacterium tuberculosis Contributes to Pathological Lung Injury of Mycobacterium smegmatis in Mice via Inducing Formation of Neutrophil Extracellular Traps. Front. Immunol. 2018, 9, 677. [Google Scholar] [CrossRef]
- van der Meer, A.J.; Zeerleder, S.; Blok, D.C.; Kager, L.M.; Lede, I.O.; Rahman, W.; Afroz, R.; Ghose, A.; Visser, C.E.; Zahed, A.S.M.; et al. Neutrophil extracellular traps in patients with pulmonary tuberculosis. Respir. Res. 2017, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Teixeira, L.; Stimpson, P.J.; Stavropoulos, E.; Hadebe, S.; Chakravarty, P.; Ioannou, M.; Aramburu, I.V.; Herbert, E.; Priestnall, S.L.; Suarez-Bonnet, A.; et al. Type I IFN exacerbates disease in tuberculosis-susceptible mice by inducing neutrophil-mediated lung inflammation and NETosis. Nat. Commun. 2020, 11, 5566. [Google Scholar] [CrossRef]
- de Melo, M.G.M.; Mesquita, E.D.D.; Oliveira, M.M.; da Silva-Monteiro, C.; Silveira, A.K.A.; Malaquias, T.S.; Dutra, T.C.P.; Galliez, R.M.; Kritski, A.L.; Silva, E.C.; et al. Imbalance of NET and Alpha-1-Antitrypsin in Tuberculosis Patients Is Related with Hyper Inflammation and Severe Lung Tissue Damage. Front. Immunol. 2019, 9, 3147. [Google Scholar] [CrossRef] [PubMed]
- Braian, C.; Hogea, V.; Stendahl, O. Mycobacterium tuberculosis-Induced Neutrophil Extracellular Traps Activate Human Macrophages. J. Innate Immun. 2013, 5, 591–602. [Google Scholar] [CrossRef]
- Su, R.; Peng, Y.P.; Deng, Z.; Deng, Y.T.; Ye, J.Q.; Guo, Y.; Li, J.M. Mycobacterium tuberculosis infection induces low-density granulocyte generation by promoting neutrophil extracellular trap formation via ROS pathway. Front. Microbiol. 2019, 10, 1468. [Google Scholar] [CrossRef]
- Deng, Y.; Ye, J.; Luo, Q.; Huang, Z.; Peng, Y.; Xiong, G.; Guo, Y.; Jiang, H.; Li, J. Low-Density Granulocytes Are Elevated in Mycobacterial Infection and Associated with the Severity of Tuberculosis. PLoS ONE 2016, 11, e0153567. [Google Scholar] [CrossRef]
- Rao, J.; Su, R.; Peng, Y.; Guo, Y.; Huang, Z.; Ye, Y.; Gao, Y.; Liu, J.; Zhang, L.; Luo, Q.; et al. Low-Density Granulocytes Affect T-SPOT.TB Assay by Inhibiting the Production of Interferon-γ in T Cells via PD-L1/PD-1 Pathway. Front. Microbiol. 2021, 11, 622389. [Google Scholar] [CrossRef]
- Eum, S.-Y.; Kong, J.-H.; Hong, M.-S.; Lee, Y.-J.; Kim, J.-H.; Hwang, S.-H.; Cho, S.-N.; Via, L.E.; Barry, C.E. Neutrophils Are the Predominant Infected Phagocytic Cells in the Airways of Patients with Active Pulmonary TB. Chest 2010, 137, 122–128. [Google Scholar] [CrossRef]
- Kroon, E.E.; Coussens, A.K.; Kinnear, C.; Orlova, M.; Möller, M.; Seeger, A.; Wilkinson, R.J.; Hoal, E.G.; Schurr, E. Neutrophils: Innate Effectors of TB Resistance? Front. Immunol. 2018, 9, 2637. [Google Scholar] [CrossRef]
- Laan, M.; Cui, Z.H.; Hoshino, H.; Lötvall, J.; Sjöstrand, M.; Gruenert, D.C.; Skoogh, B.E.; Lindén, A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999, 162, 2347–2352. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Prause, O.; Sjöstrand, M.; Laan, M.; Lötvall, J.; Lindén, A. Endogenous IL-17 as a Mediator of Neutrophil Recruitment Caused by Endotoxin Exposure in Mouse Airways. J. Immunol. 2003, 170, 4665–4672. [Google Scholar] [CrossRef]
- Sugawara, I.; Udagawa, T.; Yamada, H. Rat Neutrophils Prevent the Development of Tuberculosis. Infect. Immun. 2004, 72, 1804–1806. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, J.; Saunders, B.M.; Appelberg, R.; Orme, I.M.; Silva, M.T.; Cooper, A.M. Neutrophils Play a Protective Nonphagocytic Role in Systemic Mycobacterium tuberculosis Infection of Mice. Infect. Immun. 2000, 68, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-T.; Cambier, C.; Davis, J.M.; Hall, C.J.; Crosier, P.S.; Ramakrishnan, L. Neutrophils Exert Protection in the Early Tuberculous Granuloma by Oxidative Killing of Mycobacteria Phagocytosed from Infected Macrophages. Cell Host Microbe 2012, 12, 301–312. [Google Scholar] [CrossRef]
- Barry, C.E., III; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, R.; Young, D. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J.; Lin, P.L. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011, 4, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 170077. [Google Scholar] [CrossRef] [PubMed]
- Gideon, H.P.; Phuah, J.; Junecko, B.A.; Mattila, J.T. Neutrophils express pro- and anti-inflammatory cytokines in granulomas from Mycobacterium tuberculosis-infected cynomolgus macaques. Mucosal Immunol. 2019, 12, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Belton, M.; Brilha, S.; Manavaki, R.; Mauri, F.; Nijran, K.; Hong, Y.T.; Patel, N.H.; Dembek, M.; Tezera, L.; Green, J.; et al. Hypoxia and Tissue Destruction in Pulmonary TB. Thorax 2016, 71, 1145–1153. [Google Scholar] [CrossRef]
- Elks, P.; Van Eeden, F.J.; Dixon, G.; Wang, X.; Reyes-Aldasoro, C.C.; Ingham, P.W.; Whyte, M.K.B.; Walmsley, S.; Renshaw, S.A. Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood 2011, 118, 712–722. [Google Scholar] [CrossRef]
- Elks, P.M.; Brizee, S.; Van Der Vaart, M.; Walmsley, S.R.; Van Eeden, F.J.; Renshaw, S.A.; Meijer, A.H. Hypoxia Inducible Factor Signaling Modulates Susceptibility to Mycobacterial Infection via a Nitric Oxide Dependent Mechanism. PLoS Pathog. 2013, 9, e1003789. [Google Scholar] [CrossRef]
- Walmsley, S.R.; Print, C.; Farahi, N.; Peyssonnaux, C.; Johnson, R.S.; Cramer, T.; Sobolewski, A.; Condliffe, A.M.; Cowburn, A.S.; Johnson, N.; et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α–dependent NF-κB activity. J. Exp. Med. 2005, 201, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, T.A.; Best, T.M.; Merrick, M.A. The Dual Roles of Neutrophils and Macrophages in Inflammation: A Critical Balance Between Tissue Damage and Repair. J. Athl. Train. 2006, 41, 457–465. [Google Scholar]
- Lyadova, I.V. Neutrophils in Tuberculosis: Heterogeneity Shapes the Way? Mediat. Inflamm. 2017, 2017, 8619307. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.R.; Swanson, R.V.; Al-Hammadi, N.; Domingo-Gonzalez, R.; Rangel-Moreno, J.; Kriel, B.A.; Bucsan, A.N.; Das, S.; Ahmed, M.; Mehra, S.; et al. S100A8/A9 regulates CD11b expression and neutrophil recruitment during chronic tuberculosis. J. Clin. Investig. 2020, 130, 3098–3112. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Lyadova, I.V.; Kondratieva, T.K.; Majorov, K.B.; Scheglov, I.V.; Orlova, M.O.; Apt, A.S. Neutrophil Responses to Mycobacterium tuberculosis Infection in Genetically Susceptible and Resistant Mice. Infect. Immun. 2005, 73, 1744–1753. [Google Scholar] [CrossRef]
- Ordway, D.; Palanisamy, G.; Henao-Tamayo, M.; Smith, E.E.; Shanley, C.; Orme, I.M.; Basaraba, R.J. The Cellular Immune Response to Mycobacterium tuberculosis Infection in the Guinea Pig. J. Immunol. 2007, 179, 2532–2541. [Google Scholar] [CrossRef]
- Jones, T.P.; Dabbaj, S.; Mandal, I.; Cleverley, J.; Cash, C.; Lipman, M.C.; Lowe, D.M. The Blood Neutrophil Count After 1 Month of Treatment Predicts the Radiologic Severity of Lung Disease at Treatment End. Chest 2021, 160, 2030–2041. [Google Scholar] [CrossRef]
- Lowe, D.M.; Demaret, J.; Bangani, N.; Nakiwala, J.K.; Goliath, R.; Wilkinson, K.A.; Wilkinson, R.J.; Martineau, A.R. Differential Effect of Viable Versus Necrotic Neutrophils on Mycobacterium tuberculosis Growth and Cytokine Induction in Whole Blood. Front. Immunol. 2018, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Warren, E.; Teskey, G.; Venketaraman, V. Effector Mechanisms of Neutrophils within the Innate Immune System in Response to Mycobacterium tuberculosis Infection. J. Clin. Med. 2017, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Borkute, R.; Woelke, S.; Pei, G.; Dorhoi, A. Neutrophils in Tuberculosis: Cell Biology, Cellular Networking and Multitasking in Host Defense. Int. J. Mol. Sci. 2021, 22, 4801. [Google Scholar] [CrossRef]
- BCG Vaccine Fact Sheet. 2016. Available online: https://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm#print (accessed on 2 January 2023).
- Ahmed, M.; Smith, D.M.; Hamouda, T.; Rangel-Moreno, J.; Fattom, A.; Khader, S.A. A novel nanoemulsion vaccine induces mucosal Interleukin-17 responses and confers protection upon Mycobacterium tuberculosis challenge in mice. Vaccine 2017, 35, 4983–4989. [Google Scholar] [CrossRef]
- Trentini, M.M.; de Oliveira, F.M.; Kipnis, A.; Junqueira-Kipnis, A.P. The Role of Neutrophils in the Induction of Specific Th1 and Th17 during Vaccination against Tuberculosis. Front. Microbiol. 2016, 7, 898. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcantara, C.A.; Glassman, I.; Nguyen, K.H.; Parthasarathy, A.; Venketaraman, V. Neutrophils in Mycobacterium tuberculosis. Vaccines 2023, 11, 631. https://doi.org/10.3390/vaccines11030631
Alcantara CA, Glassman I, Nguyen KH, Parthasarathy A, Venketaraman V. Neutrophils in Mycobacterium tuberculosis. Vaccines. 2023; 11(3):631. https://doi.org/10.3390/vaccines11030631
Chicago/Turabian StyleAlcantara, Cheldon Ann, Ira Glassman, Kevin H. Nguyen, Arpitha Parthasarathy, and Vishwanath Venketaraman. 2023. "Neutrophils in Mycobacterium tuberculosis" Vaccines 11, no. 3: 631. https://doi.org/10.3390/vaccines11030631
APA StyleAlcantara, C. A., Glassman, I., Nguyen, K. H., Parthasarathy, A., & Venketaraman, V. (2023). Neutrophils in Mycobacterium tuberculosis. Vaccines, 11(3), 631. https://doi.org/10.3390/vaccines11030631






