Determinants of Antibody Response to SARS-CoV-2 Vaccines in Liver Transplant Recipients: The Role of Immunosuppression Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Serological Testing
2.3. Immunosuppression Adjustment
2.4. Clinical Follow-Up
2.5. Safety
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Immunosuppression Regimens
3.3. Humoral Responses
3.4. Humoral Response by Type of Vaccination
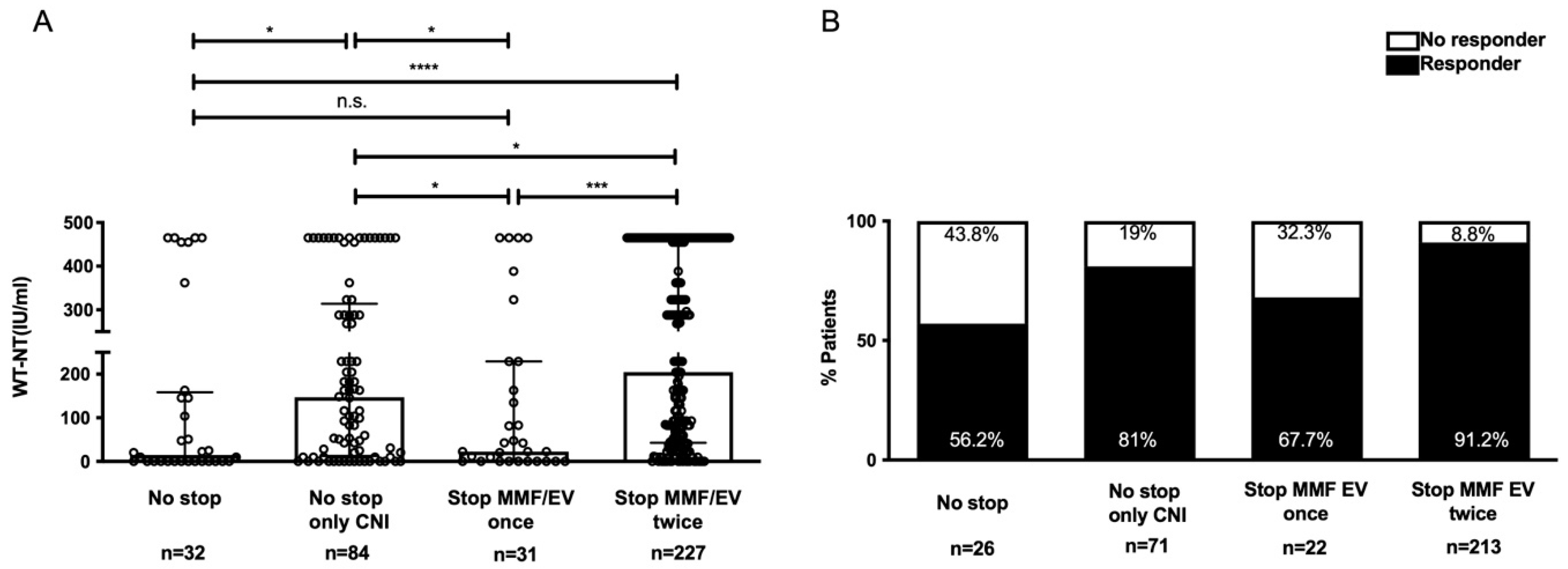
3.5. Effect of Immunosuppression Adjustment
3.6. ABOi Patients
3.7. Vaccination Safety
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Udomkarnjananun, S.; Kerr, S.J.; Townamchai, N.; Susantitaphong, P.; Tulvatana, W.; Praditpornsilpa, K.; Eiam-Ong, S.; Avihingsanon, Y. Mortality risk factors of COVID-19 infection in kidney transplantation recipients: A systematic review and meta-analysis of cohorts and clinical registries. Sci. Rep. 2021, 11, 20073. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.V.; Tevethia, H.V.; Premkumar, M.; Arab, J.P.; Candia, R.; Kumar, K.; Kumar, P.; Sharma, M.; Rao, P.N.; Reddy, D.N. Impact of COVID-19 on liver transplant recipients-A systematic review and meta-analysis. eClinicalMedicine 2021, 38, 101025. [Google Scholar] [CrossRef] [PubMed]
- Fix, O.K.; Blumberg, E.A.; Chang, K.M.; Chu, J.; Chung, R.T.; Goacher, E.K.; Hameed, B.; Kaul, D.R.; Kulik, L.M.; Kwok, R.M.; et al. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology 2021, 74, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J. Hepatol. 2021, 75, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Ruether, D.F.; Schaub, G.M.; Duengelhoef, P.M.; Haag, F.; Brehm, T.T.; Fathi, A.; Wehmeyer, M.; Jahnke-Triankowski, J.; Mayer, L.; Hoffmann, A.; et al. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin. Gastroenterol. Hepatol. 2022, 20, 162–172.e169. [Google Scholar] [CrossRef] [PubMed]
- Rashidi-Alavijeh, J.; Frey, A.; Passenberg, M.; Korth, J.; Zmudzinski, J.; Anastasiou, O.E.; Saner, F.H.; Jahn, M.; Lange, C.M.; Willuweit, K. Humoral Response to SARS-Cov-2 Vaccination in Liver Transplant Recipients-A Single-Center Experience. Vaccines 2021, 9, 738. [Google Scholar] [CrossRef]
- Curtis, J.R.; Johnson, S.R.; Anthony, D.D.; Arasaratnam, R.J.; Baden, L.R.; Bass, A.R.; Calabrese, C.; Gravallese, E.M.; Harpaz, R.; Kroger, A.; et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients With Rheumatic and Musculoskeletal Diseases: Version 4. Arthritis. Rheumatol. 2022, 74, e21–e36. [Google Scholar] [CrossRef]
- Meunier, L.; Sanavio, M.; Dumortier, J.; Meszaros, M.; Faure, S.; Ursic Bedoya, J.; Echenne, M.; Boillot, O.; Debourdeau, A.; Pageaux, G.P. Mycophenolate mofetil decreases humoral responses to three doses of SARS-CoV-2 vaccine in liver transplant recipients. Liver. Int. 2022, 42, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Thomson, T.; Prendecki, M.; Gleeson, S.; Martin, P.; Spensley, K.; De Aguiar, R.C.; Sandhu, B.; Seneschall, C.; Gan, J.; Clarke, C.L.; et al. Immune responses following 3rd and 4th doses of heterologous and homologous COVID-19 vaccines in kidney transplant recipients. eClinicalMedicine 2022, 53, 101642. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Jens, A.; Stefanski, A.L.; Hammett, C.; Osmanodja, B.; Koch, N.; Zukunft, B.; Beck, J.; Oellerich, M.; et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 2022, 7, e157836. [Google Scholar] [CrossRef]
- Regele, F.; Heinzel, A.; Hu, K.; Raab, L.; Eskandary, F.; Fae, I.; Zelzer, S.; Bohmig, G.A.; Bond, G.; Fischer, G.; et al. Stopping of Mycophenolic Acid in Kidney Transplant Recipients for 2 Weeks Peri-Vaccination Does Not Increase Response to SARS-CoV-2 Vaccination-A Non-randomized, Controlled Pilot Study. Front. Med. 2022, 9, 914424. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.G.; Lee, K.M.; Hsiao, M.J.; Yang, S.L.; Huang, P.N.; Gong, Y.N.; Hsieh, T.H.; Huang, P.W.; Lin, Y.J.; Liu, Y.C.; et al. Culture-Based Virus Isolation To Evaluate Potential Infectivity of Clinical Specimens Tested for COVID-19. J. Clin. Microbiol. 2020, 58, e01068-20. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.Y.; Lin, M.Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.J.; Liu, L.T.; Cheng, J.; Wu, Y.C.; Wu, C.C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Cheng, C.H.; Wang, Y.C.; Soong, R.S.; Wu, T.H.; Chou, H.S.; Wu, T.J.; Chan, K.M.; Lee, C.S.; Lee, W.C. Adult Living Donor Liver Transplantation Across ABO-Incompatibility. Medicine 2015, 94, e1796. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Lee, C.F.; Wang, Y.C.; Wu, T.H.; Wu, T.J.; Chou, H.S.; Chan, K.M.; Lee, W.C. ABO-Incompatible Liver Transplantation: State of Art and Future Perspectives. Curr. Pharm. Des. 2020, 26, 3406–3417. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Cheng, C.H.; Lee, C.F.; Hung, H.C.; Lee, J.C.; Wu, T.H.; Wang, Y.C.; Wu, T.J.; Chou, H.S.; Chan, K.M. Quick preparation of ABO-incompatible living donor liver transplantation for acute liver failure. Clin. Transplant. 2022, 36, e14555. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.; Starr, N.; Chan, G.; Dempsey, E.; Heffernan, E.; Newman, E.; O’Neill, J.; Hannan, M.M.; Lynch, B.; Joyce, E. Humoral response to SARS-CoV-2 adenovirus vector vaccination (ChAdOx1 nCoV-19 [AZD1222]) in heart transplant recipients aged 18 to 70 years of age. J. Heart Lung Transplant. 2022, 41, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3990–4002. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.H.; Jin, P.B.; Chen, D.Y.; Chen, Z.Y.; Li, Z.W.; Wu, J.; Lou, B.; Zhang, B.S.; Zhang, L.; Zhang, W.; et al. Evaluating the Response and Safety of Inactivated COVID-19 Vaccines in Liver Transplant Recipients. Infect. Drug Resist. 2022, 15, 2469–2474. [Google Scholar] [CrossRef]
- Herrera, S.; Colmenero, J.; Pascal, M.; Escobedo, M.; Castel, M.A.; Sole-Gonzalez, E.; Palou, E.; Egri, N.; Ruiz, P.; Mosquera, M.; et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am. J. Transplant. 2021, 21, 3971–3979. [Google Scholar] [CrossRef]
- Lai, Y.J.; Chang, H.S.; Yang, Y.P.; Lin, T.W.; Lai, W.Y.; Lin, Y.Y.; Chang, C.C. The role of micronutrient and immunomodulation effect in the vaccine era of COVID-19. J. Chin. Med. Assoc. 2021, 84, 821–826. [Google Scholar] [CrossRef]
- Murdaca, G.; Pioggia, G.; Negrini, S. Vitamin D and COVID-19: An update on evidence and potential therapeutic implications. Clin. Mol. Allergy 2020, 18, 23. [Google Scholar] [CrossRef]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef]
- Eickenberg, S.; Mickholz, E.; Jung, E.; Nofer, J.R.; Pavenstadt, H.J.; Jacobi, A.M. Mycophenolic acid counteracts B cell proliferation and plasmablast formation in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14, R110. [Google Scholar] [CrossRef] [PubMed]
- Struijk, G.H.; Minnee, R.C.; Koch, S.D.; Zwinderman, A.H.; van Donselaar-van der Pant, K.A.; Idu, M.M.; ten Berge, I.J.; Bemelman, F.J. Maintenance immunosuppressive therapy with everolimus preserves humoral immune responses. Kidney Int. 2010, 78, 934–940. [Google Scholar] [CrossRef]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021, 21, 2727–2739. [Google Scholar] [CrossRef]
- Rozen-Zvi, B.; Yahav, D.; Agur, T.; Zingerman, B.; Ben-Zvi, H.; Atamna, A.; Tau, N.; Mashraki, T.; Nesher, E.; Rahamimov, R. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin. Microbiol. Infect. 2021, 27, 1173.e1–1173.e4. [Google Scholar] [CrossRef] [PubMed]
- Impact of Immunosuppression Adjustment on COVID-19 Vaccination Response in Kidney Transplant Recipients. Available online: https://ClinicalTrials.gov/show/NCT05060991 (accessed on 6 October 2022).
- Pilot Trial on Immunosuppression Modulation to Increase SARS-CoV-2 Vaccine Response in Kidney Transplant Recipients. Available online: https://ClinicalTrials.gov/show/NCT05338177 (accessed on 6 October 2022).
- McLaughlin, P.; Grillo-Lopez, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Cholankeril, G.; Al-Hillan, A.; Tarlow, B.; Abrams, D.; Jacobs, J.S.; Flores, N.P.; Rana, A.; Kanwal, F.; Goss, J.A. Clinical Factors Associated With Lack of Serological Response to SARS-CoV-2 Messenger RNA Vaccine in Liver Transplantation Recipients. Liver Transpl. 2022, 28, 123–126. [Google Scholar] [CrossRef] [PubMed]



| Parameters | All Recipients (n = 374) | Responders (n = 314) | Nonresponders (n = 60) | p-Value |
|---|---|---|---|---|
| Age (years) | 62 (55–67.2) | 62 (55–67) | 63 (52.2–68) | 0.993 |
| Gender | 0.310 | |||
| Male | 287 (76.7) | 244 (77.7) | 43 (71.7) | |
| Female | 87 (23.3) | 70 (22.3) | 17 (28.3) | |
| Etiology | ||||
| HCC | 153 (40.9) | 132 (42) | 21 (35) | 0.310 |
| Viral hepatitis | 221 (59.1) | 190 (60.5) | 31 (51.7) | 0.335 |
| HBV | 58 (15.5) | 45 (14.3) | 13 (21.7) | 0.202 |
| HCV | 13 (3.5) | 12 (3.8) | 1 (1.7) | 0.150 |
| HBV + HCV | 73 (19.5) | 55 (17.5) | 18 (30) | 0.404 |
| Alcohol | 18 (4.8) | 16 (5.1) | 2 (3.3) | 0.025 |
| Autoimmune | 0.749 | |||
| Type of LT | 0.461 | |||
| DDLT | 86 (23) | 70 (22.3) | 16 (26.7) | |
| LDLT | 288 (77) | 244 (77.7) | 44 (73.3) | |
| Time from LT to vaccination (months) | 73 (39–112.5) | 72.5 (40.7–119.2) | 75.5(38–91.7) | 0.334 |
| Interval between Vaccinations (days) | 84 (44–97) | 84 (44–97.2) | 84 (43–95.5) | 0.455 |
| ABOi | 13 (3.5) | 7 (2.2) | 6 (10) | 0.009 |
| EGFR (mL/min/1.73 m2) | 35.6 (20–66.2) | 35.2 (20.3–66.4) | 39 (16.2–63.7) | 0.631 |
| Total bilirubin (mg/dL) | 0.7 (0.5–1.1) | 0.7 (0.5–1) | 0.7 (0.5–1.2) | 0.540 |
| AST (U/L) | 20 (16–27) | 20 (16–26.2) | 23 (17–37.7) | 0.050 |
| ALT (U/L) | 21 (14–35) | 20 (14–33) | 22.5 (14–49) | 0.164 |
| WBC (/uL) | 5700 (4600–6900) | 5900 (4700–7000) | 5100 (3800–6475) | 0.004 |
| Lymphocyte (%) | 28.1 (22.3–34.5) | 28.5 (22.8–34.7) | 25.4 (17.5–32.3) | 0.030 |
| Tacrolimus trough level (ng/mL) | 4.7 (3.5–6.4) | 4.6 (3.5–6.4) | 5.5 (3.2–8.8) | 0.208 |
| Age > 65 (years) | 135 (36.1) | 109 (34.7) | 26 (43.3) | 0.203 |
| Time from LT to vaccination < 12 (months) | 20 (5.3) | 17 (5.4) | 3 (5) | 1.000 |
| History of cancer | 162 (43.3) | 138 (44.1)) | 24 (40) | 0.559 |
| WBC < 4000 (/uL) | 55 (14.7) | 38 (12.1) | 17 (28.3) | 0.001 |
| Lymphocytes < 20 (%) | 68 (18.2) | 49 (15.6) | 19 (31.7) | 0.003 |
| NLR < 2.25 | 190 (50.8) | 167 (53.2) | 23 (38.3) | 0.035 |
| EGFR <30 (mL/min/1.73 m2) | 167 (44.7) | 140 (44.6) | 27 (45) | 0.953 |
| Hemodialysis | 26 (7) | 18 (5.7) | 8 (13.3) | 0.049 |
| Tacrolimus trough level >6.5 (ng/mL) | 87 (23.3) | 66 (21) | 39 (65) | 0.019 |
| No immunosuppressants stopped (CNI + MMF/EV) | 32 (8.6) | 18 (5.7) | 14 (23.3) | 0.000 |
| No immunosuppressant stopped (CNI only) | 84 (22.5) | 68 (21.7) | 16 (26.7) | 0.394 |
| Stopped once (MMF/EV) | 31 (8.3) | 21 (6.7) | 10 (16.7) | 0.018 |
| Stopped twice (MMF/EV) | 227 (60.7) | 207 (65.9) | 20 (33.3) | 0.000 |
| Triple immunotherapy | 6 (1.6) | 3 (1) | 3 (5) | 0.055 |
| Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age (years) >65 vs. ≤ 65 | 0.695 | 0.397–1.218 | 0.204 | |||
| Type of LT DDLT vs. LDLT | 1.268 | 0.674–2.382 | 1.268 | |||
| Alcohol Yes vs. No | 2.018 | 1.081–3.767 | 0.027 | |||
| ABOi Yes vs. No | 5.704 | 1.774–18.340 | 0.003 | 6.053 | 1.533–23.904 | 0.010 |
| WBC count (uL) <4000 vs. ≥4000 | 2.871 | 1.490–5.533 | 0.002 | 2.841 | 1.350–5.979 | 0.006 |
| Lymphocyte count (%) <20 vs. ≥20 | 2.506 | 1.343–4.675 | 0.004 | 2.648 | 1.162–6.038 | 0.021 |
| NLR <2.25 vs. ≥2.25 | 1.828 | 1.038–3.215 | 0.037 | |||
| Hemodialysis Yes vs. No | 2.530 | 1.046–6.120 | 0.039 | |||
| Tacrolimus trough level >6.5 (ng/mL) Yes vs. No | 2.023 | 1.115–3.672 | 0.020 | 2.182 | 1.056–4.509 | 0.035 |
| No immunosuppressants stopped (CNI + MMF/EV) Yes vs. No | 5.005 | 2.330–10.749 | 0.000 | 5.026 | 1.837–13.754 | 0.002 |
| No immunosuppressant stopped (CNI only) Yes vs. No | 1.316 | 0.699–2.475 | 0.395 | |||
| Stopped once (MMF/EV) Yes vs. No | 2.790 | 1.241–6.276 | 0.013 | |||
| Stopped twice (MMF/EV) Yes vs. No | 0.258 | 0.144–0.464 | 0.000 | |||
| Triple immunotherapy Yes vs. No | 5.456 | 1.074–27.709 | 0.041 | |||
| Parameters | MRNA (n = 332) | AZ (n = 22) | KT (n = 20) | p-Value |
|---|---|---|---|---|
| Age (years) | 63 (56–68) | 58.5 (53.2–68.2) | 52.5 (49.2–61.7) | 0.003 |
| Gender | 0.082 | |||
| Male | 249 (75) | 20 (90.9) | 18 (90) | |
| Female | 83 (25) | 2 (9.1) | 2 (10) | |
| Etiology | ||||
| HCC | 140 (42.2) | 6 (27.3) | 7 (35) | 0.333 |
| Viral hepatitis | 199 (59.9) | 10 (45.5) | 12 (60) | 0.407 |
| HBV | 52 (15.7) | 4 (18.2) | 2 (10) | 0.745 |
| HCV | 13 (3.5) | 12 (3.8) | 1 (1.7) | 1.000 |
| HBV + HCV | 52 (15.7) | 13 (59.1) | 8 (40) | 0.000 |
| Alcohol | 18 (5.4) | 0 | 0 | 0.584 |
| Autoimmune | ||||
| Type of LT | 0.277 | |||
| DDLT | 79 (23.8) | 2 (9.1) | 5 (25) | |
| LDLT | 253 (76.2) | 20 (90.9) | 15 (75) | |
| Time from LT to vaccination (months) | 71 (38.2–110.2) | 78.5 (58.2–108) | 97 (53.5–137.5) | 0.206 |
| Interval between Vaccinations (days) | 85.5 (46–98) | 88 (79.7–96.2) | 38.5 (35–40) | 0.000 |
| ABOi | 8 (2.4) | 4 (18.2) | 1 (5) | 0.008 |
| EGFR (mL/min/1.73 m2) | 34.5 (20.1–64.8) | 30.6 (5–88.9) | 66.5 (29.4–78.7) | 0.040 |
| Total bilirubin (mg/dL) | 0.7 (0.5–1) | 0.7 (0.4–1.3) | 0.9 (0.5–1.3) | 0.513 |
| AST (U/L) | 20 (16–27) | 19.5 (16.7–36) | 21.5 (16.2–26) | 0.963 |
| ALT (U/L) | 21 (14–34) | 21 (11.7–45) | 24 (13–43.7) | 0.842 |
| WBC (/uL) | 5750 (4600–6900) | 5450 (3750–6800) | 5750 (4900–6675) | 0.614 |
| Lymphocyte (%) | 28.2 (22.6–34.5) | 25.8 (14.4–29.4) | 29.9 (19.3–33.6) | 0.235 |
| Tacrolimus trough level (ng/mL) | 4.7 (3.6–6.4) | 3.6 (2.3–5.2) | 5 (2.6–6.7) | 0.081 |
| Age >65 (years) | 128 (38.6) | 6 (27.3) | 1 (5) | 0.007 |
| Time from LT to vaccination <12 (months) | 19 (5.7) | 1 (4.5) | 0 | 0.853 |
| History of cancer | 145 (43.8) | 10 (45.5)) | 7 (35) | 0.728 |
| WBC <4000 (/uL) | 46 (13.9) | 7 (31.8) | 2 (10) | 0.082 |
| Lymphocytes <20 (%) | 56 (16.9) | 7 (31.8) | 5 (25) | 0.113 |
| NLR <2.25 | 169 (50.9) | 10 (45.5) | 11 (55) | 0.821 |
| EGFR <30 (mL/min/1.73 m2) | 151 (45.5) | 11 (50) | 5 (25) | 0.176 |
| Hemodialysis | 20 (6) | 4 (18.2) | 2 (10) | 0.054 |
| Tacrolimus trough level >6.5 (ng/mL) | 77 (23.2) | 4 (18.2) | 6 (30) | 0.661 |
| No immunosuppressants stopped (CNI + MMF/EV) | 26 (7.8) | 5 (22.7) | 1 (5) | 0.059 |
| No immunosuppressant stopped (CNI only) | 71 (21.4) | 9 (40.9) | 4 (20) | 0.109 |
| Stopped once (MMF/EV) | 22 (6.6) | 7 (31.8) | 2 (10) | 0.002 |
| Stopped twice (MMF/EV) | 213 (64.2) | 1 (4.5) | 13 (65) | 0.000 |
| Triple immunotherapy | 6 (1.8) | - | - | 1.000 |
| Parameters | ABOi (n = 13) | ABOc (n = 361) | p-Value |
|---|---|---|---|
| Age (years) | 56 (48–65) | 63 (55–68) | 0.096 |
| Gender | 1.000 | ||
| Male | 10 (76.9) | 277 (74.1) | |
| Female | 3 (23.1) | 84 (23.3) | |
| Time from LT to vaccination (months) | 40 (24–81.5) | 73 (41–117.5) | 0.067 |
| Interval between Vaccinations (days) | 45 (37.5–90.5) | 85 (45–97) | 0.111 |
| Etiology | |||
| HCC | 4 (30.8) | 149 (41.3) | 0.449 |
| Viral hepatitis | 9 (69.2) | 212 (58.7) | 0.449 |
| HBV | 1 (7.7) | 57 (15.8) | 0.701 |
| HCV | 0 (0) | 13 (3.6) | 1.000 |
| HBV + HCV | 5 (38.5) | 68 (18.8) | 0.144 |
| Alcohol | 1 (5.6) | 17 (4.7) | 0.479 |
| Autoimmune | |||
| EGFR (mL/min/1.73 m2) | 21 (11.6–53.9) | 37.1 (20.1–66.3) | 0.111 |
| Total bilirubin (mg/dL) | 1 (0.7–1.6) | 0.7 (0.5–1) | 0.027 |
| AST (U/L) | 23 (17.5–55) | 20 (16–27) | 0.135 |
| ALT (U/L) | 29 (14–63) | 21 (14–34) | 0.274 |
| WBC (/uL) | 5500 (3650–6700) | 5700 (4650–6900) | 0.219 |
| Lymphocyte (%) | 32 (27.7–37) | 27.8 (21.9–34.5) | 0.045 |
| Tacrolimus trough level (ng/mL) | 6.8 (4.3–8.5) | 4.7 (3.5–6.2) | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-H.; Hung, H.-C.; Lee, J.-C.; Huang, P.-W.; Gu, P.-W.; Lai, Y.; Wang, Y.-C.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; et al. Determinants of Antibody Response to SARS-CoV-2 Vaccines in Liver Transplant Recipients: The Role of Immunosuppression Reduction. Vaccines 2022, 10, 1827. https://doi.org/10.3390/vaccines10111827
Cheng C-H, Hung H-C, Lee J-C, Huang P-W, Gu P-W, Lai Y, Wang Y-C, Wu T-H, Lee C-F, Wu T-J, et al. Determinants of Antibody Response to SARS-CoV-2 Vaccines in Liver Transplant Recipients: The Role of Immunosuppression Reduction. Vaccines. 2022; 10(11):1827. https://doi.org/10.3390/vaccines10111827
Chicago/Turabian StyleCheng, Chih-Hsien, Hao-Chien Hung, Jin-Chiao Lee, Po-Wei Huang, Po-Wen Gu, Yin Lai, Yu-Chao Wang, Tsung-Han Wu, Chen-Fang Lee, Ting-Jung Wu, and et al. 2022. "Determinants of Antibody Response to SARS-CoV-2 Vaccines in Liver Transplant Recipients: The Role of Immunosuppression Reduction" Vaccines 10, no. 11: 1827. https://doi.org/10.3390/vaccines10111827
APA StyleCheng, C.-H., Hung, H.-C., Lee, J.-C., Huang, P.-W., Gu, P.-W., Lai, Y., Wang, Y.-C., Wu, T.-H., Lee, C.-F., Wu, T.-J., Chou, H.-S., Chan, K.-M., Huang, C.-G., & Lee, W.-C. (2022). Determinants of Antibody Response to SARS-CoV-2 Vaccines in Liver Transplant Recipients: The Role of Immunosuppression Reduction. Vaccines, 10(11), 1827. https://doi.org/10.3390/vaccines10111827





