Effectiveness of the BNT162b2 (Pfizer-BioNTech) Vaccine in Children and Adolescents: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Selection Strategy
2.3. Data Extraction/Collection
2.4. Data Synthesis and Analysis
2.5. Quality Appraisal
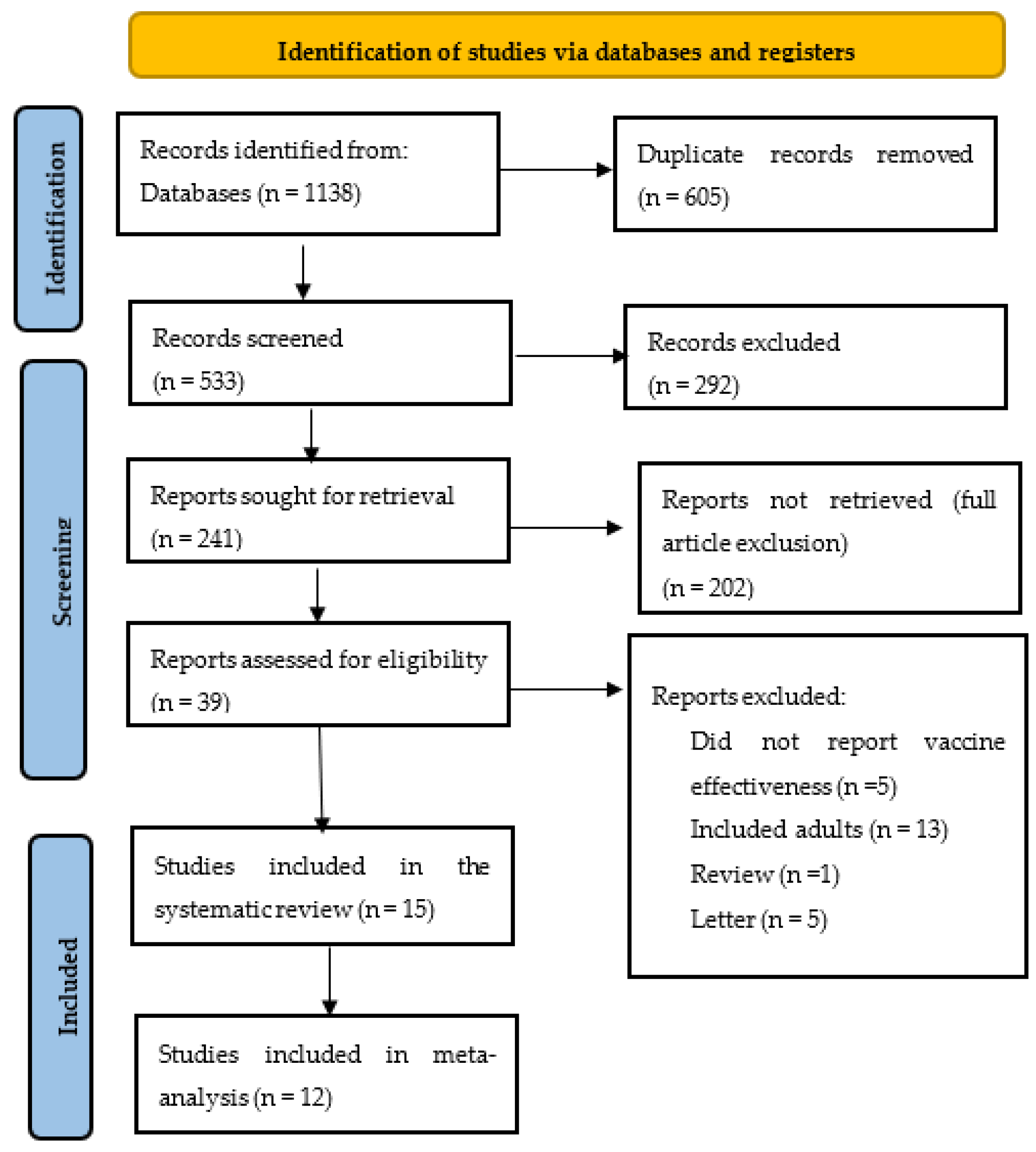
3. Results
3.1. Characteristics of Included Studies
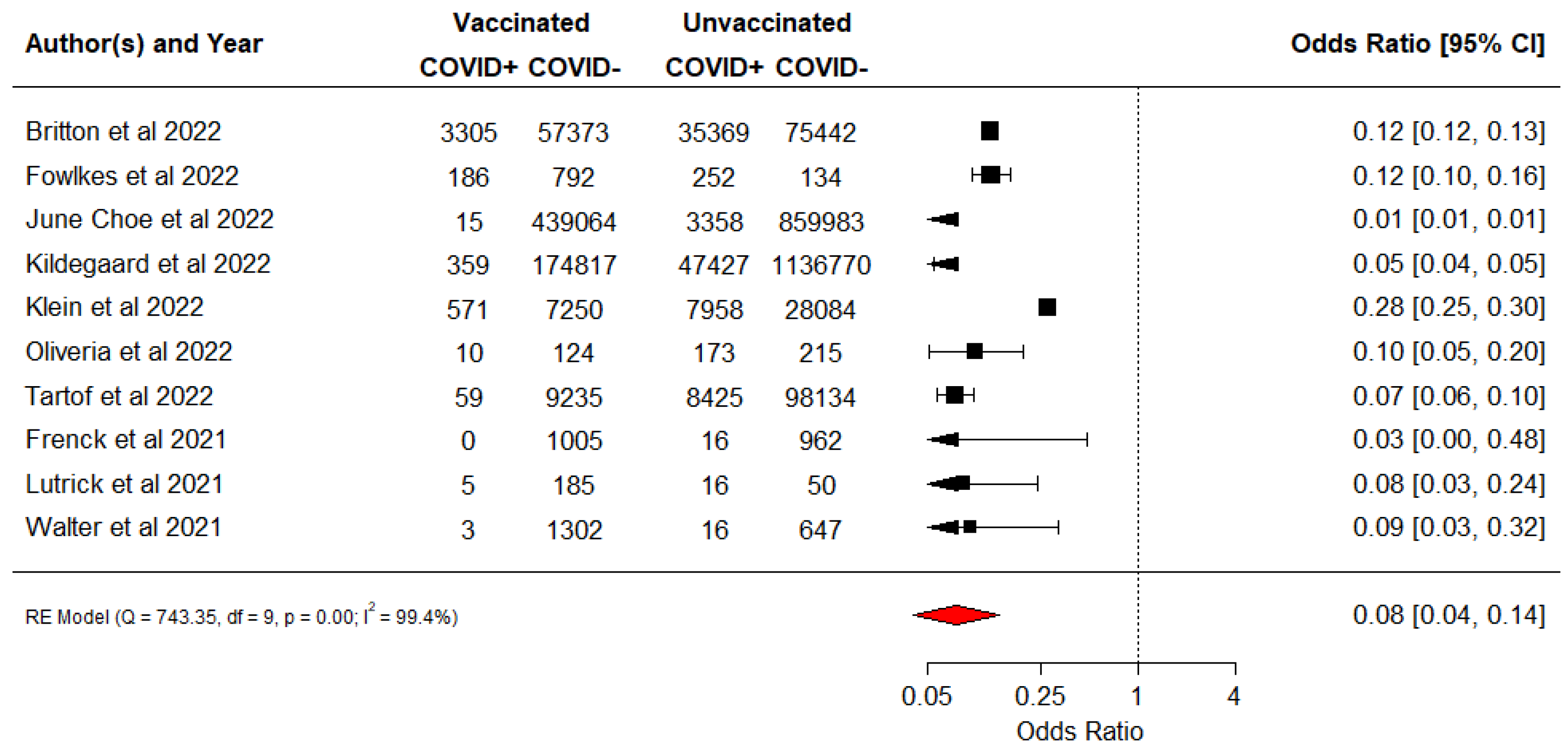
3.2. Vaccine Effectiveness against Documented COVID-19 Infection
3.3. Vaccine Effectiveness against Documented Hospitalisation
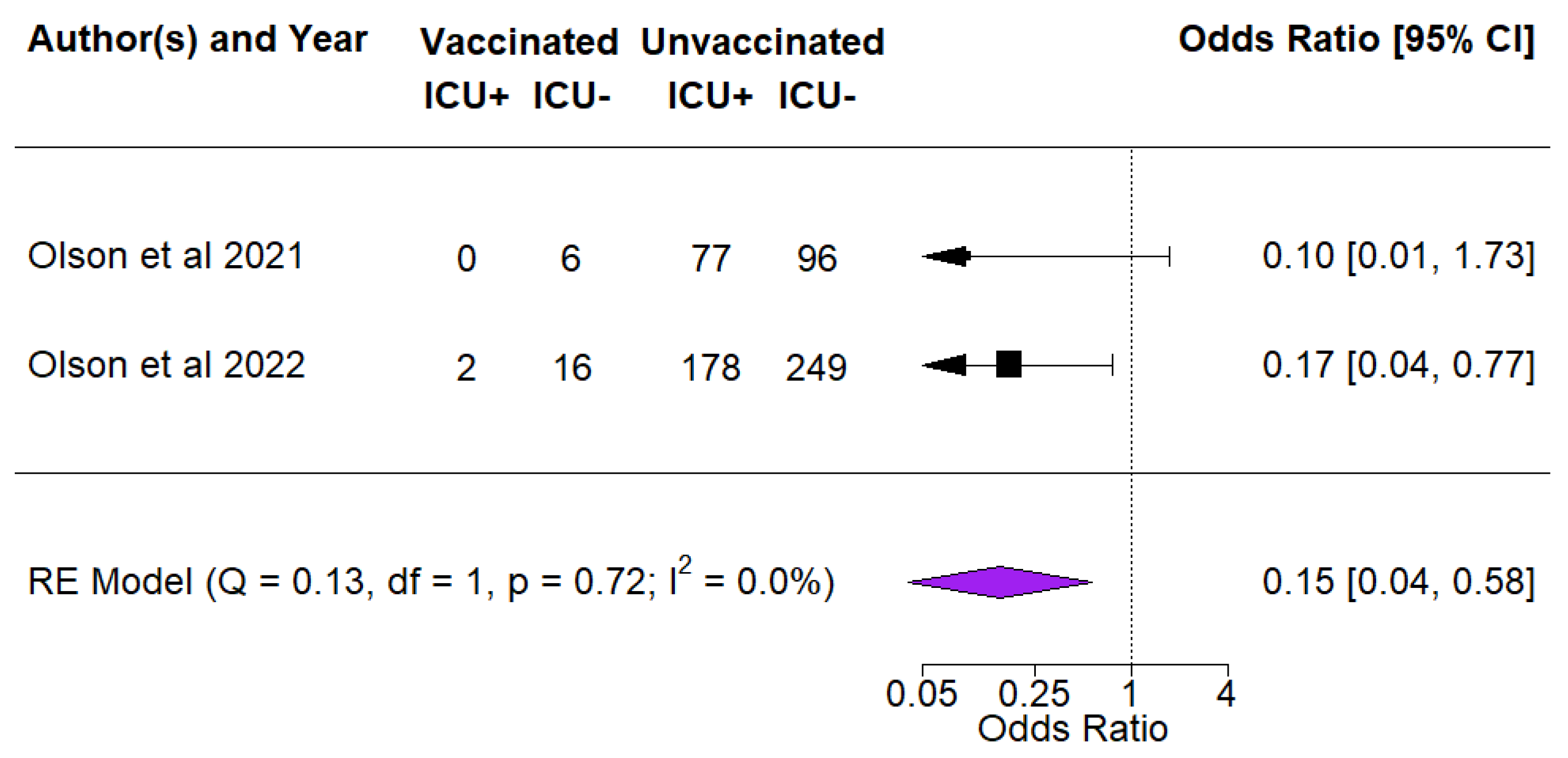
3.4. Vaccine Effectiveness against ICU Admissions among COVID Cases
3.5. Vaccine Effectiveness by Time from Immunisation or Vaccination
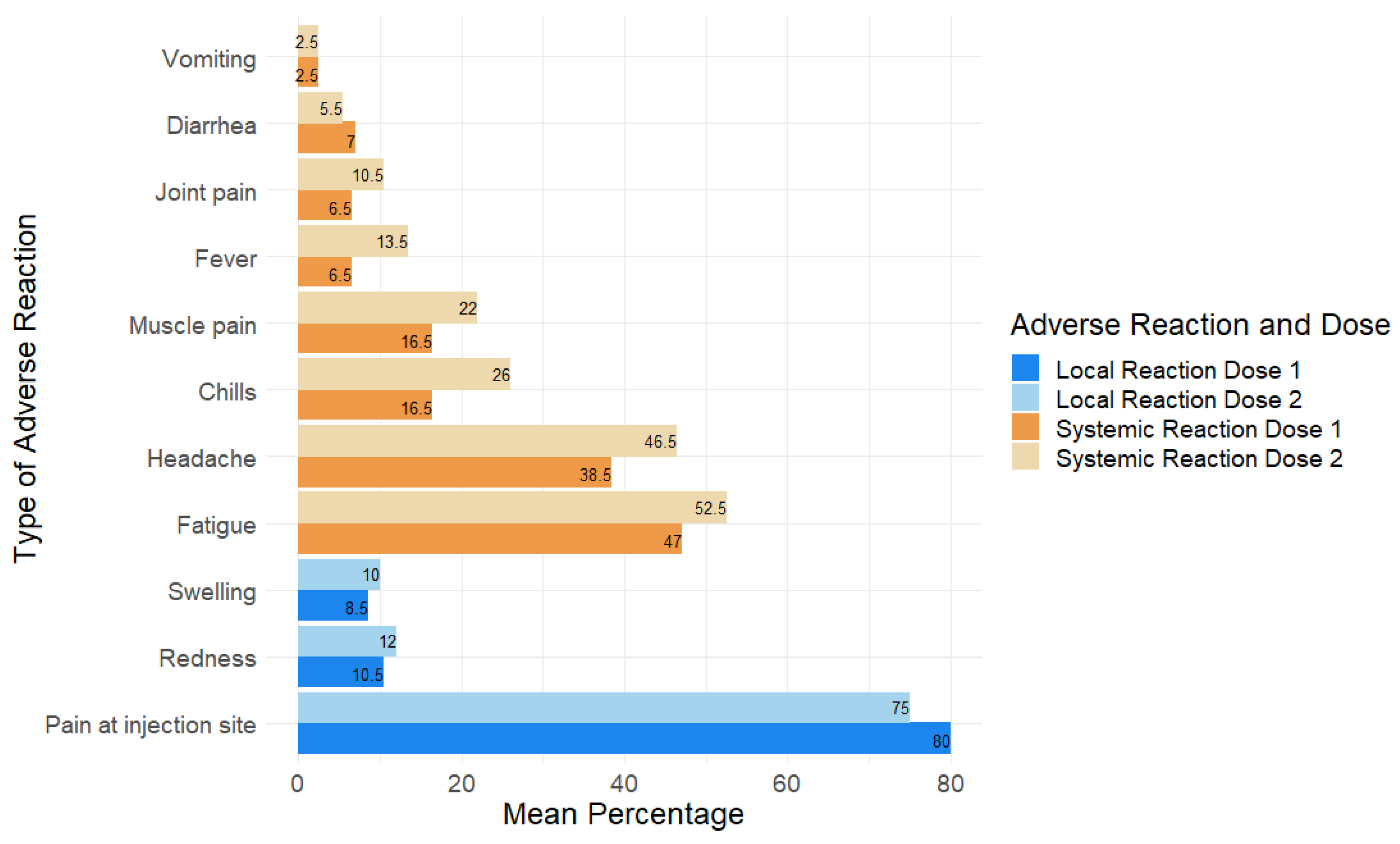
3.6. Adverse Reactions Associated with Pfizer Vaccine in Children and Adolescents
3.7. Quality Assessment of the Studies
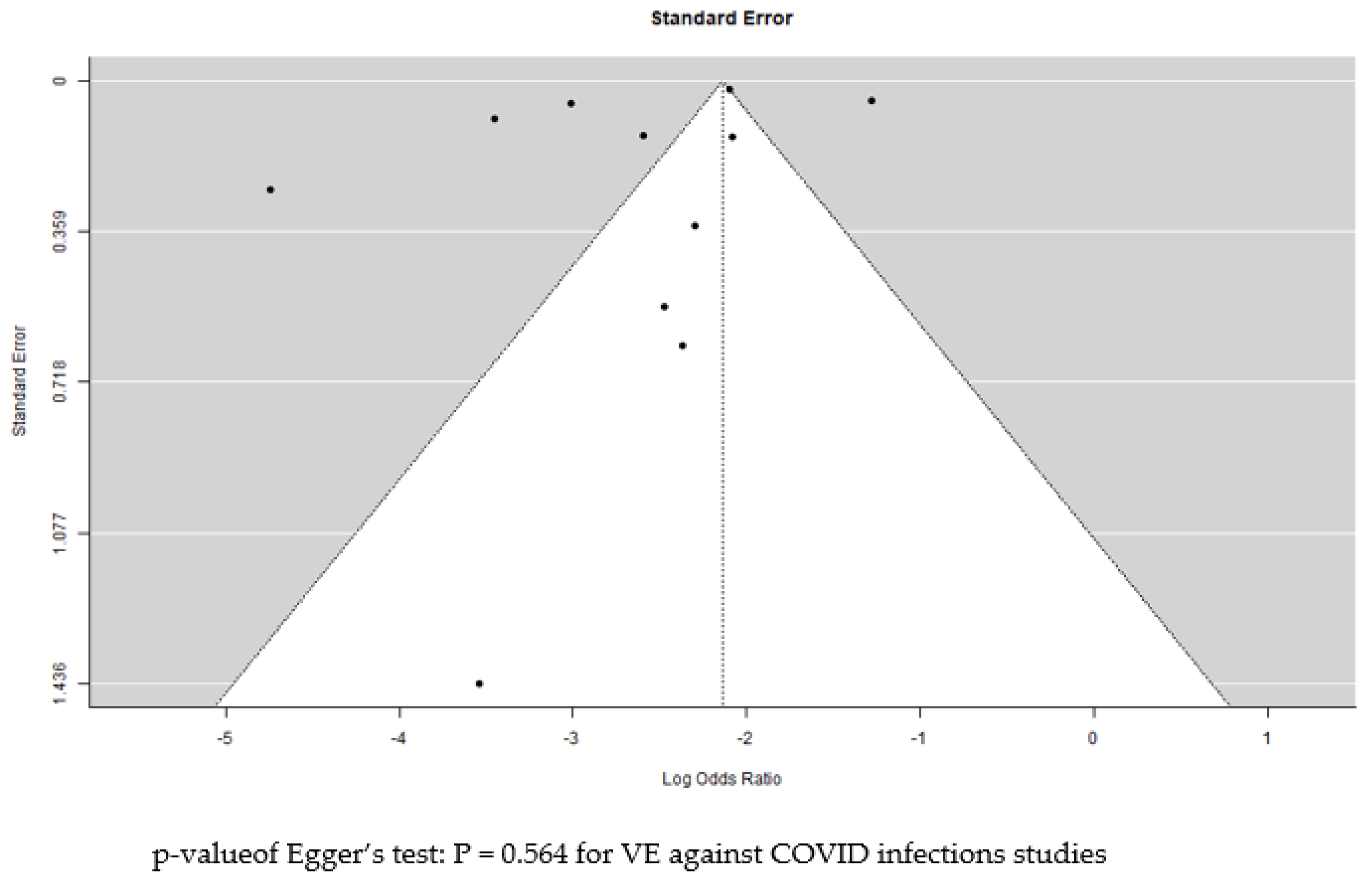
3.8. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baloch, S.; Baloch, M.A.; Zheng, T.; Pei, X. The Coronavirus Disease 2019 (COVID-19) Pandemic. Tohoku J. Exp. Med. 2020, 250, 271–278. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 23 July 2022).
- United Nations Children’s Fund (UNICEF). COVID-19 Confirmed Cases and Deaths: Age- and Sex-Disaggregated Dat. Available online: https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dashboard/ (accessed on 22 July 2022).
- Yu, C.-J.; Wang, Z.-X.; Xu, Y.; Hu, M.-X.; Chen, K.; Qin, G. Assessment of basic reproductive number for COVID-19 at global level: A meta-analysis. Medicine 2021, 100, e25837. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child 2020. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Engzell, P.; Frey, A.; Verhagen, M.D. Learning loss due to school closures during the COVID-19 pandemic. Proc. Natl. Acad. Sci. USA 2021, 118, e2022376118. [Google Scholar] [CrossRef]
- Meherali, S.; Punjani, N.; Louie-Poon, S.; Abdul Rahim, K.; Das, J.K.; Salam, R.A.; Lassi, Z.S. Mental Health of Children and Adolescents Amidst COVID-19 and Past Pandemics: A Rapid Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 3432. [Google Scholar] [CrossRef]
- Gadermann, A.C.; Thomson, K.C.; Richardson, C.G.; Gagné, M.; McAuliffe, C.; Hirani, S.; Jenkins, E. Examining the impacts of the COVID-19 pandemic on family mental health in Canada: Findings from a national cross-sectional study. BMJ Open 2021, 11, e042871. [Google Scholar] [CrossRef]
- Lotfi, M.; Hamblin, M.R.; Rezaei, N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta 2020, 508, 254–266. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19): Vaccines. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (accessed on 23 July 2022).
- Esposito, S.; Principi, N. Multisystem Inflammatory Syndrome in Children Related to SARS-CoV-2. Paediatr. Drugs 2021, 23, 119–129. [Google Scholar] [CrossRef]
- World Health Organization (WHO). COVID-19 Advice for the Public: Getting Vaccinated. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 23 July 2022).
- World Health Organization (WHO). The Pfizer BioNTech (BNT162b2) COVID-19 Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine-what-you-need-to-know (accessed on 23 July 2022).
- World Health Organization (WHO). Background Document on the mRNA Vaccine BNT162b2 (Pfizer-BioNTech) Against COVID-19. 2021. Available online: https://apps.who.int/iris/handle/10665/338671 (accessed on 30 September 2022).
- Centers for Disease Control and Prevention. Overview of COVID-19 Vaccines. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/overview-COVID-19-vaccines.html (accessed on 30 September 2022).
- World Health Organization (WHO). The Moderna COVID-19 (mRNA-1273) Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know (accessed on 30 September 2022).
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. How COVID unlocked the power of RNA vaccines. Nature 2021, 589, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother 2022, 18, 2027160. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. npj Vaccines 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Bmj 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Sirriyeh, R.; Lawton, R.; Gardner, P.; Armitage, G. Reviewing studies with diverse designs: The development and evaluation of a new tool. J. Eval. Clin. Pract. 2012, 18, 746–752. [Google Scholar] [CrossRef]
- Britton, A.; Fleming- Dutra, K.E.; Shang, N.; Smith, Z.R.; Dorji, T.; Derado, G.; Accorsi, E.K.; Ajani, U.A.; Miller, J.; Schrag, S.J.; et al. Association of COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection by Time Since Vaccination and Delta Variant Predominance. Jama 2022, 327, 1032–1041. [Google Scholar] [CrossRef]
- Fowlkes, A.L.; Yoon, S.K.; Lutrick, K.; Gwynn, L.; Burns, J.; Grant, L.; Phillips, A.L.; Ellingson, K.; Ferraris, M.V.; LeClair, L.B.; et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in Preventing SARS-CoV-2 Infection Among Children Aged 5–11 Years and Adolescents Aged 12–15 Years—PROTECT Cohort, July 2021–February 2022. MMWR Recomm. Rep. 2022, 71, 422–428. [Google Scholar] [CrossRef]
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Glatman-Freedman, A.; Hershkovitz, Y.; Kaufman, Z.; Dichtiar, R.; Keinan-Boker, L.; Bromberg, M. Eff ectiveness of bnt162b2 vaccine in adolescents during outbreak of sars-cov-2 delta variant infection, israel, 2021. Emerg. Infect. Dis. 2021, 27, 2919–2922. [Google Scholar] [CrossRef]
- June Choe, Y.; Yi, S.; Hwang, I.; Kim, J.; Park, Y.J.; Cho, E.; Jo, M.; Lee, H.; Hwa Choi, E. Safety and effectiveness of BNT162b2 mRNA COVID-19 vaccine in adolescents. Vaccine 2022, 40, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Kildegaard, H.; Lund, L.C.; Hojlund, M.; Stensballe, L.G.; Pottegard, A. Risk of adverse events after covid-19 in Danish children and adolescents and effectiveness of BNT162b2 in adolescents: Cohort study. Bmj 2022, 377, e068898. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Stockwell, M.S.; Demarco, M.; Gaglani, M.; Kharbanda, A.B.; Irving, S.A.; Rao, S.; Grannis, S.J.; Dascomb, K.; Murthy, K.; et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA Vaccination in Preventing COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Nonimmunocompromised Children and Adolescents Aged 5-17 Years - VISION Network, 10 States, April 2021-January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.K.; Moore, Z.; Zeng, D. Effectiveness of Covid-19 Vaccines over a 9-Month Period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef]
- Lutrick, K.; Rivers, P.; Yoo, Y.M.; Grant, L.; Hollister, J.; Jovel, K.; Khan, S.; Lowe, A.; Baccam, Z.; Hanson, H.; et al. Interim Estimate of Vaccine Effectiveness of BNT162b2 (Pfizer-BioNTech) Vaccine in Preventing SARS-CoV-2 Infection Among Adolescents Aged 12-17 Years - Arizona, July-December 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1761–1765. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Niccolai, L.M.; Sheikha, H.; Elmansy, L.; Kalinich, C.C.; Grubaugh, N.D.; Shapiro, E.D.; Yale, S.-C.-G.S.I. Assessment of Clinical Effectiveness of BNT162b2 COVID-19 Vaccine in US Adolescents. JAMA netw 2022, 5, e220935. [Google Scholar] [CrossRef]
- Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Irby, K.; Walker, T.C.; Schwartz, S.P.; Pannaraj, P.S.; et al. Effectiveness of Pfizer-BioNTech mRNA Vaccination Against COVID-19 Hospitalization Among Persons Aged 12–18 Years–United States, June-September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1483–1488. [Google Scholar] [CrossRef]
- Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Pannaraj, P.S.; Irby, K.; Walker, T.C.; Schwartz, S.P.; et al. Effectiveness of BNT162b2 Vaccine against Critical Covid-19 in Adolescents. N. Engl. J. Med. 2022, 386, 713–723. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2021, 386, 35–46. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Kamidani, S.; Tarquinio, K.M.; Maddux, A.B.; et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12-18 Years -- United States, July-December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Amlôt, R.; Weinman, J.; Yiend, J.; Rubin, G.J. A systematic review of factors affecting vaccine uptake in young children. Vaccine 2017, 35, 6059–6069. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choi, S.H.; Yun, K.W. Risk Factors for Severe COVID-19 in Children: A Systematic Review and Meta-Analysis. J. Korean Med. Sci. 2022, 37, e35. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, L.; Shi, Y. Safety, Immunogenicity, and Efficacy of COVID-19 Vaccines in Adolescents, Children, and Infants: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Kalantari, Y.; Shokri, S.; Fallahpour, M.; Nafissi, N.; Goodarzi, A.; Valizadeh, R. Immunologic response, Efficacy, and Safety of Vaccines against COVID-19 Infection in Healthy and immunosuppressed Children and Adolescents Aged 2–21 years old: A Systematic Review and Meta-analysis. J. Clin. Virol. 2022, 153, 105196. [Google Scholar] [CrossRef]
- Lv, M.; Luo, X.; Shen, Q.; Lei, R.; Liu, X.; Liu, E.; Li, Q.; Chen, Y. Safety, Immunogenicity, and Efficacy of COVID-19 Vaccines in Children and Adolescents: A Systematic Review. Vaccines 2021, 9, 1102. [Google Scholar] [CrossRef]
- Rahmani, K.; Shavaleh, R.; Forouhi, M.; Disfani, H.F.; Kamandi, M.; Oskooi, R.K.; Foogerdi, M.; Soltani, M.; Rahchamani, M.; Mohaddespour, M.; et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front. Public Health 2022, 10, 873596. [Google Scholar] [CrossRef]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020, 109, 1088–1095. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 11 August 2022).
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. Jama 2022, 327, 2210–2219. [Google Scholar] [CrossRef]
- Price, A.M.; Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Boom, J.A.; Sahni, L.C.; Pannaraj, P.S.; Irby, K.; Bline, K.E.; Maddux, A.B.; et al. BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N. Engl. J. Med. 2022, 386, 1899–1909. [Google Scholar] [CrossRef]
- Higdon, M.M.; Baidya, A.; Walter, K.K.; Patel, M.K.; Issa, H.; Espié, E.; Feikin, D.R.; Knoll, M.D. Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect. Dis. 2022, 22, 1114–1116. [Google Scholar] [CrossRef]
- Thompson, M.G. Effectiveness of a third dose of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1291. [Google Scholar]
- Xia, S.; Wang, L.; Zhu, Y.; Lu, L.; Jiang, S. Origin, virological features, immune evasion and intervention of SARS-CoV-2 Omicron sublineages. Signal Transduct. Target. Ther. 2022, 7, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, S.; Wu, B.; Yang, Q.; Chen, A.; Li, Y.; Zhang, Y.; Pan, T.; Zhang, H.; He, X. SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct. Target. Ther. 2021, 6, 430. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 2022, 22, 1002–1010. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events after COVID-19 mRNA Vaccination. Jama 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Hawkes, M.T.; Good, M.F. Vaccinating Children against COVID-19: Commentary and Mathematical Modeling. mBio 2022, 13, e0378921. [Google Scholar] [CrossRef]

| Author and Year | Country | Study Design | Population Characteristics | SARS-CoV-2 Variant under Investigation |
|---|---|---|---|---|
| Britton, A. et al., 2022 [25] | United States of America | Test-negative case-control | 180,112 adolescents aged 12–19 years (39,422 cases and 140,690 controls). Females accounted for 52.6% of the cases (20,696) and 54.3% (76,108) of the controls, respectively *. | Delta |
| Fowlkes, A.L. et al., 2022 [26] | United States of America | Prospective cohort study | 1364 children and adolescents aged 5–15 years. 1052 were children 5–11 years and 312 were adolescents 12–15 years. Females accounted for 52.3% (713) of the population. | Delta, Omicron |
| Frenck, R.W., Jr. et al., 2021 [27] | United States of America | Randomised control trial | 2260 adolescents aged 12–15 years. Males accounted for 51% of the total population. | Not indicated |
| Glatman-Freedman, A. et al., 2021 [28] | Israel | Retrospective cohort study | Adolescents aged 12–15 years | Delta |
| June Choe, Y. et al., 2021 [29] | South Korea | Retrospective cohort study | 454,876 12th-grade high-school adolescents | Delta |
| Kildegaard, H. et al., 2022 [30] | Denmark | Cohort study | 991,682 children and adolescents < 18 years of age | Delta |
| Klein, N. P. et al., 2022 [31] | United States of America | Case-control | 39,217 children and adolescents aged 5–17 years. Females accounted for 51.7% (20,310) of the total population | Pre-delta, Delta and Omicron |
| Lin, D.Y. et al., 2022 [32] | United States of America | Retrospective cohort study † | 806,634 adolescents aged 12–17 years * | Delta |
| Lutrick, K. et al., 2021 [33] | United States of America | Prospective cohort study | 243 adolescents aged 12–17 years. Females accounted for 43.6% (106) of the total population. | Delta |
| Oliveira, C.R. et al., 2022 [34] | United States of America | Case-control | 542 adolescents aged 12–18 years. The median age was 14 years (IQR, 13–16 years). Females accounted for 48% (262) of the total population. | Delta |
| Olson, Samantha M. et al., 2021 [35] | United States of America | Test-negative case-control | 464 hospitalised adolescents aged 12–18 years. The median age was 15 years (IQR, 14–17 years). Females accounted for 45.3% (210) of the total population. | Delta |
| Olson, Samantha M. et al., 2022 [36] | United States of America | Test-negative case-control | 1222 adolescents aged 12–18 years. The median age for the cases was 16 years and the median age for the controls was 15 years. Females accounted for 49.5% (605) of the total population. | Delta |
| Tartof, S.Y. et al., 2021 [37] | United States of America | Retrospective cohort study | 201,622 adolescents aged 12–15 years * | Delta, other variants |
| Walter, E.B. et al., 2022 [38] | United States of America, Spain, Finland and Poland | Randomised control trial | 2285 children aged 5–11 years. The mean age was 8.2 years and males accounted for 52% (1182) of the total population. | Not indicated |
| Zambrano, Laura D. et al., 2022 [39] | United States of America | Test-negative case-control | 283 hospitalised adolescents aged 12–18 years. The median age was 14.5 years (IQR 13.4–15.9) and females accounted for 46.6% (132) of the total population. | Delta |
| Duration after Vaccination (Weeks) | Strain | Mean VE |
|---|---|---|
| 1–4 | Delta | 91.14 |
| 5–8 | Delta | 91.5 |
| 9–12 | Delta | 91 |
| 13–17 | Delta | 85.3 |
| 18–21 | Delta | 88.4 |
| 22–25 | Delta | 80.2 |
| 2–20 | Omicron | 44 |
| ≥21 | Omicron | 18 |
| QATSDD/ Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Total Score | % of Total Score | Grade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Britton et al., 2022 [25] | 2 | 1 | 2 | 0 | 2 | 3 | 0 | 2 | 0 | 3 | 3 | 1 | 2 | 21 | 53.85% | Good |
| Fowlkes et al., 2022 [26] | 1 | 0 | 1 | 1 | 1 | 3 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 13 | 33.33% | Low |
| Frenck et al., 2021 [27] | 1 | 2 | 1 | 1 | 1 | 3 | 1 | 0 | 1 | 2 | 2 | 1 | 2 | 18 | 46.15% | Low |
| Glatman-Freedman et al., 2021 [28] | 0 | 1 | 2 | 0 | 3 | 1 | 0 | 1 | 0 | 3 | 2 | 0 | 1 | 14 | 35.90% | Low |
| June Choe et al., 2021 [29] | 2 | 1 | 2 | 0 | 2 | 3 | 1 | 1 | 0 | 2 | 2 | 1 | 1 | 18 | 46.15% | Low |
| Kildegaard et al., 2022 [30] | 3 | 2 | 3 | 0 | 2 | 2 | 1 | 2 | 1 | 3 | 3 | 2 | 3 | 27 | 69.23% | Good |
| Klein et al., 2022 [31] | 1 | 1 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 18 | 46.15% | Low |
| Lin et al., 2022 [32] | 2 | 2 | 1 | 0 | 3 | 2 | 1 | 1 | 1 | 3 | 3 | 2 | 2 | 23 | 58.97% | Good |
| Lutrick et al., 2021 [33] | 1 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 3 | 2 | 1 | 2 | 17 | 43.59% | Low |
| Oliveira et al., 2022 [34] | 3 | 3 | 3 | 1 | 3 | 3 | 0 | 3 | 0 | 3 | 3 | 3 | 2 | 30 | 76.92% | Good |
| Olson et al., 2021 [35] | 1 | 2 | 3 | 0 | 2 | 3 | 0 | 2 | 0 | 3 | 3 | 2 | 2 | 23 | 58.97% | Good |
| Olson et al., 2022 [36] | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 2 | 0 | 3 | 3 | 2 | 2 | 23 | 58.97% | Good |
| Tartof et al., 2021 [37] | 3 | 3 | 3 | 1 | 2 | 3 | 1 | 1 | 0 | 3 | 3 | 3 | 2 | 28 | 71.79% | Good |
| Walter et al., 2022 [38] | 2 | 1 | 0 | 3 | 3 | 3 | 1 | 1 | 0 | 3 | 3 | 3 | 2 | 25 | 64.10% | Good |
| Zambrano et al., 2022 [39] | 1 | 1 | 2 | 0 | 1 | 3 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 14 | 35.90% | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabu, J.M.; Zahid, I.; Jacob, N.; Alele, F.O.; Malau-Aduli, B.S. Effectiveness of the BNT162b2 (Pfizer-BioNTech) Vaccine in Children and Adolescents: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1880. https://doi.org/10.3390/vaccines10111880
Sabu JM, Zahid I, Jacob N, Alele FO, Malau-Aduli BS. Effectiveness of the BNT162b2 (Pfizer-BioNTech) Vaccine in Children and Adolescents: A Systematic Review and Meta-Analysis. Vaccines. 2022; 10(11):1880. https://doi.org/10.3390/vaccines10111880
Chicago/Turabian StyleSabu, Jewel Maria, Izza Zahid, Namitha Jacob, Faith O. Alele, and Bunmi S. Malau-Aduli. 2022. "Effectiveness of the BNT162b2 (Pfizer-BioNTech) Vaccine in Children and Adolescents: A Systematic Review and Meta-Analysis" Vaccines 10, no. 11: 1880. https://doi.org/10.3390/vaccines10111880
APA StyleSabu, J. M., Zahid, I., Jacob, N., Alele, F. O., & Malau-Aduli, B. S. (2022). Effectiveness of the BNT162b2 (Pfizer-BioNTech) Vaccine in Children and Adolescents: A Systematic Review and Meta-Analysis. Vaccines, 10(11), 1880. https://doi.org/10.3390/vaccines10111880






