Differences in Demographics of Vaccinees, Access to, and Satisfaction with SARS-CoV-2 Vaccination Procedures between German General Practices and Mass Vaccination Centers
Abstract
1. Introduction
2. Methods
2.1. Sampling and Design
2.2. Questionnaire
2.3. Ethics Statement
2.4. Statistical Analyses
3. Results
3.1. Sample Characteristics
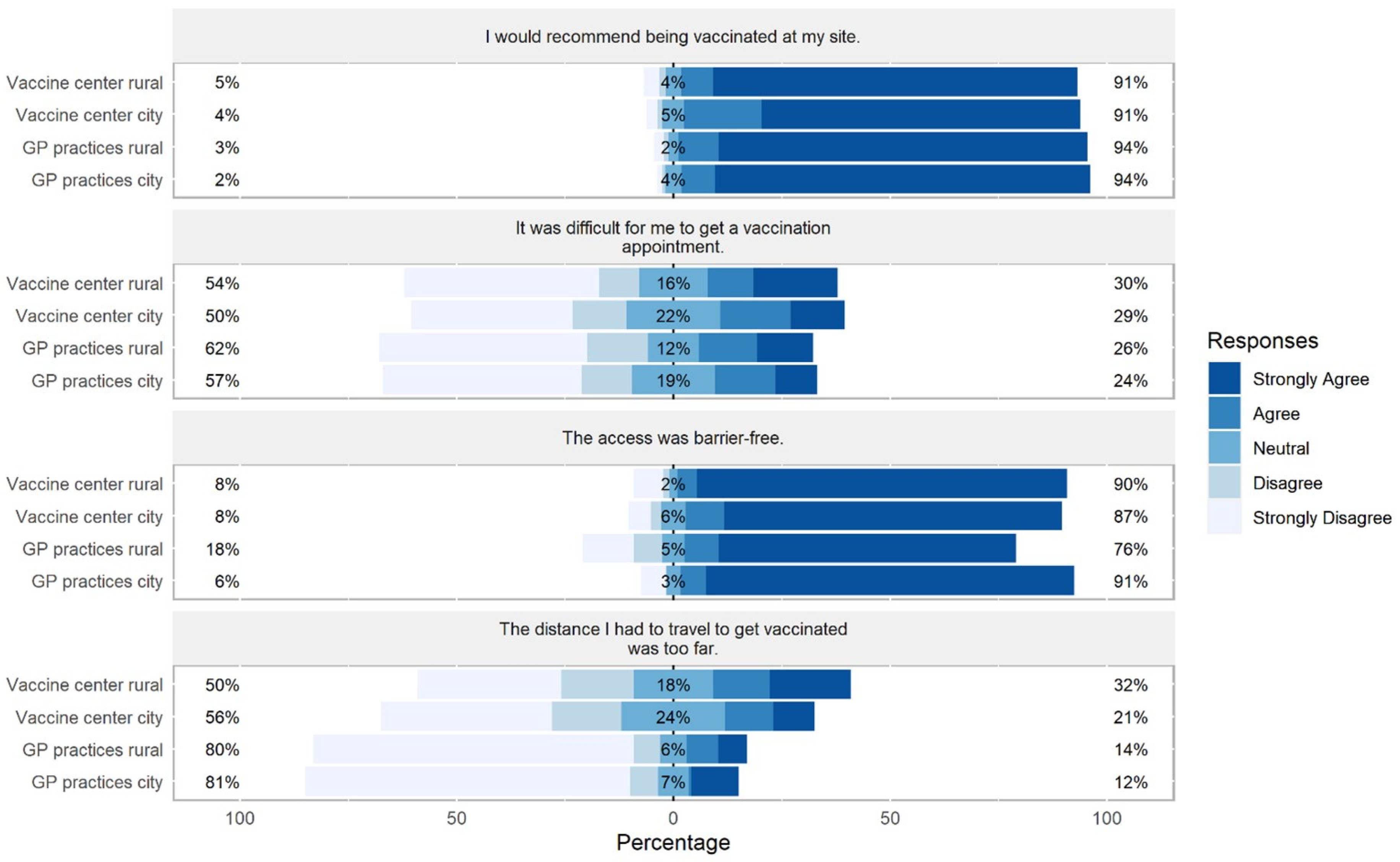
3.2. Differences between Sites in Preference, Administering of, and Access to Vaccines
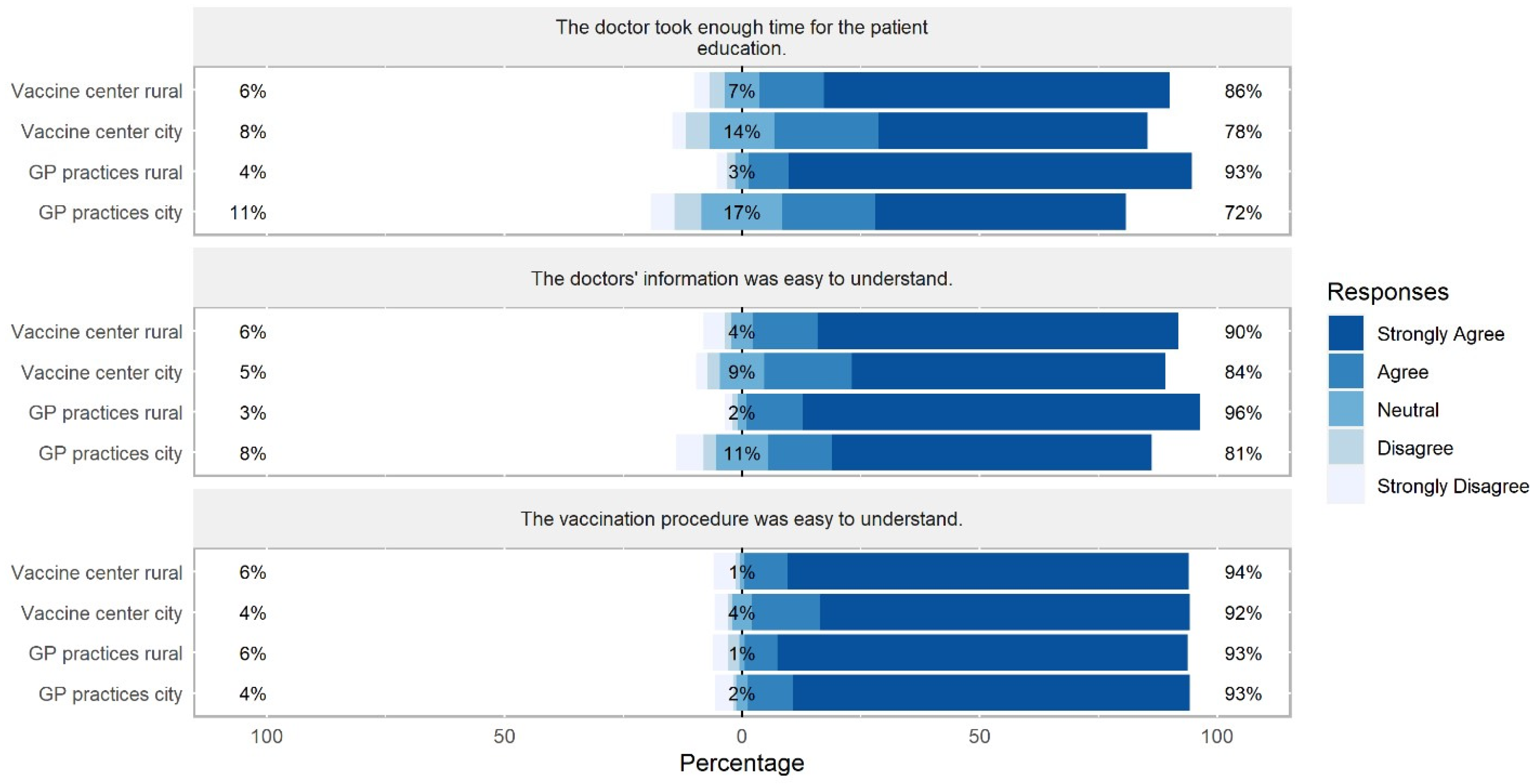
3.3. Differences between Sites in Physicians’ Provision of Vaccination Information
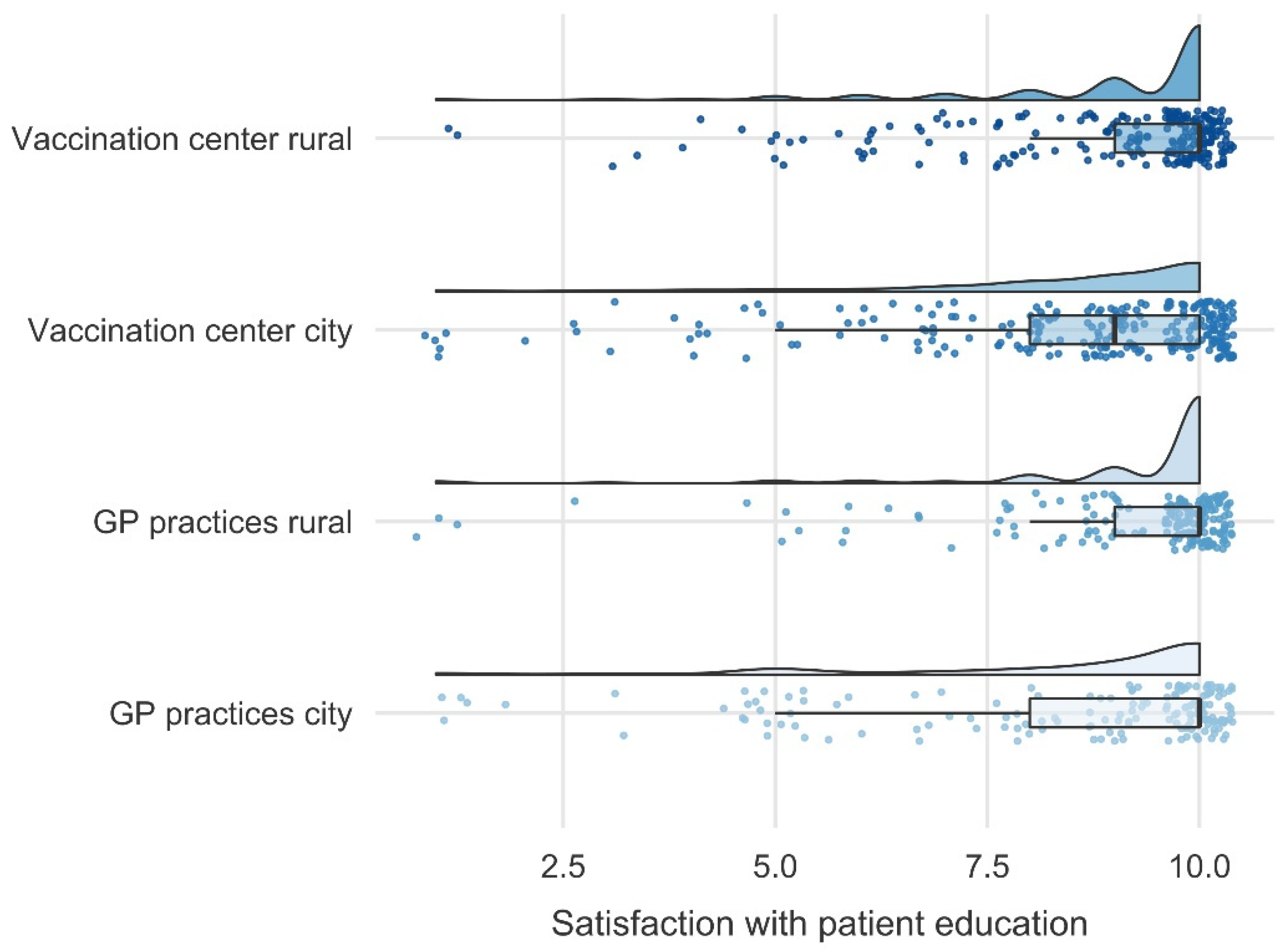
3.4. Satisfaction with Patient Education and Recommendation of Vaccination at Patients’ Vaccination Site
4. Discussion
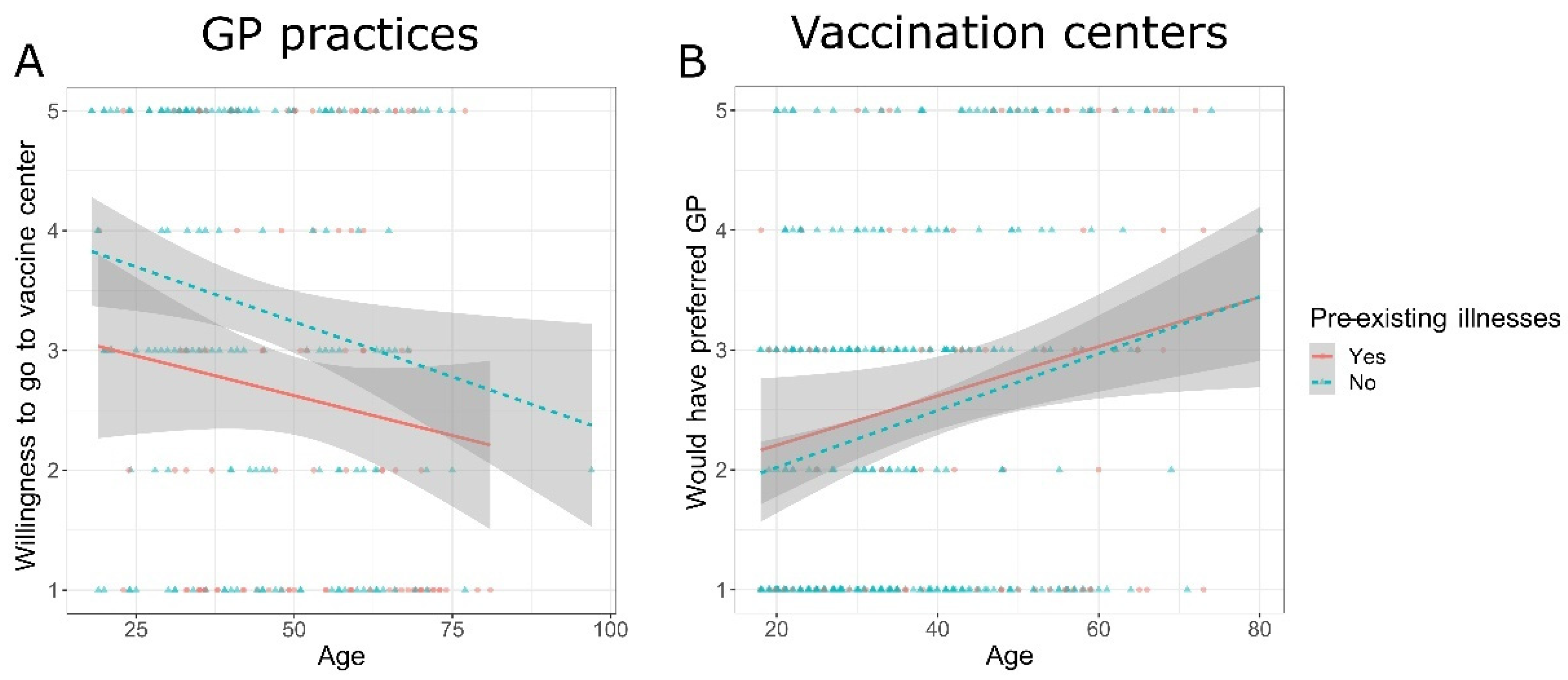
4.1. Participants’ Socio-Demographic Characteristics and Their Influence on Preference
4.2. Access to Vaccination Site
4.3. Received Vaccine Type and Dose Intervals
4.4. Physicians’ Provision of Vaccination Information
4.5. Satisfaction with Patient Education
4.6. Recommendation of Vaccination Site
4.7. Limitations
4.8. Implications for Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sah, P.; Vilches, T.N.; Moghadas, S.M.; Fitzpatrick, M.C.; Singer, B.H.; Hotez, P.J.; Galvani, A.P. Accelerated vaccine rollout is imperative to mitigate highly transmissible COVID-19 variants. EClinicalMedicine 2021, 35, 100865. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2021, 589, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Kuter, B.J.; Offit, P.A.; Poland, G.A. The development of COVID-19 vaccines in the United States: Why and how so fast? Vaccine 2021, 39, 2491–2495. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. COVID-19 Vaccines Authorized in the European Union (EU) to Prevent COVID-19, Following Evaluation by the European Medicines Agency (EMA); European Medicines Agency: Amsterdam, The Netherlands, 2022; Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised (accessed on 27 September 2022).
- Cylus, J.; Panteli, D.; van Ginneken, E. Who should be vaccinated first? Comparing vaccine prioritization strategies in Israel and European countries using the Covid-19 health system response monitor. Isr. J. Health Policy Res. 2021, 10, 16. [Google Scholar] [CrossRef]
- Pfeiffer-Ruiz, M.; Schroder, V. COVID-19 vaccination strategy in Germany. Clin. Soc. Work. Health Interv. 2021, 12, 31–34. [Google Scholar] [CrossRef]
- Goralnick, E.; Kaufmann, C.; Gawande, A.A. Mass-vaccination sites—An essential innovation to curb the COVID-19 pan-demic. N. Engl. J. Med. 2021, 384, e67. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Neumeier, S. Accessibility of COVID-19 vaccination centers in Germany via different means of transport. KN J. Cartogr. Geogr. Inf. 2022, 72, 41–58. [Google Scholar] [CrossRef]
- Heinrich, D.; Scheidel, H.-P. COVID-19-Pandemie: Deutschlands größtes Impfzentrum. Dtsch. Aerzteblatt Int. 2021, 118, A1858–A1862. Available online: https://www.aerzteblatt.de/archiv/221557 (accessed on 27 September 2022).
- Steiger, E.; Rass, S.; Seidel, A.; Kroll, L.; Czihal, T. COVID-19 vaccination in medical practices in Germany. Dtsch. Aerzteblatt Int. 2021, 118, 756–757. [Google Scholar] [CrossRef]
- Götz, G.; Herold, D.; Klotz, P.A.; Schäfer, J.T. Efficiency in COVID-19 vaccination campaigns—A comparison across Ger-many’s federal states. Vaccines 2021, 9, 788. [Google Scholar] [CrossRef]
- Desson, Z.; Kauer, L.; Otten, T.; Peters, J.W.; Paolucci, F. Finding the way forward: COVID-19 vaccination progress in Germany, Austria and Switzerland. Health Policy Technol. 2022, 11, 100584. [Google Scholar] [CrossRef] [PubMed]
- Vygen-Bonnet, S.; Koch, J.; Bogdan, C.; Heininger, U.; Littmann, M.; Meerpohl, J.; Meyer, H.; Mertens, T.; Schmid-Küpke, N.; Scholz, S.; et al. Beschluss der STIKO zur 7. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wis-senschaftliche Begründung. Epidemiol. Bull. 2021, 25, 3–13. [Google Scholar] [CrossRef]
- Warren, G.W.; Lofstedt, R. COVID-19 vaccine rollout management and communication in Europe: One year on. J. Risk Res. 2021, 25, 1098–1117. [Google Scholar] [CrossRef]
- Wood, R.M.; Murch, B.J.; Moss, S.J.; Tyler, J.M.B.; Thompson, A.L.; Vasilakis, C. Operational research for the safe and effective design of COVID-19 mass vaccination centres. Vaccine 2021, 39, 3537–3540. [Google Scholar] [CrossRef]
- Guhlincozzi, A.R.; Lotfata, A. Travel distance to flu and COVID-19 vaccination sites for people with disabilities and age 65 and older, Chicago metropolitan area. J. Health Res. 2021, 36, 859–866. [Google Scholar] [CrossRef]
- Parker, W.F.; Persad, G.; Peek, M.E. Fair allocation at COVID-19 mass vaccination sites. JAMA Health Forum 2021, 2, e210464. [Google Scholar] [CrossRef]
- Ratzan, S.; Schneider, E.C.; Hatch, H.; Cacchione, J. Missing the point—How primary care can overcome COVID-19 vaccine “hesitancy”. N. Engl. J. Med. 2021, 384, e100. [Google Scholar] [CrossRef]
- McPhedran, R.; Gold, N.; Bemand, C.; Weston, D.; Rosen, R.; Scott, R.; Chadborn, T.; Amlôt, R.; Mawby, M.; Toombs, B. Location, location, location: A discrete choice experiment to inform COVID-19 vaccination programme delivery in the UK. BMC Public Health 2022, 22, 431. [Google Scholar] [CrossRef]
- Brauns, H.; Scherer, S.; Steinmann, S. The CASMIN educational classification in international comparative research. In Advances in Cross-National Comparison: A European Working Book for Demographic and Socio-Economic Variables (221–244); Hoffmeyer-Zlotnik, J.H.P., Wolf, C., Eds.; Springer Science + Business Media: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Drennan, J. Cognitive interviewing: Verbal data in the design and pretesting of questionnaires. J. Adv. Nurs. 2003, 42, 57–63. [Google Scholar] [CrossRef]
- Press, V.G.; Huisingh-Scheetz, M.; Arora, V.M. Inequities in technology contribute to disparities in COVID-19 vaccine distribution. JAMA Health Forum 2021, 2, e210264. [Google Scholar] [CrossRef] [PubMed]
- Reiners, F.; Sturm, J.; Bouw, L.J.W.; Wouters, E.J.M. Sociodemographic factors influencing the use of eHealth in people with chronic diseases. Int. J. Environ. Res. Public Health 2019, 16, 645. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Robards, F.; Luscombe, G.; Sanci, L.; Usherwood, T. The relationship between having a regular general practi-tioner (GP) and the experience of healthcare barriers: A cross-sectional study among young people in NSW, Australia, with oversampling from marginalised groups. BMC Fam. Pract. 2020, 21, 220. [Google Scholar] [CrossRef]
- Moos, M. From gentrification to youthification? The increasing importance of young age in delineating high-density living. Urban Stud. 2016, 53, 2903–2920. [Google Scholar] [CrossRef]
- OECD. Education at a Glance 2022: OECD Indicators; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Kelly, B.J.; Southwell, B.G.; McCormack, L.A.; Bann, C.M.; MacDonald, P.D.M.; Frasier, A.M.; Bevc, C.A.; Brewer, N.T.; Squiers, L.B. Predictors of willingness to get a COVID-19 vaccine in the US. BMC Infect. Dis. 2021, 21, 338. [Google Scholar] [CrossRef]
- Lazarus, J.v.; Wyka, K.; Rauh, L.; Rabin, K.; Ratzan, S.; Gostin, L.O.; Larson, H.J.; El-Mohandes, A. Hesitant or not? The association of age, gender, and education with potential acceptance of a COVID-19 vaccine: A country-level analysis. J. Health Commun. 2020, 25, 799–807. [Google Scholar] [CrossRef]
- Rijken, M.; Stüssgen, R.; Leemrijse, C.; Bogerd, M.J.L.; Korevaar, J.C. Priorities and preferences for care of people with multiple chronic conditions. Health Expect 2021, 24, 1300–1311. [Google Scholar] [CrossRef]
- Goldberg, S.A.; Callaway, D.; Resnick-Ault, D.; Mandavia, S.; Martinez, R.; Bass, M.; Goralnick, E. Critical concepts for COVID-19 mass vaccination site operations. Disaster Med. Public Health Prep. 2021, 1–7. [Google Scholar] [CrossRef]
- Piraveenan, M.; Sawleshwarkar, S.; Walsh, M.; Zablotska, I.; Bhattacharyya, S.; Farooqui, H.H.; Bhatnagar, T.; Karan, A.; Murhekar, M.; Zodpey, S.; et al. Optimal governance and implementation of vaccination programmes to contain the COVID-19 pandemic. R. Soc. Open Sci. 2021, 8, 210429. [Google Scholar] [CrossRef]
- Duffy, C.; Newing, A.; Górska, J. Evaluating the geographical accessibility and equity of COVID-19 vaccination sites in England. Vaccines 2021, 10, 50. [Google Scholar] [CrossRef]
- Rosen, B.; Waitzberg, R.; Israeli, A.; Hartal, M.; Davidovitch, N. Addressing vaccine hesitancy and access barriers to achieve persistent progress in Israel’s COVID-19 vaccination program. Isr. J. Health Policy Res. 2021, 10, 43. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Pro-tection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef]
- Payne, P.R.; Longet, S.; Austin, A.J.; Skelly, T.D.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, C.S.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Mohammadnezhad, M.; Ward, P. Trust and communication in a doctor-patient relationship: A literature review. J. Healthc. Commun. 2018, 3, 36. [Google Scholar] [CrossRef]
- Ridda, I.; MacIntyre, C.R.; Lindley, R.I. A qualitative study to assess the perceived benefits and barriers to the pneumococcal vaccine in hospitalised older people. Vaccine 2009, 27, 3775–3779. [Google Scholar] [CrossRef]
- Leung, K.C.; Mui, C.; Chiu, W.Y.; Ng, Y.Y.; Chen, M.H.Y.; Ho, P.H.; Kwok, C.P.; Lam, S.S.M.; Wong, C.Y.; Wong, K.Y.; et al. Impact of patient education on influenza vaccine uptake among community-dwelling elderly: A randomized controlled trial. Health Educ. Res. 2017, 32, 455–464. [Google Scholar] [CrossRef]
- Pohontsch, N.J.; Hansen, H.; Schäfer, I.; Scherer, M. General practitioners’ perception of being a doctor in urban vs. rural regions in Germany—A focus group study. Fam. Pract. 2018, 35, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.; Hinds, K.; Richards, H.; Godden, D. Urban versus rural populations’ views of health care in Scotland. J. Health Serv. Res. Policy 2005, 10, 212–219. [Google Scholar] [CrossRef]
- Clever, S.L.; Jin, L.; Levinson, W.; Meltzer, D.O. Does doctor–patient communication affect patient satisfaction with hospital care? Results of an analysis with a novel instrumental variable. Health Serv. Res. 2008, 43, 1505–1519. [Google Scholar] [CrossRef]
- Kornides, M.L.; Fontenot, H.B.; McRee, A.L.; Panozzo, C.A.; Gilkey, M.B. Associations between parents’ satisfaction with provider communication and HPV vaccination behaviors. Vaccine 2018, 36, 2637–2642. [Google Scholar] [CrossRef]
- Riedl, D.; Schüßler, G. The influence of doctor-patient communication on health outcomes: A systematic review. Z. Für Psychosom. Med. Psychother. 2017, 63, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.M. Age-related differences in doctor-patient interaction and patient satisfaction. Curr. Gerontol. Geriatr. Res. 2011, 2011, 137492. [Google Scholar] [CrossRef] [PubMed]
- Bleustein, C.; Rothschild, D.B.; Valen, A.; Valaitis, E.; Schweitzer, L.; Jones, R. Wait times, patient satisfaction scores, and the perception of care. Am. J. Manag. Care 2014, 20, 393–400. [Google Scholar] [PubMed]
- Kreitz, T.M.; Winters, B.S.; Pedowitz, D.I. The influence of wait time on patient satisfaction in the orthopedic clinic. J. Patient Exp. 2016, 3, 39–42. [Google Scholar] [CrossRef]
- Fustino, N.J.; Wohlfeil, M.; Smith, H.L. Determination of key drivers of patient experience in a midsize pediatric hema-tology-oncology ambulatory clinic. Ochsner J. 2018, 18, 332–338. [Google Scholar] [CrossRef]
- Hojat, M.; Louis, D.Z.; Maxwell, K.; Markham, F.W.; Wender, R.C.; Gonnella, J.S. A brief instrument to measure patients’ overall satisfaction with primary care physicians. Fam. Med. 2011, 43, 412. [Google Scholar] [PubMed]
- Wong, S.T.; Watson, D.E.; Young, E.; Regan, S. What do people think is important about primary healthcare? Healthc. Policy 2008, 3, 89–104. [Google Scholar] [CrossRef]
- Kleeberg, U.R.; Feyer, P.; Günther, W.; Behrens, M. Patient satisfaction in outpatient cancer care: A prospective survey using the PASQOC® questionnaire. Support. Care Cancer 2008, 16, 947–954. [Google Scholar] [CrossRef]
- Gianfredi, V.; Pennisi, F.; Lume, A.; Ricciardi, G.E.; Minerva, M.; Riccò, M.; Odone, A.; Signorelli, C. Challenges and op-portunities of mass vaccination centers in COVID-19 times: A rapid review of literature. Vaccines 2021, 9, 574. [Google Scholar] [CrossRef]
- Piraux, A.; Cavillon, M.; Ramond-Roquin, A.; Faure, S. Assessment of satisfaction with pharmacist-administered COVID-19 vaccinations in France: PharmaCoVax. Vaccines 2022, 10, 440. [Google Scholar] [CrossRef]
- Rose, O.; Erzkamp, S.; Schöbel, W.; Grajeda, M.; Köberlein–Neu, J. COVID-19 vaccinations in German pharmacies: A survey on patient and provider satisfaction. Vaccine 2022, 40, 5207–5212. [Google Scholar] [CrossRef] [PubMed]

| Total | Vaccine Center City | Vaccine Center Rural | GP Practices City | GP Practices Rural | Comparison between Sites | |
|---|---|---|---|---|---|---|
| n | 838 | 246 | 228 | 169 | 195 | |
| Age | 42.5 ± 16.1 | 36.2 ± 14.1 | 40.5 ± 14.4 | 48.5 ± 17.6 | 48.1 ± 15.6 | F(3, 711) = 27.663 |
| p < 0.001 | ||||||
| ηp2 = 0.105 | ||||||
| Gender | ||||||
| female | 406 (51.4) | 119 (50.0) | 107 (50.2) | 93 (57.8) | 87 (48.9) | χ2(6) = 5.574 |
| male | 380 (48.1) | 117 (49.2) | 104 (48.8) | 68 (42.2) | 91 (51.1) | p = 0.411 |
| diverse | 4 (0.5) | 2 (0.8) | 2 (0.9) | 0 (0.0) | 0 (0.0) | V = 0.063 |
| Education | ||||||
| Primary | 10 (1.2) | 1 (0.4) | 3 (1.3) | 2 (1.2) | 4 (2.2) | χ2(6) = 24.960 |
| Secondary | 560 (68.4) | 143 (58.4) | 159 (70.7) | 130 (78.8) | 128 (69.6) | p < 0.001 |
| Tertiary | 249 (30.4) | 101 (41.2) | 63 (28.0) | 33 (20.0) | 52 (28.3) | V = 0.124 |
| Employment | ||||||
| Employed | 563 (68.1) | 176 (71.5) | 175 (77.1) | 99 (59.3) | 113 (60.8) | χ2(6) = 54.942 |
| Unemployed | 132 (16.0) | 45 (18.3) | 38 (16.7) | 18 (10.8) | 31 (16.7) | p < 0.001 |
| Retired | 131 (15.9) | 25 (10.2) | 14 (6.2) | 50 (29.9) | 42 (22.6) | V = 0.182 |
| Pre-existing illness(es) | 235 (28.8) | 44 (18.0) | 52 (23.4) | 75 (45.7) | 64 (34.4) | χ2(3) = 42.985 |
| p < 0.001 | ||||||
| V = 0.229 | ||||||
| Recently infected by COVID-19 | χ2(3) = 14.211 | |||||
| 29 (3.5) | 5 (2.0) | 4 (1.8) | 4 (2.4) | 16 (8.4) | p = 0.002 | |
| V = 0.147 | ||||||
| COVID-19 infection of friends/family | 538 (65.1) | 167 (68.2) | 161 (70.9) | 90 (53.9) | 120 (63.8) | χ2(3) = 13.760 |
| p = 0.003 | ||||||
| V = 0.129 |
| Total | Vaccine Center City | Vaccine Center Rural | GP Practices City | GP Practices Rural | Comparison between Sites | |
|---|---|---|---|---|---|---|
| Vaccination no. | χ2(3) = 29.187 | |||||
| 1 | 147 (18.0) | 51 (21.2) | 31 (13.8) | 12 (7.4) | 53 (27.9) | p < 0.001 |
| 2 | 670 (82.0) | 190 (78.8) | 193 (86.2) | 150 (92.6) | 137 (72.1) | V = 0.189 |
| Vaccine received today | ||||||
| BioNTech | 600 (73.7) | 125 (52.1) | 189 (83.6) | 141 (86.5) | 145 (78.4) | χ2(9) = 267.673 |
| AstraZeneca | 58 (7.1) | 3 (1.3) | 0 | 17 (10.4) | 38 (20.5) | p < 0.001 |
| Moderna | 153 (18.8) | 112 (46.6) | 36 (15.9) | 3 (1.8) | 2 (1.1) | V = 0.326 |
| J&J | 3 (0.4) | 0 | 1 (0.4) | 2 (1.2) | 0 | |
| Vaccine received 1st time (if applicable) | ||||||
| BioNTech | 419 (63.7) | 83 (43.9) | 150 (80.2) | 126 (82.9) | 60 (46.2) | χ2(6) = 206.724 |
| AstraZeneca | 155 (23.6) | 52 (27.5) | 8 (4.3) | 25 (16.4) | 70 (53.8) | p < 0.001 |
| Moderna | 84 (12.8) | 54 (28.6) | 29 (15.5) | 1 (0.7) | 0 | V = 0.381 |
| Same vaccine 1st and 2nd time | χ2(3) = 64.341 | |||||
| 529 (82.5) | 136 (72.7) | 177 (95.7) | 132 (91.7) | 84 (67.2) | p < 0.001 | |
| V = 0.314 | ||||||
| Days between 1st and 2nd dose | 38.3 ± 21.6 | 36.2 ± 19.0 | 24.6 ± 9.3 | 42.4 ± 20.8 | 58.8 ± 23.2 | F(3, 610) = 88.658 |
| p < 0.001 | ||||||
| ηp2 = 0.304 | ||||||
| Days between 1st and 2nd dose depending on vaccine type | ||||||
| Both mRNA | 27.6 ± 9.1 | 26.1 ± 6.3 | 23.2 ± 7.0 | 33.8 ± 9.5 | 34.9 ± 10.0 | F(3, 591) = 9.042 |
| At least one AstraZeneca | 70.6 ± 16.1 | 62.3 ± 15.7 | 50.0 ± 9.0 | 79.1 ± 14.7 | 77.2 ± 11.5 | p < 0.001 |
| ηp2 = 0.044 | ||||||
| Registration at vaccine center | n.a. | n.a. | n.a. | |||
| Online | 189 (78.4) | 156 (70.0) | χ2(4) = 8.605 | |||
| Via phone | 20 (8.3) | 25 (11.2) | p = 0.069 | |||
| By friends or family | 12 (5.0) | 21 (9.4) | V = 0.137 | |||
| Initiative by center | 2 (0.8) | 7 (3.1) | ||||
| Other | 18 (7.5) | 14 (6.3) | ||||
| Vaccination initiative by | n.a. | n.a. | n.a. | |||
| GP | 20 (12.8) | 31 (16.8) | χ2(3) = 8.734 | |||
| Myself | 105 (67.3) | 120 (64.9) | p = 0.033 | |||
| Family/ | 18 (11.5) | 30 (16.2) | V = 0.160 | |||
| friends | ||||||
| Other | 13 (8.3) | 4 (2.2) | ||||
| Time between scheduling and vaccination date | ||||||
| <1 week | 234 (29.5) | 71 (30.3) | 71 (32.6) | 52 (32.5) | 40 (22.1) | χ2(12) = 13.471 |
| 1–2 weeks | 230 (29.0) | 69 (29.5) | 63 (28.9) | 41 (25.6) | 57 (31.5) | p = 0.337 |
| 3–4 weeks | 180 (22.7) | 56 (23.9) | 48 (22.0) | 34 (21.3) | 42 (23.2) | V = 0.075 |
| 1–2 months | 84 (10.6) | 20 (8.5) | 17 (7.8) | 23 (14.4) | 24 (13.3) | |
| >2 months | 65 (8.2) | 18 (7.7) | 19 (8.7) | 10 (6.3) | 18 (9.9) | |
| Wait time at vaccine site | ||||||
| <10 min | 565 (70.4) | 200 (83.7) | 178 (80.5) | 98 (62.4) | 89 (47.8) | χ2(9) = 85.529 |
| 10–30 min | 196 (24.4) | 36 (15.1) | 36 (16.3) | 50 (31.8) | 74 (39.8) | p < 0.001 |
| 31–60 min | 36 (4.5) | 2 (0.8) | 6 (2.7) | 8 (5.1) | 20 (10.8) | V = 0.191 |
| >60 min | 6 (0.7) | 1 (0.4) | 1 (0.5) | 1 (0.6) | 3 (1.6) | |
| Travel distance | ||||||
| <5 min | 94 (11.4) | 3 (1.2) | 6 (2.6) | 44 (26.8) | 41 (21.6) | χ2(12) = 344.726 |
| 5–10 min | 155 (18.9) | 21 (8.7) | 10 (4.4) | 53 (32.3) | 71 (37.4) | p < 0.001 |
| 11–20 min | 186 (22.6) | 68 (28.2) | 43 (18.9) | 40 (24.4) | 35 (18.4) | V = 0.366 |
| 21–30 min | 142 (17.3) | 69 (28.6) | 34 (15.0) | 19 (11.6) | 20 (10.5) | |
| >30 min | 245 (29.8) | 80 (33.2) | 134 (59.0) | 8 (4.9) | 23 (12.1) | |
| Arrival at site | χ2(3) = 44.975 | |||||
| Alone | 495 (62.1) | 141 (60.5) | 100 (45.7) | 117 (72.7) | 137 (74.5) | p < 0.001 |
| Accompanied | 302 (37.9) | 92 (39.5) | 119 (54.3) | 44 (27.3) | 47 (25.5) | V = 0.238 |
| Transport | ||||||
| Walking | 95 (11.6) | 1 (0.4) | 1 (0.5) | 53 (32.3) | 40 (20.9) | χ2(12) = 314.261 |
| Bike | 57 (7.0) | 20 (8.3) | 3 (1.3) | 11 (6.7) | 23 (12.0) | p < 0.001 |
| Car | 550 (67.1) | 138 (57.6) | 214 (95.5) | 79 (48.2) | 119 (62.3) | V = 0.349 |
| Public transport | 110 (13.4) | 80 (33.3) | 1 (0.5) | 20 (12.2) | 9 (4.7) | |
| Other | 7 (0.9) | 1 (0.4) | 5 (2.2) | 1 (0.6) | 0 (0.0) |
| Total | Vaccine Center City | Vaccine Center Rural | GP Practices | GP Practices | Comparison between Sites | |
|---|---|---|---|---|---|---|
| City | Rural | |||||
| Information given by doctor | ||||||
| χ2(3) = 8.642 | ||||||
| Vaccine type information | 503 (62.4) | 137 (58.3) | 131 (58.2) | 104 (65.4) | 131 (70.1) | p = 0.035 |
| V = 0.104 | ||||||
| χ2(3) = 23.866 | ||||||
| Vaccine benefits | 283 (35.1) | 69 (29.4) | 60 (26.7) | 71 (44.7) | 83 (44.4) | p < 0.001 |
| V = 0.172 | ||||||
| χ2(3) = 28.294 | ||||||
| Vaccine effectiveness | 423 (52.5) | 104 (44.3) | 104 (46.2) | 88 (55.3) | 127 (67.9) | p < 0.001 |
| V = 0.187 | ||||||
| χ2(3) = 1.072 | ||||||
| Behavior before/after vaccination | 455 (56.5) | 139 (59.1) | 126 (56.0) | 88 (55.3) | 102 (54.5) | p = 0.785 |
| V = 0.036 | ||||||
| χ2(3) = 7.975 | ||||||
| Common vaccination reactions | 727 (90.2) | 211 (89.8) | 206 (91.6) | 135 (84.9) | 175 (93.6) | p = 0.053 |
| V = 0.099 | ||||||
| χ2(3) = 3.958 | ||||||
| Potential complications | 371 (46.0) | 97 (41.3) | 106 (47.1) | 73 (45.9) | 95 (50.8) | p = 0.273 |
| V = 0.070 | ||||||
| χ2(3) = 3.597 | ||||||
| Other | 13 (1.6) | 2 (0.9) | 4 (1.8) | 5 (3.1) | 2 (1.1) | p = 0.321 |
| V = 0.067 | ||||||
| Quantity of vaccine information given | 3.3± 1.8 | 3.1 ± 1.7 | 3.2 ±1.6 | 3.4 ± 2.0 | 3.7 ± 2.0 | F(3, 834) = 3.741 |
| p = 0.011 | ||||||
| ηp2 = 0.013 | ||||||
| Duration of patient | ||||||
| education | ||||||
| <2 min | 122 (15.5) | 54 (22.9) | 31 (13.9) | 26 (17.0) | 11 (6.3) | χ2(9) = 85.069 |
| 2–5 min | 424 (53.8) | 146 (61.9) | 133 (59.6) | 52 (34.0) | 93 (52.8) | p < 0.001 |
| 6–10 min | 206 (26.1) | 33 (14.0) | 51 (22.9) | 58 (37.9) | 64 (36.4) | V = 0.188 |
| >10 min | 36 (4.6) | 3 (1.3) | 8 (3.6) | 17 (11.1) | 8 (4.5) | |
| Possibility to ask questions after vaccination | ||||||
| Yes | 298 (36.6) | 74 (30.8) | 73 (32.3) | 54 (33.5) | 97 (51.9) | χ2(9) = 33.622 |
| Yes, but not needed | 411 (50.5) | 135 (56.3) | 122 (54.0) | 80 (49.7) | 74 (39.6) | p < 0.001 |
| Not known | 58 (7.1) | 23 (9.6) | 14 (6.2) | 13 (8.1) | 8 (4.3) | V = 0.117 |
| No | 47 (5.8) | 8 (3.3) | 17 (7.5) | 14 (8.7) | 8 (4.3) |
| Predictor | B | SE B | β | R2 |
|---|---|---|---|---|
| 0.371 | ||||
| Constant | 4.214 | 0.510 | ||
| Age | 0.015 | 0.004 | 0.126 ** | |
| Education | −0.311 | 0.120 | −0.082 * | |
| Vaccine center/GP practice | −0.118 | 0.128 | −0.031 | |
| Rural/city | −0.244 | 0.119 | −0.065 | |
| Comprehensibility of physicians’ information | 0.527 | 0.104 | 0.263 ** | |
| Perceived sufficiency of patient education duration | 0.496 | 0.101 | 0.265 ** | |
| Duration of patient education | 0.175 | 0.090 | 0.070 | |
| Quantity of vaccine information given | 0.076 | 0.037 | 0.070 * | |
| Predictor | B | SE B | β | R2 |
|---|---|---|---|---|
| 0.534 | ||||
| Constant | 1.221 | 0.240 | ||
| Age | 0.000 | 0.001 | 0.006 | |
| Education | −0.068 | 0.044 | −0.045 | |
| Vaccine center/GP practice | 0.122 | 0.050 | 0.079 * | |
| Rural/city | 0.056 | 0.044 | 0.037 | |
| Perceived distance to vaccination site | −0.008 | 0.016 | −0.016 | |
| Access to site was barrier-free | 0.038 | 0.023 | 0.055 | |
| Wait time at vaccine site | −0.010 | 0.039 | −0.008 | |
| Comprehensibility of the vaccination procedure | 0.340 | 0.033 | 0.368 ** | |
| Perceived sufficiency of patient education duration | 0.093 | 0.039 | 0.123 * | |
| Duration of patient education | −0.062 | 0.034 | −0.062 | |
| Comprehensibility of physicians’ information | 0.263 | 0.040 | 0.327 ** | |
| Quantity of vaccine information given | −0.012 | 0.014 | −0.027 | |
| Perception of the post-vaccination waiting period | 0.026 | 0.014 | 0.055 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jentzsch, A.; Geier, A.-K.; Bleckwenn, M.; Schrimpf, A. Differences in Demographics of Vaccinees, Access to, and Satisfaction with SARS-CoV-2 Vaccination Procedures between German General Practices and Mass Vaccination Centers. Vaccines 2022, 10, 1823. https://doi.org/10.3390/vaccines10111823
Jentzsch A, Geier A-K, Bleckwenn M, Schrimpf A. Differences in Demographics of Vaccinees, Access to, and Satisfaction with SARS-CoV-2 Vaccination Procedures between German General Practices and Mass Vaccination Centers. Vaccines. 2022; 10(11):1823. https://doi.org/10.3390/vaccines10111823
Chicago/Turabian StyleJentzsch, Anne, Anne-Kathrin Geier, Markus Bleckwenn, and Anne Schrimpf. 2022. "Differences in Demographics of Vaccinees, Access to, and Satisfaction with SARS-CoV-2 Vaccination Procedures between German General Practices and Mass Vaccination Centers" Vaccines 10, no. 11: 1823. https://doi.org/10.3390/vaccines10111823
APA StyleJentzsch, A., Geier, A.-K., Bleckwenn, M., & Schrimpf, A. (2022). Differences in Demographics of Vaccinees, Access to, and Satisfaction with SARS-CoV-2 Vaccination Procedures between German General Practices and Mass Vaccination Centers. Vaccines, 10(11), 1823. https://doi.org/10.3390/vaccines10111823






