Real-Time Monitoring of the Effectiveness of Six COVID-19 Vaccines against Laboratory-Confirmed COVID-19 in Hungary in 2021 Using the Screening Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Data Sources
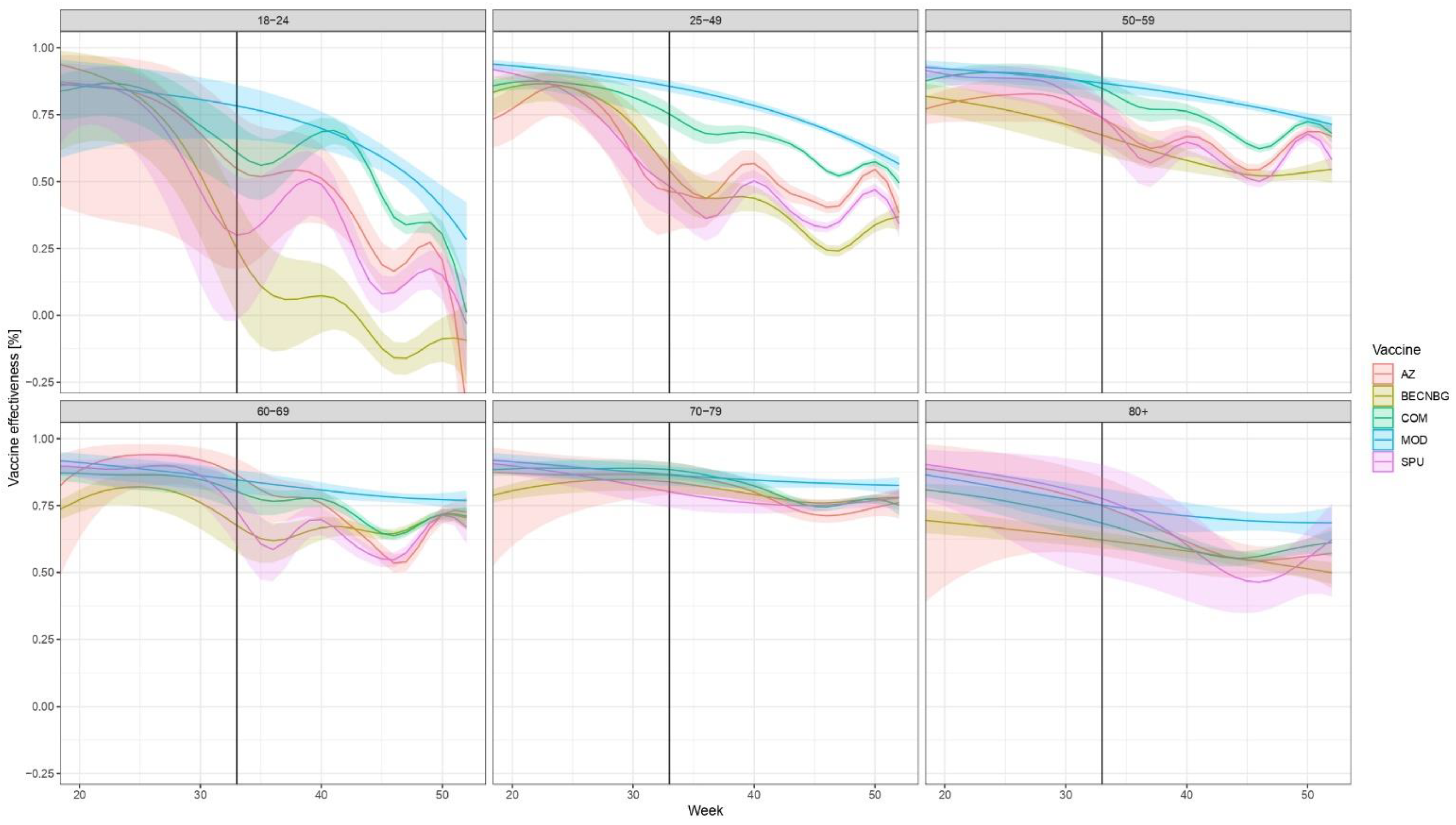
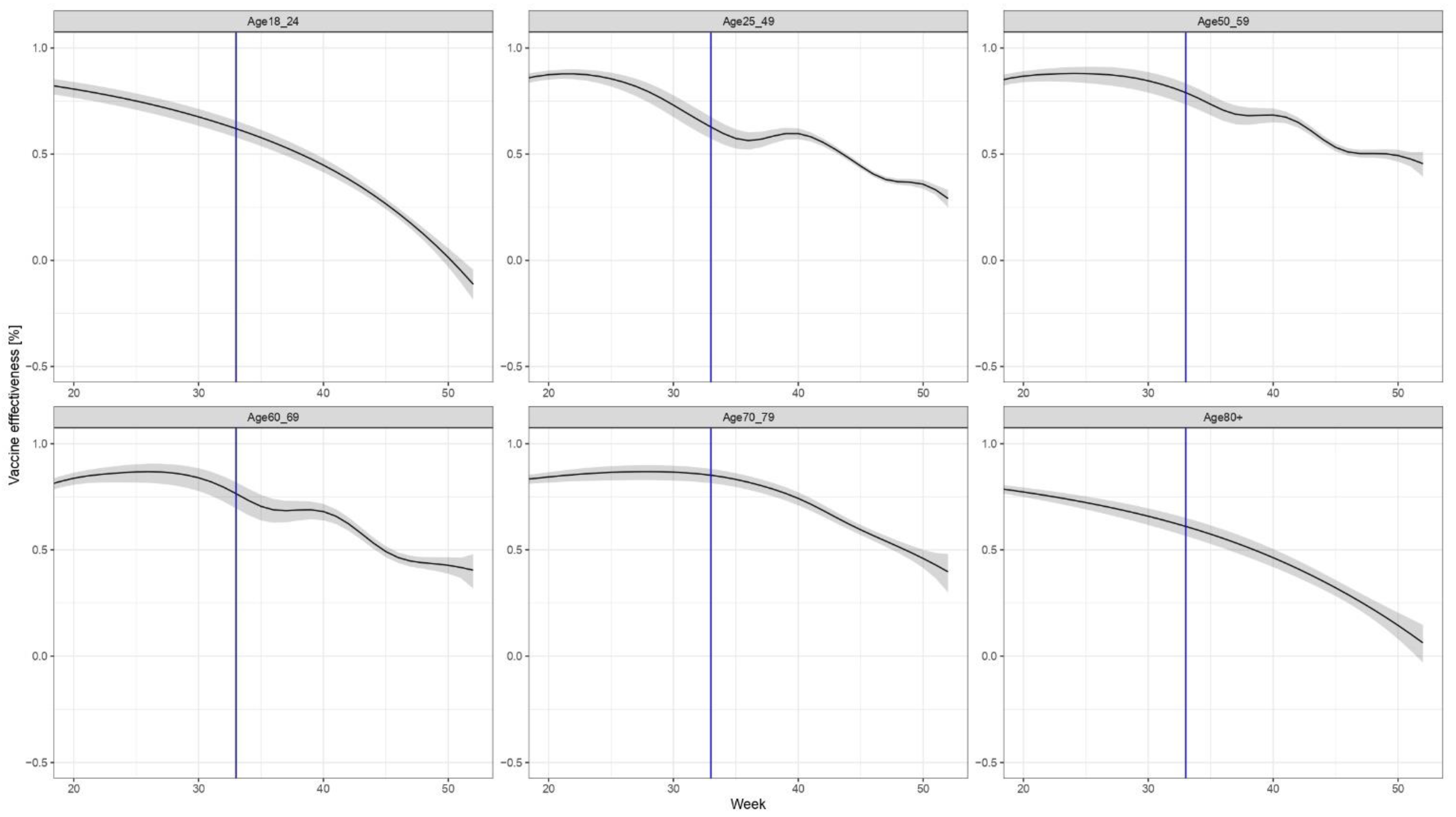
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Guidance on Conducting Vaccine Effectiveness Evaluations in the Setting of New SARS-CoV-2 Variants: Interim Guidance, 22 July 2021. Addendum to Evaluation of COVID-19 Vaccine Effectiveness. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-vaccine_effectiveness-variants-2021.1 (accessed on 22 August 2021).
- University of Oxford Our World in Data Database. Available online: https://ourworldindata.org/explorers/coronavirus-data-explorer (accessed on 22 August 2022).
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Israel, A.; Merzon, E.; Schäffer, A.A.; Shenhar, Y.; Green, I.; Golan-Cohen, A.; Ruppin, E.; Magen, E. Vinker, S. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection: Test negative design study. BMJ 2021, 375, e067873. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, D.J.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Kissling, E.; Hooiveld, M.; Martín, V.S.; Martínez-Baz, I.; William, N.; Vilcu, A.-M.; Mazagatos, C.; Domegan, L.; de Lusignan, S.; Meijer, A.; et al. Vaccine effectiveness against symptomatic SARS-CoV-2 infection in adults aged 65 years and older in primary care: I-MOVE-COVID-19 project, Europe, December 2020 to May 2021. Eurosurveillance 2021, 26, 2100670. [Google Scholar] [CrossRef]
- Suah, J.L.; Tok, P.S.K.; Ong, S.M.; Husin, M.; Tng, B.H.; Sivasampu, S.; Thevananthan, T.; Appannan, M.R.; Zin, F.M.; Zin, S.M.; et al. PICK-ing Malaysias Epidemic Apart: Effectiveness of a Diverse COVID-19 Vaccine Portfolio. Vaccines 2021, 9, 1381. [Google Scholar] [CrossRef]
- The World Bank. Hungary Country Overview. Available online: https://data.worldbank.org/country/HU (accessed on 22 August 2021).
- OECD/European Observatory on Health Systems and Policies. Hungary: Country Health Profile 2021; OECD Publishing: Paris, France, 2021. [Google Scholar]
- European Centre for Disease Prevention and Control. Data on COVID-19 Vaccination in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/data-covid-19-vaccination-eu-eea (accessed on 22 December 2021).
- Farrington, C.P. Estimation of Vaccine Effectiveness Using the Screening Method. Int. J. Epidemiol. 1993, 22, 742–746. [Google Scholar] [CrossRef]
- Orenstein, W.A.; Bernier, R.H.; Dondero, T.J.; Hinman, A.R.; Marks, J.S.; Bart, K.J.; Sirotkin, B. Field evaluation of vaccine efficacy. Bull. World Health Organ. 1985, 63, 1055–1068. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Measles Vaccine Efficacy—United States. Morb. Mortal. Wkly. Rep. 1980, 29, 470–472. [Google Scholar]
- Wood, S.N. Thin plate regression splines: Thin Plate Regression Splines. J. R. Stat. Soc. Ser. B 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Orenstein, E.W.; De Serres, G.; Haber, M.J.; Shay, D.K.; Bridges, C.B.; Gargiullo, P.; Orenstein, W.A. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int. J. Epidemiol. 2007, 36, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.A.; Fine, P.E.M. An assessment of methods for routine local monitoring of vaccine efficacy, with particular reference to measles and pertussis. Epidemiol. Infect. 1987, 99, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.; Vasileiou, E.; Robertson, C.; Sheikh, A. COVID-19 vaccine effectiveness against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes from Delta AY.4.2: Cohort and test-negative study of 5.4 million individuals in Scotland. J. Glob. Health 2022, 12, 05025. [Google Scholar] [CrossRef] [PubMed]
- Lytras, T.; Kontopidou, F.; Lambrou, A.; Tsiodras, S. Comparative effectiveness and durability of COVID-19 vaccination against death and severe disease in an ongoing nationwide mass vaccination campaign. J. Med. Virol. 2022, 94, 5044–5050. [Google Scholar] [CrossRef]
- Ghosh, S.; Shankar, S.; Chatterjee, K.; Chatterjee, K.; Yadav, A.K.; Pandya, K.; Suryam, V.; Agrawal, S.; Ray, S.; Phutane, V.; et al. COVISHIELD (AZD1222) VaccINe effectiveness among healthcare and frontline Workers of INdian Armed Forces: Interim results of VIN-WIN cohort study. Med. J. Armed. Forces India 2021, 77, S264–S270. [Google Scholar] [CrossRef]
- Vokó, Z.; Kiss, Z.; Surján, G.; Surján, O.; Barcza, Z.; Pályi, B.; Formanek-Balku, E.; Molnár, G.A.; Herczeg, R.; Gyenesei, A.; et al. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary—The HUN-VE study. Clin. Microbiol. Infect. 2022, 28, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Kiss, Z.; Wittmann, I.; Polivka, L.; Surján, G.; Surján, O.; Barcza, Z.; Molnár, G.A.; Nagy, D.; Müller, V.; Bogos, K.; et al. Nationwide Effectiveness of First and Second SARS-CoV2 Booster Vaccines During the Delta and Omicron Pandemic Waves in Hungary (HUN-VE 2 Study). Front. Immunol. 2022, 13, 905585. [Google Scholar] [CrossRef]
- Vokó, Z.; Kiss, Z.; Surján, G.; Surján, O.; Barcza, Z.; Wittmann, I.; Molnár, G.A.; Nagy, D.; Müller, V.; Bogos, K.; et al. Effectiveness and Waning of Protection With Different SARS-CoV-2 Primary and Booster Vaccines During the Delta Pandemic Wave in 2021 in Hungary (HUN-VE 3 Study). Front. Immunol. 2022, 13, 919408. [Google Scholar] [CrossRef]
- Müller, V.; Polivka, L.; Valyi-Nagy, I.; Nagy, A.; Szekanecz, Z.; Bogos, K.; Vago, H.; Kamondi, A.; Fekete, F.; Szlavik, J.; et al. Booster Vaccination Decreases 28-Day All-Cause Mortality of the Elderly Hospitalized Due to SARS-CoV-2 Delta Variant. Vaccines 2022, 10, 986. [Google Scholar] [CrossRef]
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults without Immunocompromising Conditions-United States, March–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1337–1343. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.A.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Sy, L.S.; Qian, L.; Ackerson, B.K.; Luo, Y.; Lee, G.S.; Tian, Y.; Florea, A.; Aragones, M.; Tubert, J.E.; et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: Test negative case-control study. BMJ 2021, 375, e068848. [Google Scholar] [CrossRef]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to Covid-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hermán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, B.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, J.K.; Ferenci, T.; Ferenczi, A.; Túri, G.; Röst, G.; Oroszi, B. Real-Time Monitoring of the Effectiveness of Six COVID-19 Vaccines against Laboratory-Confirmed COVID-19 in Hungary in 2021 Using the Screening Method. Vaccines 2022, 10, 1824. https://doi.org/10.3390/vaccines10111824
Horváth JK, Ferenci T, Ferenczi A, Túri G, Röst G, Oroszi B. Real-Time Monitoring of the Effectiveness of Six COVID-19 Vaccines against Laboratory-Confirmed COVID-19 in Hungary in 2021 Using the Screening Method. Vaccines. 2022; 10(11):1824. https://doi.org/10.3390/vaccines10111824
Chicago/Turabian StyleHorváth, Judit K., Tamás Ferenci, Annamária Ferenczi, Gergő Túri, Gergely Röst, and Beatrix Oroszi. 2022. "Real-Time Monitoring of the Effectiveness of Six COVID-19 Vaccines against Laboratory-Confirmed COVID-19 in Hungary in 2021 Using the Screening Method" Vaccines 10, no. 11: 1824. https://doi.org/10.3390/vaccines10111824
APA StyleHorváth, J. K., Ferenci, T., Ferenczi, A., Túri, G., Röst, G., & Oroszi, B. (2022). Real-Time Monitoring of the Effectiveness of Six COVID-19 Vaccines against Laboratory-Confirmed COVID-19 in Hungary in 2021 Using the Screening Method. Vaccines, 10(11), 1824. https://doi.org/10.3390/vaccines10111824






