Limited T-Cell-Stimulating Effect of Cytochalasin-B-Induced Membrane Vesicles Isolated from Artificial Antigen-Presenting Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Culture Conditions
2.2. Production and Isolation of CIMVs and AP-CIMVs
2.3. Characterization of AP-CIMVs
2.4. aAPC Endogenously Expressing CMV pp65 Antigen
2.5. Flow Cytometry
2.6. Generation and Stimulation of A*02:01 Specific Binding TCR Reporter Jurkat
2.7. Statistical Analysis
3. Results
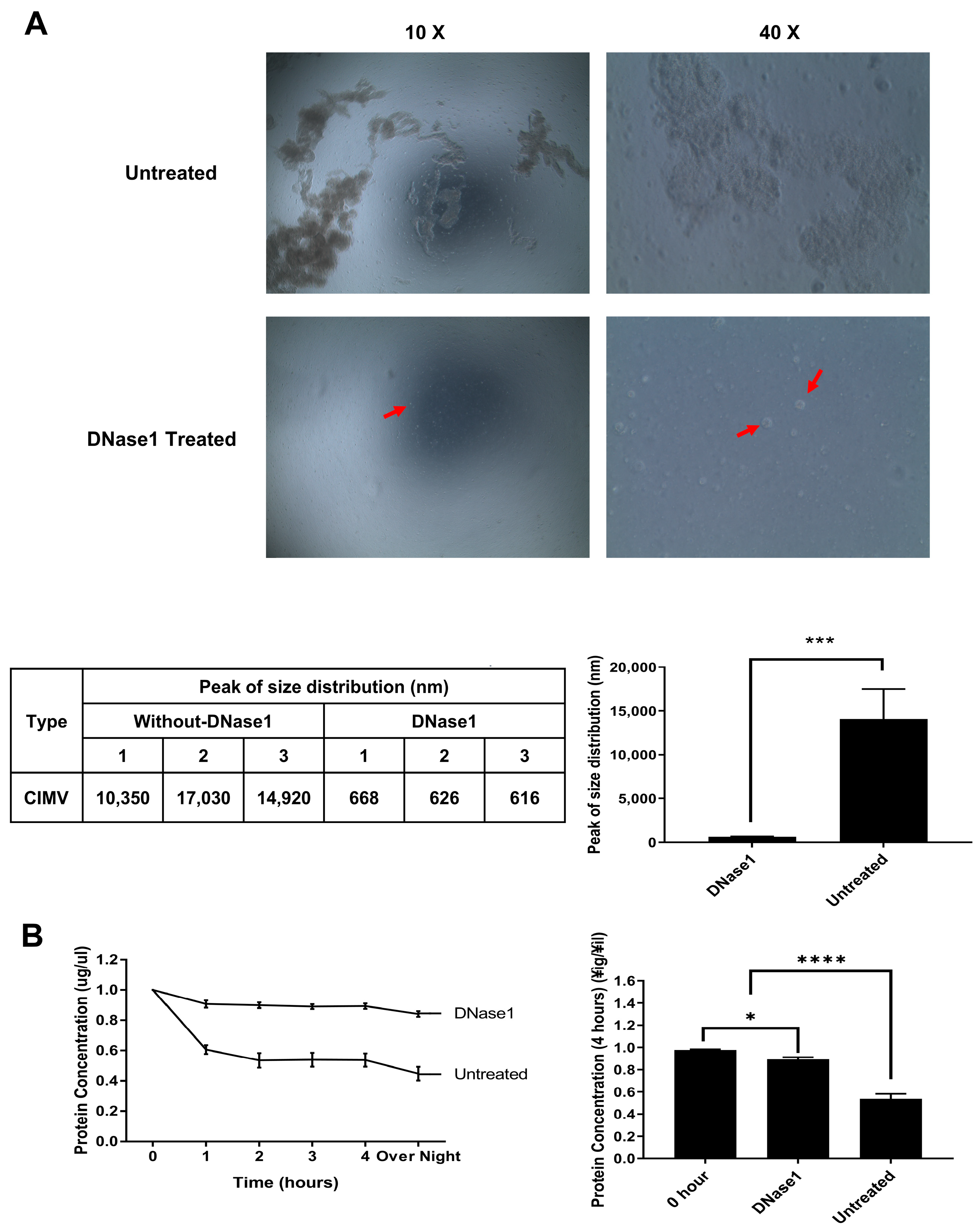
3.1. Effect of DNase1 Treatment on Isolated AP-CIMVs
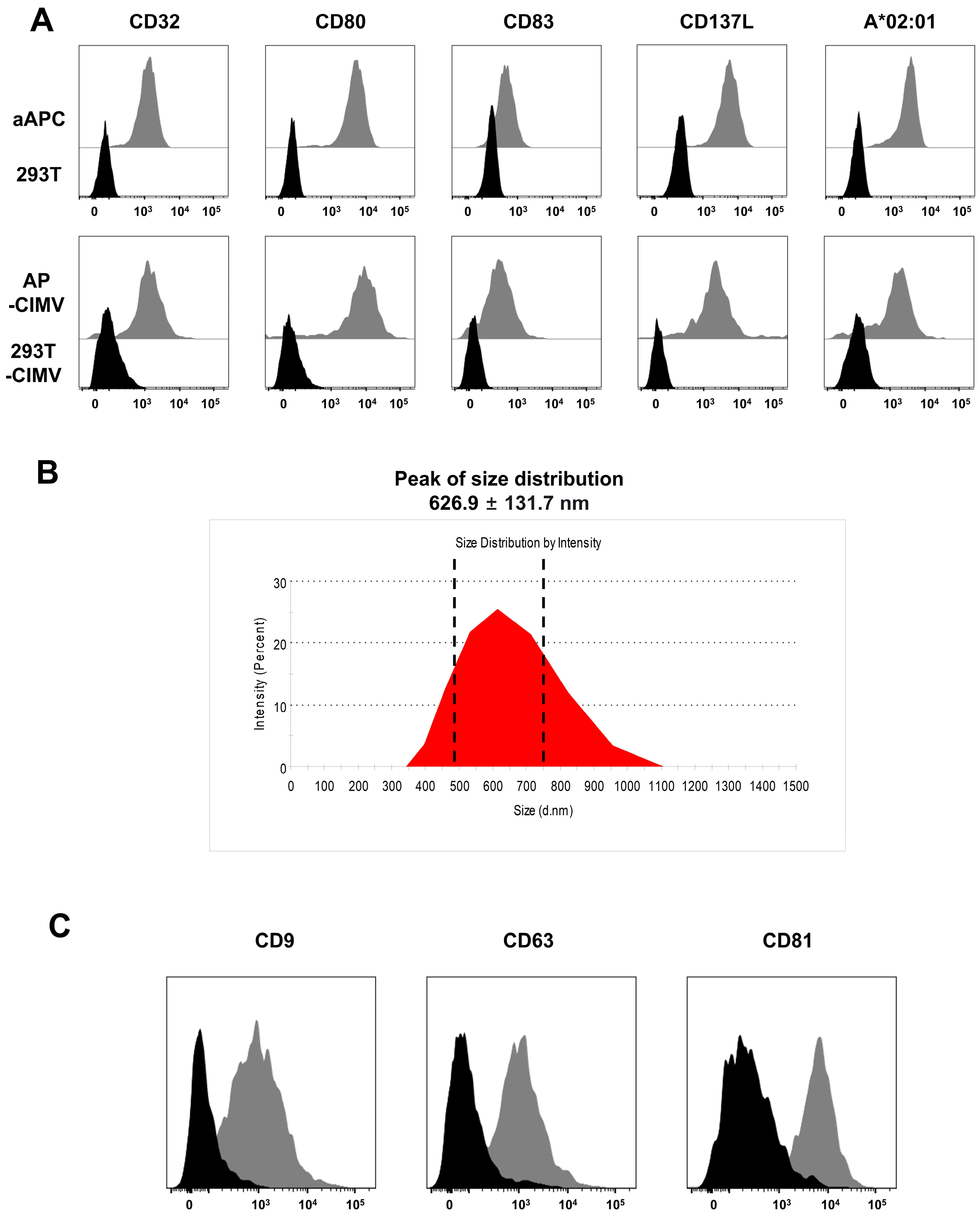
3.2. Immunological Characteristics of AP-CIMVs
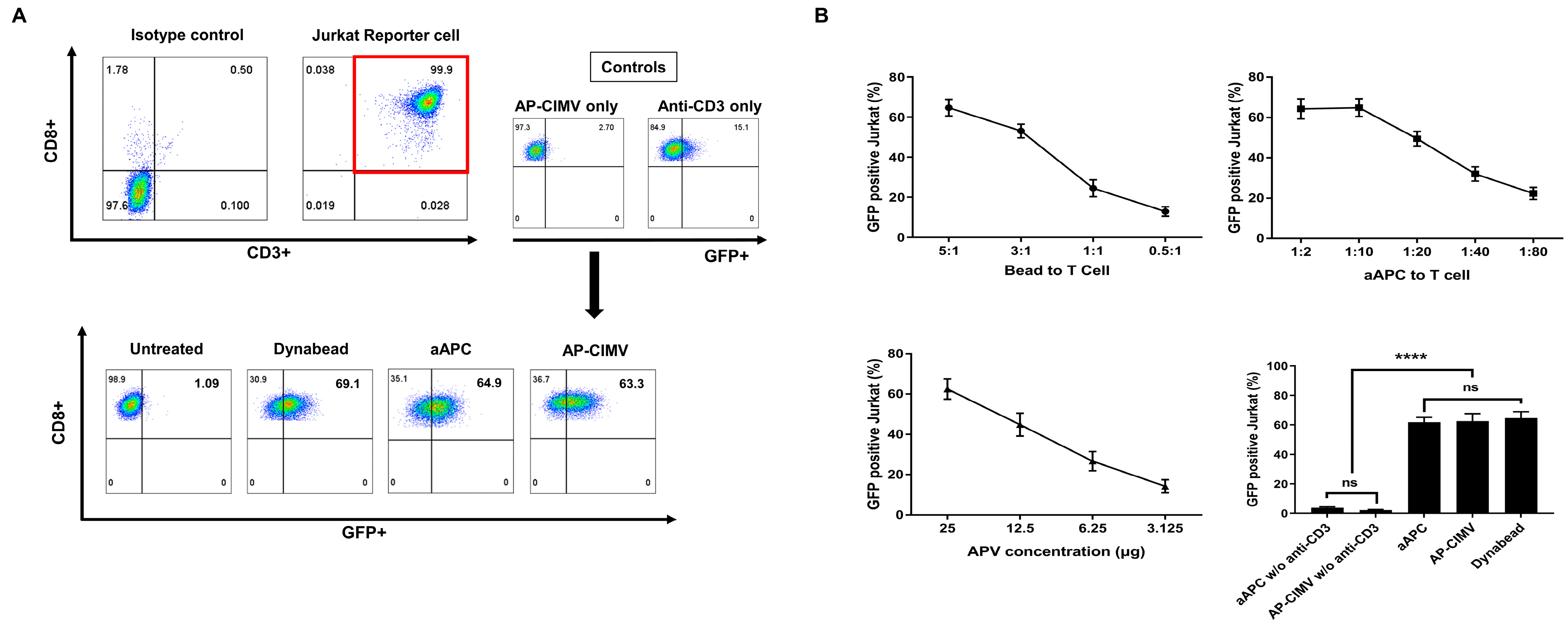
3.3. T Cell Activation by AP-CIMVs Coated with Anti-CD3 Antibody
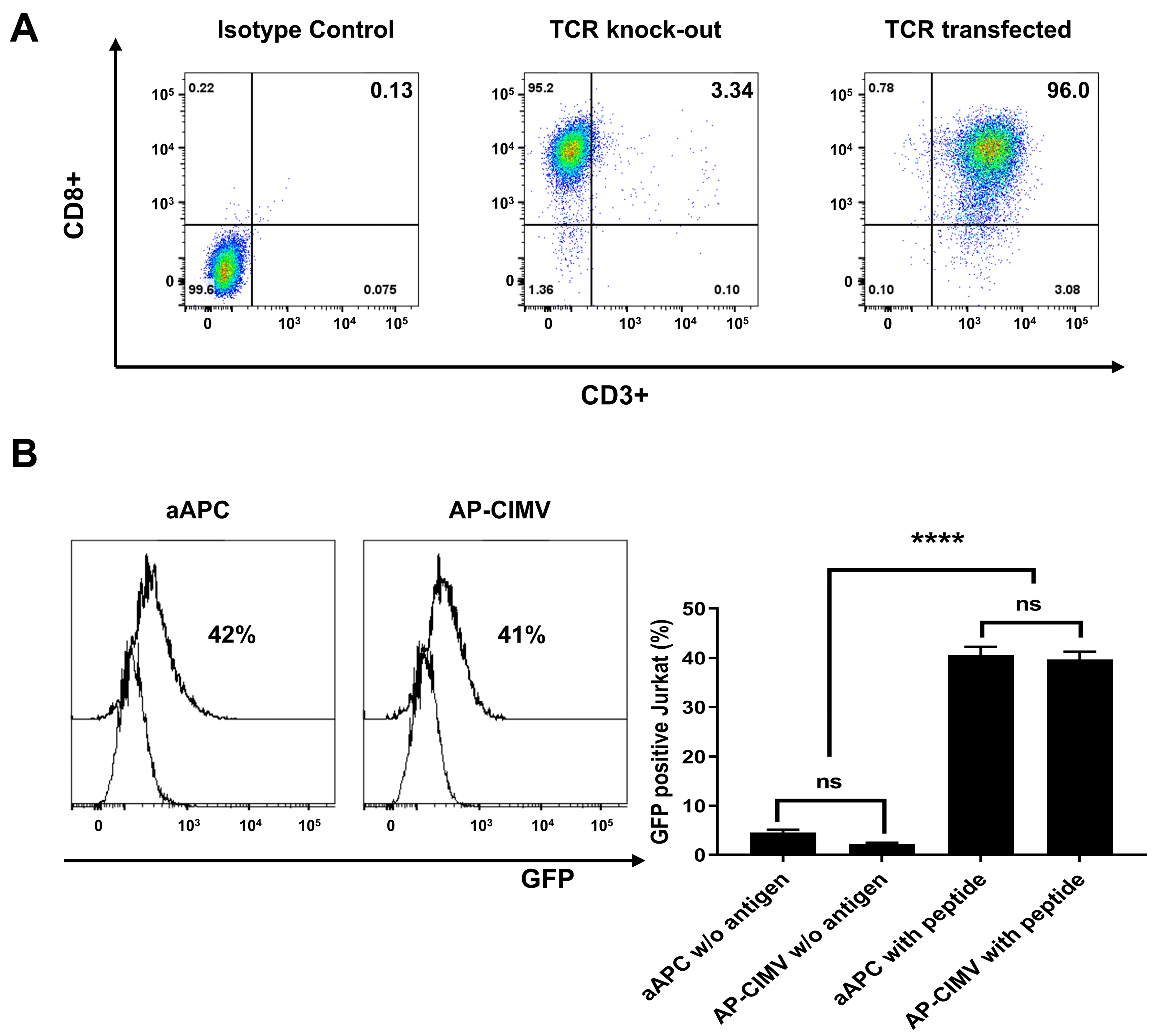
3.4. HLA-A*02:01 Restricted T Cell Activation by AP-CIMVs Loaded with CMV pp65 Peptide
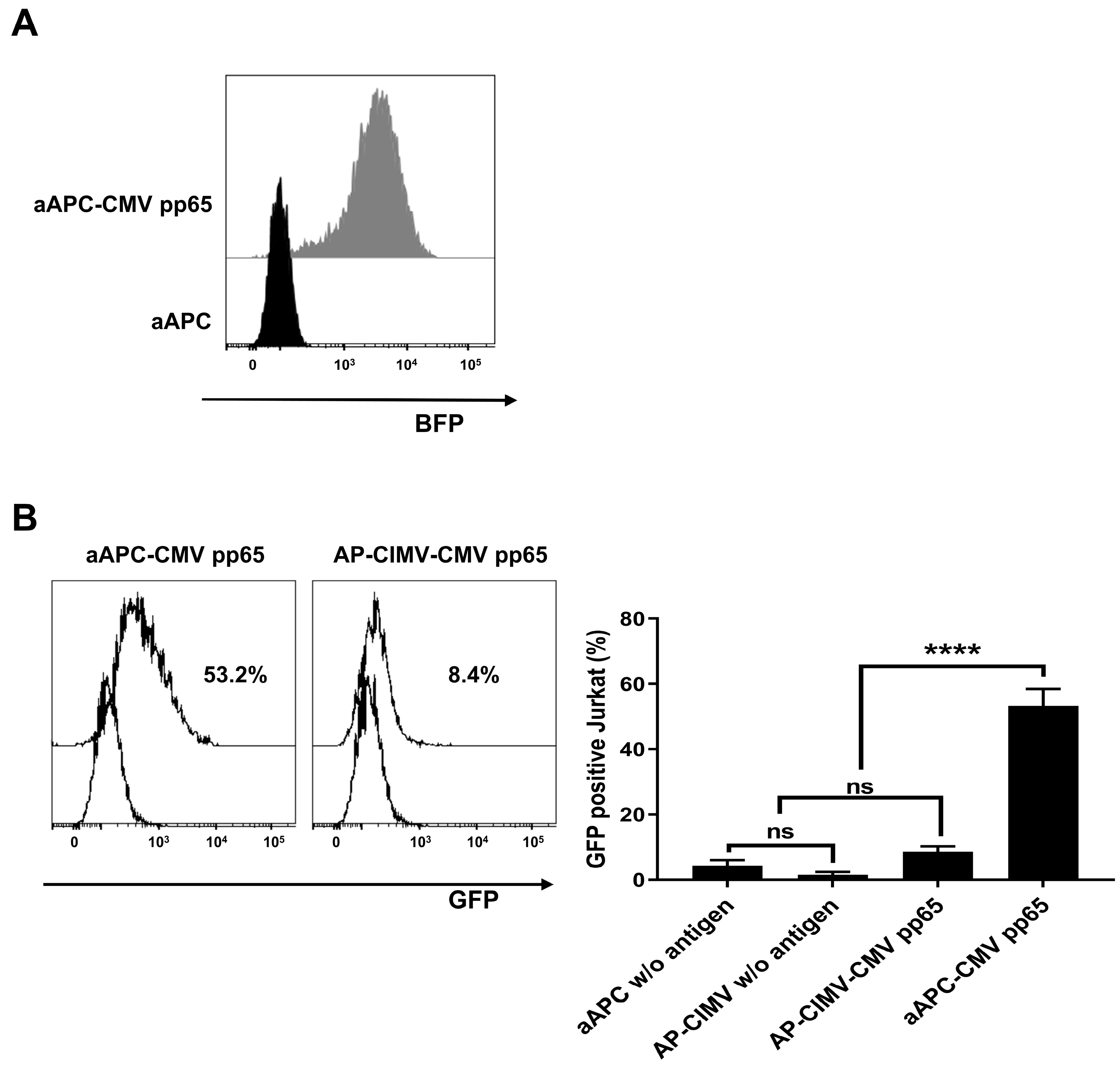
3.5. Presentation of Endogenously Processed Antigen by AP-CIMVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Rak, J. Extracellular Vesicles—Biomarkers and Effectors of the Cellular Interactome in Cancer. Front. Pharmacol. 2013, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-Ibáñez, E.; Sanz-Garcia, A.; Visakorpi, T.; Escobedo-Lucea, C.; Siljander, P.; Ayuso-Sacido, Á.; Yliperttula, M. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: Apoptotic bodies, microvesicles, and exosomes. Prostate 2014, 74, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- García-Manrique, P.; Matos, M.; Gutiérrez, G.; Pazos, C.; Blanco-López, M.C. Therapeutic biomaterials based on extracellular vesicles: Classification of bio-engineering and mimetic preparation routes. J. Extracell. Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.d.; et al. Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Steffan, A.-M.; Gendrault, J.-L.; Kirn, A. Increase in the number of fenestrae in mouse endothelial liver cells by altering the cytoskeleton with cytochalasin B. Hepatology 1987, 7, 1230–1238. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; Zhuravleva, M.N.; Miftakhova, R.R.; Arkhipova, S.S.; Evtugin, V.G.; Khaiboullina, S.F.; Kiyasov, A.P.; Persson, J.L.; Mongan, N.P.; Pestell, R.G.; et al. Cytochalasin B-induced membrane vesicles convey angiogenic activity of parental cells. Oncotarget 2017, 8, 70496–70507. [Google Scholar] [CrossRef]
- Oshchepkova, A.; Neumestova, A.; Matveeva, V.; Artemyeva, L.; Morozova, K.; Kiseleva, E.; Zenkova, M.; Vlassov, V. Cytochalasin-B-Inducible Nanovesicle Mimics of Natural Extracellular Vesicles That Are Capable of Nucleic Acid Transfer. Micromachines 2019, 10, 750. [Google Scholar] [CrossRef]
- Inaba, K.; Metlay, J.P.; Crowley, M.T.; Witmer-Pack, M.; Steinman, R.M. Dendritic cells as antigen presenting cells in vivo. Int. Rev. Immunol. 1990, 6, 197–206. [Google Scholar] [CrossRef]
- Paulis, L.E.; Mandal, S.; Kreutz, M.; Figdor, C.G. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr. Opin. Immunol. 2013, 25, 389–395. [Google Scholar] [CrossRef]
- Suhoski, M.M.; Golovina, T.N.; Aqui, N.A.; Tai, V.C.; Varela-Rohena, A.; Milone, M.C.; Carroll, R.G.; Riley, J.L.; June, C.H. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol. Ther. 2007, 15, 981–988. [Google Scholar] [CrossRef]
- Li, H.; Shao, S.; Cai, J.; Burner, D.; Lu, L.; Chen, Q.; Minev, B.; Ma, W. Artificial human antigen-presenting cells are superior to dendritic cells at inducing cytotoxic T-cell responses. Immunology 2017, 152, 462–471. [Google Scholar] [CrossRef]
- Kim, S.; Sohn, H.-J.; Lee, H.-J.; Sohn, D.-H.; Hyun, S.-J.; Cho, H.-I.; Kim, T.-G. Use of engineered Exosomes expressing HLA and Costimulatory molecules to generate antigen-specific CD8+ T cells for adoptive cell therapy. J. Immunother. 2017, 40, 83–93. [Google Scholar] [CrossRef]
- Ukrainskaya, V.; Rubtsov, Y.; Pershin, D.; Podoplelova, N.; Terekhov, S.; Yaroshevich, I.; Sokolova, A.; Bagrov, D.; Kulakovskaya, E.; Shipunova, V.; et al. Antigen-Specific Stimulation and Expansion of CAR-T Cells Using Membrane Vesicles as Target Cell Surrogates. Small 2021, 17, 2102643. [Google Scholar] [CrossRef]
- Humeniuk, P.; Geiselhart, S.; Battin, C.; Webb, T.; Steinberger, P.; Paster, W.; Hoffmann-Sommergruber, K. Generation of a Jurkat-based fluorescent reporter cell line to evaluate lipid antigen interaction with the human iNKT cell receptor. Sci. Rep. 2019, 9, 7426. [Google Scholar] [CrossRef]
- Hong, C.-H.; Sohn, H.-J.; Lee, H.-J.; Cho, H.-I.; Kim, T.-G. Antigen presentation by individually transferred HLA class I genes in HLA-A, HLA-B, HLA-C null human cell line generated using the multiplex CRISPR-Cas9 system. J. Immunother. 2017, 40, 201–210. [Google Scholar] [CrossRef]
- Pyo, H.-S.; Hong, C.-H.; Choi, H.; Baek, I.-C.; Kim, T.-G. Identification of Naturally Processed Epitope Region Using Artificial APC Expressing a Single HLA Class I Allotype and mRNA of HCMV pp65 Antigen Fragments. Vaccines 2022, 10, 787. [Google Scholar] [CrossRef]
- Hong, C.-H.; Pyo, H.-S.; Baek, I.-C.; Kim, T.-G. Rapid identification of CMV-specific TCRs via reverse TCR cloning based on bulk TCR repertoire data. Front. Immunol. 2022; submitted. [Google Scholar]
- Rogers, M.A.; Buffolo, F.; Schlotter, F.; Atkins, S.K.; Lee, L.H.; Halu, A.; Blaser, M.C.; Tsolaki, E.; Higashi, H.; Luther, K.; et al. Annexin A1–dependent tethering promotes extracellular vesicle aggregation revealed with single–extracellular vesicle analysis. Sci. Adv. 2020, 6, eabb1244. [Google Scholar] [CrossRef]
- Kletukhina, S.K.; Neustroeva, O.A.; Kurbangaleeva, S.V.; Salafutdinov, I.I.; Rogov, A.M.; James, V.; Rizvanov, A.A.; Gomzikova, M.O. Storage stability and delivery potential of cytochalasin B induced membrane vesicles. Biotechnol. Rep. 2021, 30, e00616. [Google Scholar] [CrossRef]
- Jutz, S.; Hennig, A.; Paster, W.; Asrak, Ö.; Dijanovic, D.; Kellner, F.; Pickl, W.F.; Huppa, J.B.; Leitner, J.; Steinberger, P. A cellular platform for the evaluation of immune checkpoint molecules. Oncotarget 2017, 8, 64892. [Google Scholar] [CrossRef]
- Hirano, N.; Butler, M.O.; Xia, Z.; Berezovskaya, A.; Murray, A.P.; Ansén, S.; Nadler, L.M. Efficient Presentation of Naturally Processed HLA Class I Peptides by Artificial Antigen-Presenting Cells for the Generation of Effective Antitumor Responses. Clin. Cancer Res. 2006, 12, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.M.; Dustin, M.L. What is the importance of the immunological synapse? Trends Immunol. 2004, 25, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Monks, C.R.; Freiberg, B.A.; Kupfer, H.; Sciaky, N.; Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998, 395, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Curado, S.; Kumari, S.; Dustin, M.L. Cell Biology Meets Physiology: Functional Organization of Vertebrate Plasma Membranes—The Immunological Synapse. In Current Topics in Membranes; Elsevier: Amsterdam, The Netherlands, 2013; Volume 72, pp. 313–346. [Google Scholar]
- Yang, X.; Annaert, W. The Nanoscopic Organization of Synapse Structures: A Common Basis for Cell Communication. Membranes 2021, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, J.; Mellody, M.P.; Hou, A.J.; Desai, R.P.; Fung, A.W.; Pham, A.H.T.; Chen, Y.Y.; Zhao, W. CAR-T design: Elements and their synergistic function. eBioMedicine 2020, 58, 102931. [Google Scholar] [CrossRef]
- Weinkove, R.; George, P.; Dasyam, N.; McLellan, A.D. Selecting costimulatory domains for chimeric antigen receptors: Functional and clinical considerations. Clin. Transl. Immunol. 2019, 8, e1049. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, S.; Hong, C.-H.; Hyun, Y.-S.; Baek, I.-C.; Kim, T.-G. Limited T-Cell-Stimulating Effect of Cytochalasin-B-Induced Membrane Vesicles Isolated from Artificial Antigen-Presenting Cells. Vaccines 2022, 10, 1877. https://doi.org/10.3390/vaccines10111877
Kim Y, Kim S, Hong C-H, Hyun Y-S, Baek I-C, Kim T-G. Limited T-Cell-Stimulating Effect of Cytochalasin-B-Induced Membrane Vesicles Isolated from Artificial Antigen-Presenting Cells. Vaccines. 2022; 10(11):1877. https://doi.org/10.3390/vaccines10111877
Chicago/Turabian StyleKim, Yeongwon, Sueon Kim, Cheol-Hwa Hong, You-Seok Hyun, In-Cheol Baek, and Tai-Gyu Kim. 2022. "Limited T-Cell-Stimulating Effect of Cytochalasin-B-Induced Membrane Vesicles Isolated from Artificial Antigen-Presenting Cells" Vaccines 10, no. 11: 1877. https://doi.org/10.3390/vaccines10111877
APA StyleKim, Y., Kim, S., Hong, C.-H., Hyun, Y.-S., Baek, I.-C., & Kim, T.-G. (2022). Limited T-Cell-Stimulating Effect of Cytochalasin-B-Induced Membrane Vesicles Isolated from Artificial Antigen-Presenting Cells. Vaccines, 10(11), 1877. https://doi.org/10.3390/vaccines10111877




