Sputnik Light and Sputnik V Vaccination Is Effective at Protecting Medical Personnel from COVID-19 during the Period of Delta Variant Dominance
Abstract
1. Introduction
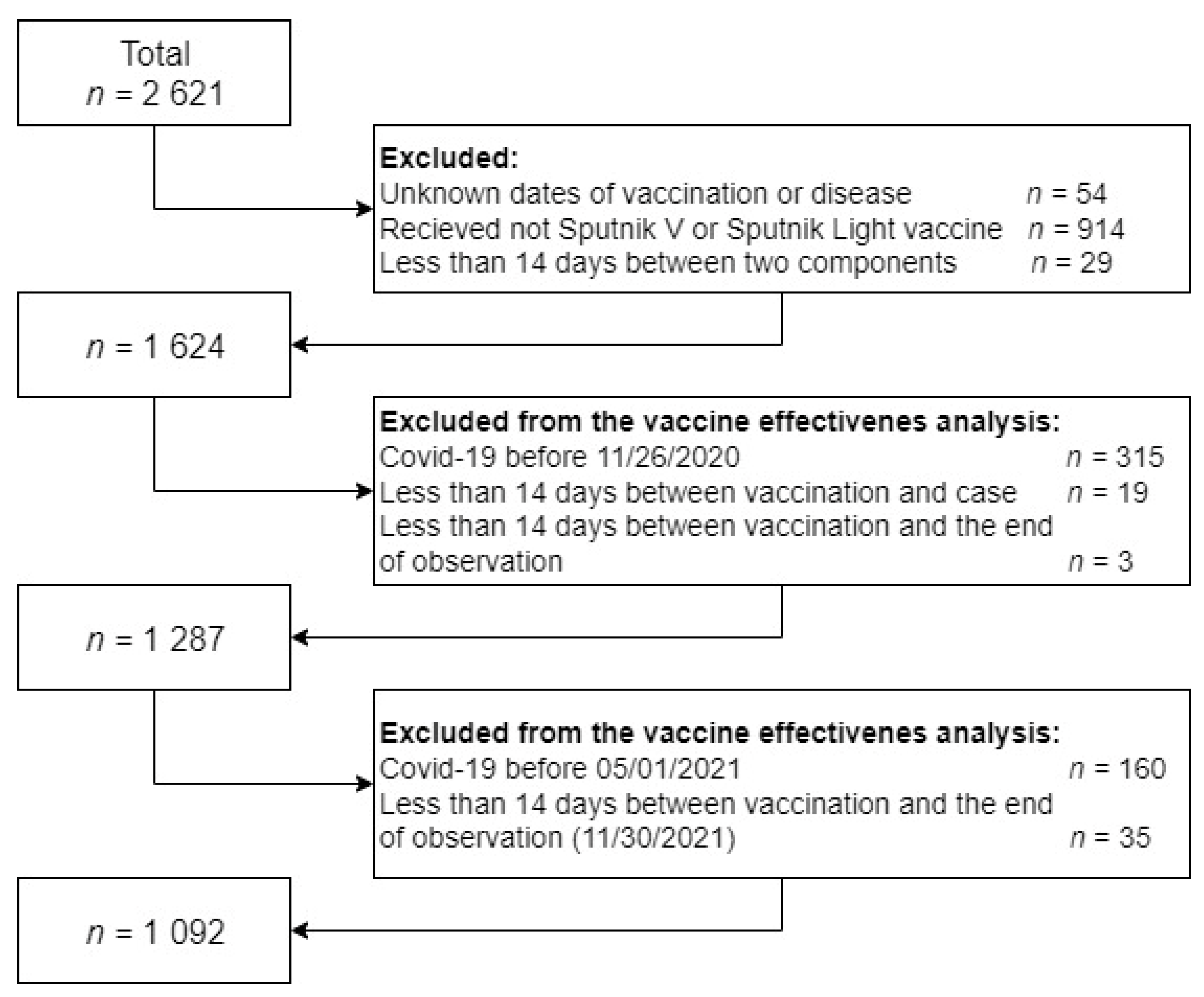
2. Materials and Methods
2.1. Database Preparation for Epidemiological Analysis
2.2. Statistical Analysis
2.3. Characteristics of the Genetic Landscape of SARS-CoV-2 Lines
3. Results
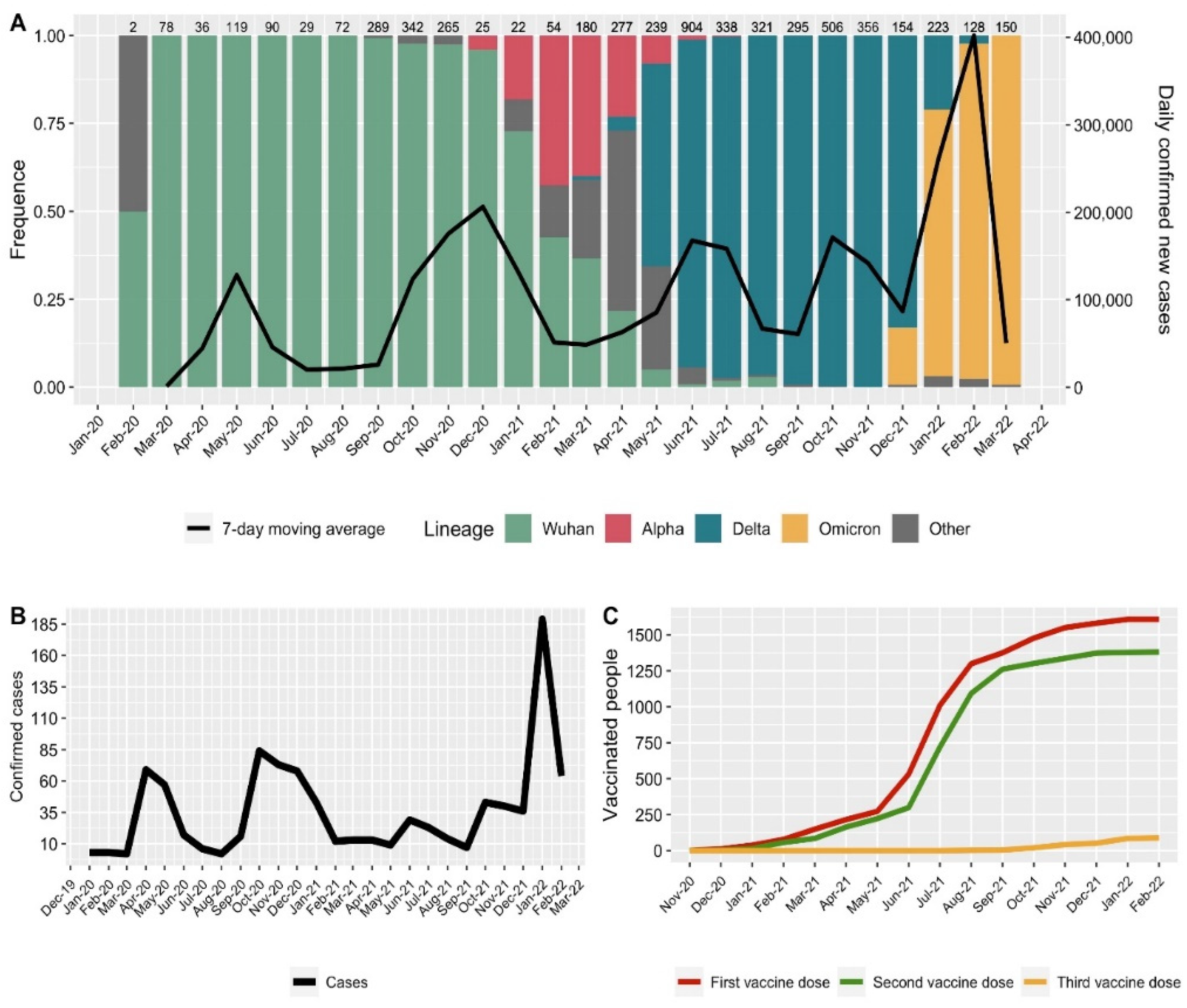
3.1. Circulating Lines SARS-CoV-2 Virus
3.2. Vaccination and Cases of the Disease among the Medical Center Personnel
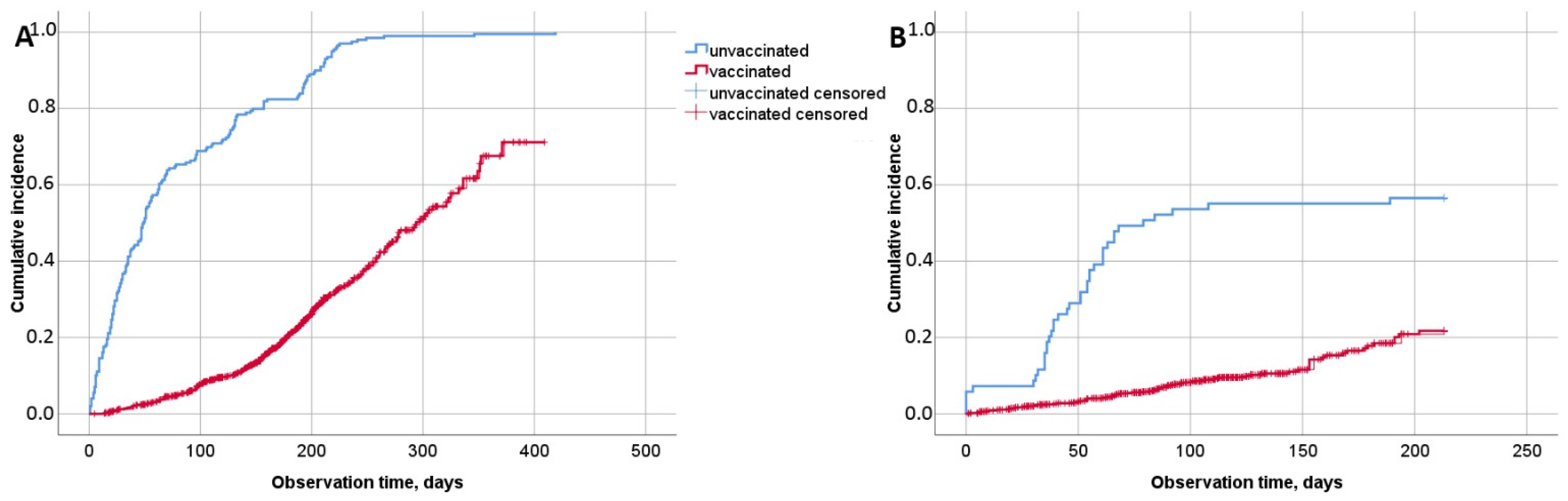
3.3. Effectiveness of Sputnik V in the Medical Personnel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ren, L.L.; Wang, Y.M.; Wu, Z.Q.; Xiang, Z.C.; Guo, L.; Xu, T.; Jiang, Y.Z.; Xiong, Y.; Li, Y.J.; Li, X.W.; et al. Identification of a Novel Coronavirus Causing Severe Pneumonia in Human: A Descriptive Study. Chin. Med. J. 2020, 133, 1015–1024. [Google Scholar] [CrossRef]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The Origin, Transmission and Clinical Therapies on Coronavirus Disease 2019 (COVID-19) Outbreak- A n Update on the Status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef]
- Garralda Fernandez, J.; Molero Vilches, I.; Bermejo Rodríguez, A.; Cano Torres, I.; Colino Romay, E.I.; García Arata, I.; Jaqueti Aroca, J.; Lillo Rodríguez, R.; López Lacomba, D.; Mazón Cuadrado, L.; et al. Impact of SARS-CoV-2 Pandemic among Health Care Workers in a Secondary Teaching Hospital in Spain. PLoS ONE 2021, 16, e0245001. [Google Scholar] [CrossRef]
- Huete-Pérez, J.A.; Cabezas-Robelo, C.; Páiz-Medina, L.; Hernández-Álvarez, C.A.; Quant-Durán, C.; McKerrow, J.H. First Report on Prevalence of SARS-CoV-2 Infection among Health-Care Workers in Nicaragua. PLoS ONE 2021, 16, e0246084. [Google Scholar] [CrossRef]
- Nienhaus, A.; Hod, R. COVID-19 among Health Workers in Germany and Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 4881. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and Immunogenicity of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine in Two Formulations: Two Open, Non-Randomised Phase 1/2 Studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; Botikov, A.G.; et al. An Open, Non-Randomised, Phase 1/2 Trial on the Safety, Tolerability, and Immunogenicity of Single-Dose Vaccine “Sputnik Light” for Prevention of Coronavirus Infection in Healthy Adults. Lancet Reg. Health-Eur. 2021, 11, 100241. [Google Scholar] [CrossRef]
- Barchuk, A.; Cherkashin, M.; Bulina, A.; Berezina, N.; Rakova, T.; Kuplevatskaya, D.; Stanevich, O.; Skougarevskiy, D.; Okhotin, A. Vaccine Effectiveness against Referral to Hospital and Severe Lung Injury Associated with COVID-19: A Population-Based Case-Control Study in St. Petersburg, Russia. BMC Med. 2022, 20, 312. [Google Scholar] [CrossRef]
- Dolzhikova, I.V.; Iliukhina, A.A.; Kovyrshina, A.V.; Kuzina, A.V.; Gushchin, V.A.; Siniavin, A.E.; Pochtovyi, A.A.; Shidlovskaya, E.V.; Kuznetsova, N.A.; Megeryan, M.M.; et al. Sputnik Light Booster after Sputnik V Vaccination Induces Robust Neutralizing Antibody Response to B.1.1.529 (Omicron) SARS-CoV-2 Variant. medRxiv 2021. [Google Scholar] [CrossRef]
- Gushchin, V.A.; Tsyganova, E.V.; Ogarkova, D.A.; Adgamov, R.R.; Shcheblyakov, D.V.; Glukhoedova, N.V.; Zhilenkova, A.S.; Kolotii, A.G.; Zaitsev, R.D.; Logunov, D.Y.; et al. Sputnik V Protection from COVID-19 in People Living with HIV under Antiretroviral Therapy. eClinicalMedicine 2022, 46, 101360. [Google Scholar] [CrossRef]
- Hightower, A.W.; Orenstein, W.A.; Martin, S.M. Recommendations for the Use of Taylor Series Confidence Intervals for Estimates of Vaccine Efficacy. Bull. World Health Organ. 1988, 66, 99. [Google Scholar]
- dplyr: A Grammar of Data Manipulation. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 18 October 2022).
- Ginestet, C. Ggplot2: Elegant Graphics for Data Analysis. J. R. Stat. Soc. Ser. A 2011, 174. [Google Scholar] [CrossRef]
- Gushchin, V.A.; Dolzhikova, I.V.; Shchetinin, A.M.; Odintsova, A.S.; Siniavin, A.E.; Nikiforova, M.A.; Pochtovyi, A.A.; Shidlovskaya, E.V.; Kuznetsova, N.A.; Burgasova, O.A.; et al. Neutralizing Activity of Sera from Sputnik V-Vaccinated People against Variants of Concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow Endemic SARS-CoV-2 Variants. Vaccines 2021, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- GOGOV, Coronavirus Statistics in Moscow. Available online: https://gogov.ru/covid-19/msk#data (accessed on 18 October 2022).
- Yandex. Coronovirus: Statistics. Available online: https://yandex.ru/covid19/stat (accessed on 18 October 2022).
- GOGOV, Vaccination Statistics in Moscow. Available online: https://gogov.ru/articles/covid-v-stats (accessed on 18 October 2022).
- WHO. Clinical Management of COVID-19—Interim Guidance. Available online: https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf?sequence=1&isAllowed=y (accessed on 18 October 2022).
- Hrin, M.L.; Emmerich, V.K.; Ip, E.H.; Feldman, S.R. Development and Validation of a COVID-19 Vaccine Hesitancy Scale for Adults in the United States. Vaccine 2022, 40, 5764–5768. [Google Scholar] [CrossRef] [PubMed]
- Terry, E.; Cartledge, S.; Damery, S.; Greenfield, S. Factors Associated with COVID-19 Vaccine Intentions during the COVID-19 Pandemic; a Systematic Review and Meta-Analysis of Cross-Sectional Studies. BMC Public Health 2022, 22, 1667. [Google Scholar] [CrossRef] [PubMed]
- Larrauri, B.J.; Malbrán, A.; Larrauri, J.A.; Larrauri, B. Omicron and Vaccines: An Analysis on the Decline in COVID-19 Mortality. MedRxiv 2022. [Google Scholar] [CrossRef]
- Cooper, D.J.; Lear, S.; Watson, L.; Shaw, A.; Ferris, M.; Doffinger, R.; Bousfield, R.; Sharrocks, K.; Weekes, M.P.; Warne, B.; et al. A Prospective Study of Risk Factors Associated with Seroprevalence of SARS-CoV-2 Antibodies in Healthcare Workers at a Large UK Teaching Hospital. J. Infect. 2022, 85, 557–564. [Google Scholar] [CrossRef]
- Violán, C.; Torán-Monserrat, P.; Quirant, B.; Lamonja-Vicente, N.; Carrasco-Ribelles, L.A.; Chacón, C.; Manresa-Dominguez, J.M.; Ramos-Roure, F.; Dacosta-Aguayo, R.; Palacios-Fernández, C.; et al. Kinetics of Humoral Immune Response over 17 Months of COVID-19 Pandemic in a Large Cohort of Healthcare Workers in Spain: The ProHEpiC-19 Study. BMC Infect. Dis. 2022, 22, 721. [Google Scholar] [CrossRef]
- Pacutova, V.; Madarasova Geckova, A.; Majernikova, S.M.; Kizek, P.; de Winter, A.F.; Reijneveld, S.A. Job Leaving Intentions of Dentists Associated With COVID-19 Risk, Impact of Pandemic Management, and Personal Coping Resources. Int. J. Public Health 2022, 67, 146. [Google Scholar] [CrossRef]
- Klink, G.V.; Safina, K.R.; Nabieva, E.; Shvyrev, N.; Garushyants, S.; Alekseeva, E.; Komissarov, A.B.; Danilenko, D.M.; Pochtovyi, A.A.; Divisenko, E.V.; et al. The Rise and Spread of the SARS-CoV-2 AY.122 Lineage in Russia. Virus Evol. 2022, 8, veac017. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Shkoda, A.S.; Gushchin, V.A.; Ogarkova, D.A.; Stavitskaya, S.V.; Orlova, O.E.; Kuznetsova, N.A.; Keruntu, E.N.; Pochtovyi, A.A.; Pukhov, A.V.; Kleymenov, D.A.; et al. Sputnik V Effectiveness against Hospitalization with COVID-19 during Omicron Dominance. Vaccines 2022, 10, 938. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Schwartz, O. Towards SARS-CoV-2 Serotypes? Nat. Rev. Microbiol. 2022, 20, 187–188. [Google Scholar] [CrossRef]
- Komissarov, A.A.; Dolzhikova, I.V.; Efimov, G.A.; Logunov, D.Y.; Mityaeva, O.; Molodtsov, I.A.; Naigovzina, N.B.; Peshkova, I.O.; Shcheblyakov, D.V.; Volchkov, P.; et al. Boosting of the SARS-CoV-2–Specific Immune Response after Vaccination with Single-Dose Sputnik Light Vaccine. J. Immunol. 2022, 208, 1139–1145. [Google Scholar] [CrossRef]
- Real-World COVID-19 Vaccine Effectiveness in Healthcare Workers. Available online: https://www.cdc.gov/vaccines/covid-19/effectiveness-research/vaccine-effectiveness-hcp.html (accessed on 18 October 2022).
- Pilishvili, T.; Fleming-Dutra, K.E.; Farrar, J.L.; Gierke, R.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C.; et al. Vaccine Effectiveness Among Healthcare Personnel Study Team. Interim Estimates of Vaccine Effectiveness of Pfizer-BioNTech and Moderna COVID-19 Vaccines Among Health Care Personnel-33 U.S. Sites, January-March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 753–758. [Google Scholar] [CrossRef]
- Pilishvili, T.; Gierke, R.; Fleming-Dutra, K.E.; Farrar, J.L.; Mohr, N.M.; Talan, D.A.; Krishnadasan, A.; Harland, K.K.; Smithline, H.A.; Hou, P.C.; et al. Vaccine Effectiveness among Healthcare Personnel Study Team. Effectiveness of mRNA COVID-19 Vaccine among U.S. Health Care Personnel. N. Engl. J. Med. 2021, 385, e90. [Google Scholar] [CrossRef]
| Vaccination Status | Cases | Non-Cases | VE, % (95% CI) | |
|---|---|---|---|---|
| (1-RR) | (1-HR) | |||
| Unvaccinated | 39 | 30 | ||
| One dose | 6 | 113 | 91.1% (80.0–96.0%) | 78.8% (49.2–91.1%) |
| Two doses | 91 | 788 | 81.7% (75.7–86.7%) | 81.8% (73.1–87.6%) |
| Three or four doses | 0 | 25 | 100% (–) | 100% (–) |
| At least one dose | 97 | 926 | 83.2% (77.8–87.3%) | 81.7% (73.1–87.5%) |
| Vaccination Status | Average Age, Years Old M ± SD (95% CI) | With COVID-19 during the Observation Period | Without COVID-19 during the Observation Period | p (Student’s Criterion) |
|---|---|---|---|---|
| Unvaccinated (n = 64, 92.8%) | 42.98 ± 12.77 (39.79–46.17) | n = 39 (100%) 44.00 ± 12.81 (39.85–48.15) | n = 25 (83.3%) 41.40 ± 12.80 (36.12–46.68) | 0.431 |
| One dose (n = 105, 88.2%) | 42.28 ± 13.73 (42.62–47.93) | n = 6 (100%) 44.17 ± 10.83 (32.80–55.54) | n = 99 (87.6%) 45.34 ± 13.93 (42.57–48.12) | 0.840 |
| Two doses (n = 854, 97.2%) | 42.97 ± 13.31 (42.97–42.07) | n = 91 (100%) 44.14 ± 13.16 (41.40–46.88) | n = 763 (96.8%) 42.83 ± 13.33 (41.88–43.77) | 0.373 |
| Three or four doses 25 (100%) | 47.00 ± 14.35 (41.08–52.92) | – | n = 25 (100%) 47.00 ± 14.35 (41.08–52.92) | – |
| p (ANOVA) | 0.189 | 0.998 | 0.135 |
| Severity | Non-Vaccinated (n = 514) | Vaccinated (n = 285) |
|---|---|---|
| Mild | 346 (67.3%) | 221 (77.5%) |
| Moderate | 151 (29.4%) | 59 (20.7%) |
| Severe | 17 (3.3%) | 5 (1.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhikh, G.T.; Priputnevich, T.V.; Ogarkova, D.A.; Pochtovyi, A.A.; Kustova, D.D.; Zlobin, V.I.; Logunov, D.Y.; Gushchin, V.A.; Gintsburg, A.L. Sputnik Light and Sputnik V Vaccination Is Effective at Protecting Medical Personnel from COVID-19 during the Period of Delta Variant Dominance. Vaccines 2022, 10, 1804. https://doi.org/10.3390/vaccines10111804
Sukhikh GT, Priputnevich TV, Ogarkova DA, Pochtovyi AA, Kustova DD, Zlobin VI, Logunov DY, Gushchin VA, Gintsburg AL. Sputnik Light and Sputnik V Vaccination Is Effective at Protecting Medical Personnel from COVID-19 during the Period of Delta Variant Dominance. Vaccines. 2022; 10(11):1804. https://doi.org/10.3390/vaccines10111804
Chicago/Turabian StyleSukhikh, Gennady T., Tatiana V. Priputnevich, Darya A. Ogarkova, Andrei A. Pochtovyi, Daria D. Kustova, Vladimir I. Zlobin, Denis Y. Logunov, Vladimir A. Gushchin, and Alexander L. Gintsburg. 2022. "Sputnik Light and Sputnik V Vaccination Is Effective at Protecting Medical Personnel from COVID-19 during the Period of Delta Variant Dominance" Vaccines 10, no. 11: 1804. https://doi.org/10.3390/vaccines10111804
APA StyleSukhikh, G. T., Priputnevich, T. V., Ogarkova, D. A., Pochtovyi, A. A., Kustova, D. D., Zlobin, V. I., Logunov, D. Y., Gushchin, V. A., & Gintsburg, A. L. (2022). Sputnik Light and Sputnik V Vaccination Is Effective at Protecting Medical Personnel from COVID-19 during the Period of Delta Variant Dominance. Vaccines, 10(11), 1804. https://doi.org/10.3390/vaccines10111804








