An Overview of Rift Valley Fever Vaccine Development Strategies
Abstract
1. Introduction
1.1. Rift Valley Fever Epidemiology
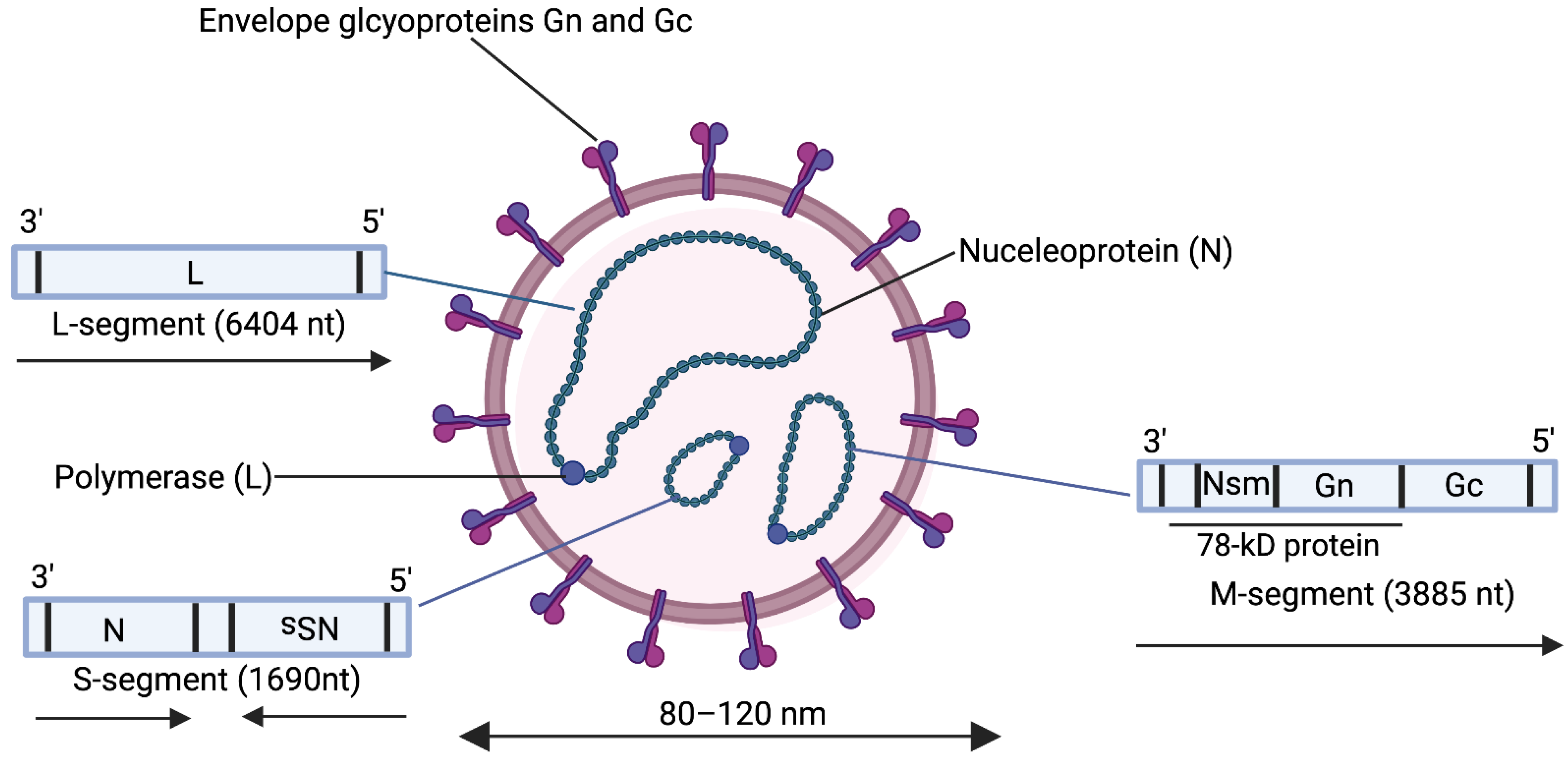
1.2. Molecular Biology of the Rift Valley Fever Virus
1.3. Immune Responses against Rift Valley Fever Virus
2. Rift Valley Fever Vaccine Development
2.1. Conventional Live Attenuated RVF Vaccines
2.1.1. Smithburn
2.1.2. MP-12
2.2. Formalin Inactivated Vaccines
2.2.1. NDBR-103
2.2.2. TSI-GSD-200
2.3. Genetically Modified Live Attenuated RVF Vaccines
2.3.1. Clone 13
2.3.2. ArMP-12ΔNSm21/384
2.3.3. RMP-12-GM50
2.3.4. R566
2.3.5. RRVF-ΔNSs:GFP-ΔNSm and ΔNSs-ΔNSm rRVFV
2.3.6. Four Segmented RVFV 4S
2.4. DNA Vaccines
2.5. Virus Vectored Vaccines
2.5.1. Lumpy Skin Disease Virus (rLSDV-RVFV)
2.5.2. Complex Adenovirus Vector (CAdax-RVF)
2.5.3. New Castle Disease Virus (NDFL-GnGC)
2.5.4. Replication-Competent Vaccinia Virus (vCOGnGc and vCOGnGcγ)
2.5.5. Replication-Deficient Chimpanzee Adenovirus (ChAdOx1-GnGc)
2.5.6. MVA Vectored (rMVA-Gn/Gc and rMVA-N)
2.5.7. Bivalent MVA Vectored (MVA-GnGc-VP2, MVA-GnGc-NS1, and MVA-GnGc-NS1-Nt)
2.5.8. Equine Herpesvirus Type 1 (rH_Gn-Gc) and Capripoxvirus Recombinant Virus (rKS1/RVFV)
2.5.9. Rabies Virus Vector (rSRV9-eGn)
2.6. Subunit Vaccines
2.7. Virus Replicon Vaccines
2.7.1. Alphavirus Replicon Vectors
2.7.2. Replication Deficient RVFV Replicons
2.8. Virus-like Particles
3. Outstanding Gaps and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daubney, R.; Hudson, J.; Garnham, P. Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep cattle and man from East Africa. J. Pathol. Bacteriol. 1931, 34, 545–579. [Google Scholar] [CrossRef]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley fever: Biology and epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Adam, I.; Karsany, M.S. Case report: Rift Valley fever with vertical transmission in a pregnant Sudanese woman. J. Med. Virol. 2008, 80, 929. [Google Scholar] [CrossRef] [PubMed]
- Wichgers Schreur, P.J.; Van Keulen, L.; Kant, J.; Oreshkova, N.; Moormann, R.J.; Kortekaas, J. Co-housing of Rift Valley fever virus infected lambs with immunocompetent or immunosuppressed lambs does not result in virus transmission. Front. Microbiol. 2016, 7, 287. [Google Scholar] [CrossRef]
- Rostal, M.K.; Evans, A.L.; Sang, R.; Gikundi, S.; Wakhule, L.; Munyua, P.; Macharia, J.; Feikin, D.R.; Breiman, R.F.; Njenga, M.K. Identification of potential vectors of and detection of antibodies against Rift Valley fever virus in livestock during interepizootic periods. Am. J. Vet. Res. 2010, 71, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Murithi, R.; Munyua, P.; Ithondeka, P.; Macharia, J.; Hightower, A.; Luman, E.; Breiman, R.; Njenga, M.K. Rift Valley fever in Kenya: History of epizootics and identification of vulnerable districts. Epidemiol. Infect. 2010, 139, 372–380. [Google Scholar] [CrossRef]
- Ikegami, T. Molecular biology and genetic diversity of Rift Valley fever virus. Antivir. Res. 2012, 95, 293–310. [Google Scholar] [CrossRef]
- Madani, T.A.; Al-Mazrou, Y.Y.; Al-Jeffri, M.H.; Mishkhas, A.A.; Al-Rabeah, A.M.; Turkistani, A.M.; Al-Sayed, M.O.; Abodahish, A.A.; Khan, A.S.; Ksiazek, T.G. Rift Valley fever epidemic in Saudi Arabia: Epidemiological, clinical, and laboratory characteristics. Clin. Infect. Dis. 2003, 37, 1084–1092. [Google Scholar] [CrossRef]
- Samy, A.M.; Peterson, A.T.; Hall, M. Phylogeography of Rift Valley fever virus in Africa and the Arabian Peninsula. PLoS Negl. Trop. Dis. 2017, 11, e0005226. [Google Scholar] [CrossRef]
- Paweska, J.T.; van Vuren, P.J. Rift Valley fever virus: A virus with potential for global emergence. In The Role of Animals in Emerging Viral Diseases; Elsevier: Amsterdam, The Netherlands, 2014; pp. 169–200. [Google Scholar]
- Mandell, R.; Flick, R. Rift Valley fever virus: A real bioterror threat. J. Bioterrorism Biodefense 2011, 2, 108. [Google Scholar] [CrossRef]
- Animal and Plant Health Inspection Service; U.S Department of Agriculture. OIE and International Standards. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/nvap/NVAP-Reference-Guide/Animal-Health-Emergency-Management/OIE-and-International-Standards (accessed on 20 October 2022).
- Federal Select Agent Program; Centers for Disease Control and Prevention; The United States Department of Agriculture. Select Agents and Toxins List. Available online: https://www.selectagents.gov/sat/list.htm (accessed on 20 October 2022).
- Evans, A.; Gakuya, F.; Paweska, J.; Rostal, M.; Akoolo, L.; Van Vuren, P.J.; Manyibe, T.; Macharia, J.; Ksiazek, T.; Feikin, D. Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol. Infect. 2008, 136, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Paweska, J.T. Rift Valley fever. Rev. Sci. Tech. 2015, 34, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A. Rift valley fever. Clin. Lab. Med. 2017, 37, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T.; Makino, S. The pathogenesis of Rift Valley fever. Viruses 2011, 3, 493–519. [Google Scholar] [CrossRef]
- World Health Organization. Rift Valley fever fact sheet:(Revised in September 2007). Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2008, 83, 17–22. [Google Scholar]
- Bouloy, M.; Weber, F. Molecular biology of Rift Valley fever virus. Open Virol. J. 2010, 4, 8. [Google Scholar] [CrossRef]
- Lopez, N.; Muller, R.; Prehaud, C.; Bouloy, M. The L protein of Rift Valley fever virus can rescue viral ribonucleoproteins and transcribe synthetic genome-like RNA molecules. J. Virol. 1995, 69, 3972–3979. [Google Scholar] [CrossRef]
- Habjan, M.; Pichlmair, A.; Elliott, R.M.; Overby, A.K.; Glatter, T.; Gstaiger, M.; Superti-Furga, G.; Unger, H.; Weber, F. NSs protein of rift valley fever virus induces the specific degradation of the double-stranded RNA-dependent protein kinase. J. Virol. 2009, 83, 4365–4375. [Google Scholar] [CrossRef]
- Ikegami, T.; Narayanan, K.; Won, S.; Kamitani, W.; Peters, C.J.; Makino, S. Rift Valley fever virus NSs protein promotes post-transcriptional downregulation of protein kinase PKR and inhibits eIF2alpha phosphorylation. PLoS Pathog. 2009, 5, e1000287. [Google Scholar] [CrossRef]
- Kalveram, B.; Lihoradova, O.; Ikegami, T. NSs protein of rift valley fever virus promotes posttranslational downregulation of the TFIIH subunit p62. J. Virol. 2011, 85, 6234–6243. [Google Scholar] [CrossRef]
- Billecocq, A.; Spiegel, M.; Vialat, P.; Kohl, A.; Weber, F.; Bouloy, M.; Haller, O. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol. 2004, 78, 9798–9806. [Google Scholar] [CrossRef] [PubMed]
- Bouloy, M.; Janzen, C.; Vialat, P.; Khun, H.; Pavlovic, J.; Huerre, M.; Haller, O. Genetic evidence for an interferon-antagonistic function of rift valley fever virus nonstructural protein NSs. J. Virol. 2001, 75, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Carnec, X.; Ermonval, M.; Kreher, F.; Flamand, M.; Bouloy, M. Role of the cytosolic tails of Rift Valley fever virus envelope glycoproteins in viral morphogenesis. Virology 2014, 448, 1–14. [Google Scholar] [CrossRef]
- Won, S.; Ikegami, T.; Peters, C.J.; Makino, S. NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis. J. Virol. 2007, 81, 13335–13345. [Google Scholar] [CrossRef]
- Weingartl, H.M.; Zhang, S.; Marszal, P.; McGreevy, A.; Burton, L.; Wilson, W.C. Rift Valley fever virus incorporates the 78 kDa glycoprotein into virions matured in mosquito C6/36 cells. PLoS ONE 2014, 9, e87385. [Google Scholar] [CrossRef]
- Lozach, P.-Y.; Kühbacher, A.; Meier, R.; Mancini, R.; Bitto, D.; Bouloy, M.; Helenius, A. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe 2011, 10, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, I.; Nishiyama, S.; Lokugamage, N.; Hill, T.; Huante, M.; Slack, O.; Carpio, V.; Freiberg, A.; Ikegami, T. N-glycans on the Rift Valley fever virus envelope glycoproteins Gn and Gc redundantly support viral infection via DC-SIGN. Viruses 2016, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.; Schudel, B.R.; Maar, D.; Kozina, C.; Ikegami, T.; Tseng, C.-T.K.; Negrete, O.A. Rift Valley fever virus strain MP-12 enters mammalian host cells via caveola-mediated endocytosis. J. Virol. 2012, 86, 12954–12970. [Google Scholar] [CrossRef]
- De Boer, S.; Kortekaas, J.; Spel, L.; Rottier, P.; Moormann, R.; Bosch, B. Acid-activated structural reorganization of the Rift Valley fever virus Gc fusion protein. J. Virol. 2012, 86, 13642–13652. [Google Scholar] [CrossRef]
- Müller, R.; Poch, O.; Delarue, M.; Bishop, D.H.; Bouloy, M. Rift Valley fever virus L segment: Correction of the sequence and possible functional role of newly identified regions conserved in RNA-dependent polymerases. J. Gen. Virol. 1994, 75, 1345–1352. [Google Scholar] [CrossRef]
- Harmon, J.R.; Barbeau, D.J.; Nichol, S.T.; Spiropoulou, C.F.; McElroy, A.K. Rift Valley fever virus vaccination induces long-lived, antigen-specific human T cell responses. NPJ Vaccines 2020, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Easterday, B.C. Rift valley fever. Adv. Vet. Sci. 1965, 10, 65–127. [Google Scholar] [PubMed]
- Faburay, B.; LaBeaud, A.D.; McVey, D.S.; Wilson, W.C.; Richt, J.A. Current status of Rift Valley fever vaccine development. Vaccines 2017, 5, 29. [Google Scholar] [CrossRef]
- Pepin, M.; Bouloy, M.; Bird, B.H.; Kemp, A.; Paweska, J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): An update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 2010, 41, 61. [Google Scholar] [CrossRef]
- Xu, W.; Watts, D.M.; Costanzo, M.C.; Tang, X.; Venegas, L.A.; Jiao, F.; Sette, A.; Sidney, J.; Sewell, A.K.; Wooldridge, L.; et al. The nucleocapsid protein of Rift Valley fever virus is a potent human CD8+ T cell antigen and elicits memory responses. PLoS ONE 2013, 8, e59210. [Google Scholar] [CrossRef] [PubMed]
- Jansen van Vuren, P.; Tiemessen, C.T.; Paweska, J.T. Anti-nucleocapsid protein immune responses counteract pathogenic effects of Rift Valley fever virus infection in mice. PLoS ONE 2011, 6, e25027. [Google Scholar] [CrossRef]
- Wonderlich, E.R.; Caroline, A.L.; McMillen, C.M.; Walters, A.W.; Reed, D.S.; Barratt-Boyes, S.M.; Hartman, A.L. Peripheral Blood Biomarkers of Disease Outcome in a Monkey Model of Rift Valley Fever Encephalitis. J. Virol. 2018, 92, e01662-17. [Google Scholar] [CrossRef]
- McElroy, A.K.; Nichol, S.T. Rift Valley fever virus inhibits a pro-inflammatory response in experimentally infected human monocyte derived macrophages and a pro-inflammatory cytokine response may be associated with patient survival during natural infection. Virology 2012, 422, 6–12. [Google Scholar] [CrossRef]
- Ikegami, T. Candidate vaccines for human Rift Valley fever. Expert Opin. Biol. Ther. 2019, 19, 1333–1342. [Google Scholar] [CrossRef]
- Alhaj, M. Safety and Efficacy Profile of Commercial Veterinary Vaccines against Rift Valley Fever: A Review Study. J. Immunol. Res. 2016, 2016, 7346294. [Google Scholar] [CrossRef]
- Smithburn, K. Rift Valley fever: The neurotropic adaptation of the virus and the experimental use of this modified virus as a vaccine. Br. J. Exp. Pathol. 1949, 30, 1–16. [Google Scholar] [PubMed]
- Smithburn, K.C.; Haddow, A.J.; Gillett, J.D. Rift Valley fever; isolation of the virus from wild mosquitoes. Br. J. Exp. Pathol. 1948, 29, 107–121. [Google Scholar] [PubMed]
- von Teichman, B.; Engelbrecht, A.; Zulu, G.; Dungu, B.; Pardini, A.; Bouloy, M. Safety and efficacy of Rift Valley fever Smithburn and Clone 13 vaccines in calves. Vaccine 2011, 29, 5771–5777. [Google Scholar] [CrossRef]
- Anthony, T.; van Schalkwyk, A.; Romito, M.; Odendaal, L.; Clift, S.J.; Davis, A.S. Vaccination with Rift Valley fever virus live attenuated vaccine strain Smithburn caused meningoencephalitis in alpacas. J. Vet. Diagn. Investig. 2021, 33, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Botros, B.; Omar, A.; Elian, K.; Mohamed, G.; Soliman, A.; Salib, A.; Salman, D.; Saad, M.; Earhart, K. Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J. Med. Virol. 2006, 78, 787–791. [Google Scholar] [CrossRef]
- Kamal, S.A. Pathological studies on postvaccinal reactions of Rift Valley fever in goats. Virol. J. 2009, 6, 94. [Google Scholar] [CrossRef]
- Dungu, B.; Lubisi, B.A.; Ikegami, T. Rift Valley fever vaccines: Current and future needs. Curr. Opin. Virol. 2018, 29, 8–15. [Google Scholar] [CrossRef]
- Grobbelaar, A.A.; Weyer, J.; Leman, P.A.; Kemp, A.; Paweska, J.T.; Swanepoel, R. Molecular epidemiology of Rift Valley fever virus. Emerg. Infect. Dis. 2011, 17, 2270–2276. [Google Scholar] [CrossRef]
- Vialat, P.; Muller, R.; Vu, T.H.; Prehaud, C.; Bouloy, M. Mapping of the mutations present in the genome of the Rift Valley fever virus attenuated MP12 strain and their putative role in attenuation. Virus Res. 1997, 52, 43–50. [Google Scholar] [CrossRef]
- Morrill, J.C.; Carpenter, L.; Taylor, D.; Ramsburg, H.H.; Quance, J.; Peters, C.J. Further evaluation of a mutagen-attenuated Rift Valley fever vaccine in sheep. Vaccine 1991, 9, 35–41. [Google Scholar] [CrossRef]
- Wilson, W.C.; Bawa, B.; Drolet, B.S.; Lehiy, C.; Faburay, B.; Jasperson, D.C.; Reister, L.; Gaudreault, N.N.; Carlson, J.; Ma, W. Evaluation of lamb and calf responses to Rift Valley fever MP-12 vaccination. Vet. Microbiol. 2014, 172, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.M.; Bennett, K.E.; Drolet, B.S.; Lindsay, R.; Mecham, J.O.; Reeves, W.K.; Weingartl, H.M.; Wilson, W.C. Evaluation of the Efficacy, Potential for Vector Transmission, and Duration of Immunity of MP-12, an Attenuated Rift Valley Fever Virus Vaccine Candidate, in Sheep. Clin. Vaccine Immunol. 2015, 22, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Morrill, J.C.; Mebus, C.A.; Peters, C.J. Safety and efficacy of a mutagen-attenuated Rift Valley fever virus vaccine in cattle. Am. J. Vet. Res. 1997, 58, 1104–1109. [Google Scholar] [PubMed]
- Morrill, J.C.; Peters, C.J. Pathogenicity and neurovirulence of a mutagen-attenuated Rift Valley fever vaccine in rhesus monkeys. Vaccine 2003, 21, 2994–3002. [Google Scholar] [CrossRef]
- Pittman, P.R.; Norris, S.L.; Brown, E.S.; Ranadive, M.V.; Schibly, B.A.; Bettinger, G.E.; Lokugamage, N.; Korman, L.; Morrill, J.C.; Peters, C.J. Rift Valley fever MP-12 vaccine Phase 2 clinical trial: Safety, immunogenicity, and genetic characterization of virus isolates. Vaccine 2016, 34, 523–530. [Google Scholar] [CrossRef]
- Hunter, P.; Erasmus, B.J.; Vorster, J.H. Teratogenicity of a mutagenised Rift Valley fever virus (MVP 12) in sheep. Onderstepoort J. Vet. Res. 2002, 69, 95–98. [Google Scholar] [PubMed]
- Morrill, J.C.; Peters, C.J. Mucosal immunization of rhesus macaques with Rift Valley Fever MP-12 vaccine. J. Infect. Dis. 2011, 204, 617–625. [Google Scholar] [CrossRef]
- Morrill, J.C.; Peters, C.J. Protection of MP-12-vaccinated rhesus macaques against parenteral and aerosol challenge with virulent rift valley fever virus. J. Infect. Dis. 2011, 204, 229–236. [Google Scholar] [CrossRef]
- Randall, R.; Gibbs, C.J., Jr.; Aulisio, C.G.; Binn, L.N.; Harrison, V.R. The development of a formalin-killed Rift Valley fever virus vaccine for use in man. J. Immunol. 1962, 89, 660–671. [Google Scholar]
- Randall, R.; Binn, L.N.; Harrison, V.R. Immunization against rift valley fever virus: Studies on the immunogenicity of lyophilized formalin-inactivated vaccine. J. Immunol. 1964, 93, 293–299. [Google Scholar]
- Niklasson, B.; Peters, C.J.; Bengtsson, E.; Norrby, E. Rift Valley fever virus vaccine trial: Study of neutralizing antibody response in humans. Vaccine 1985, 3, 123–127. [Google Scholar] [CrossRef]
- Meadors, G.F., 3rd; Gibbs, P.H.; Peters, C.J. Evaluation of a new Rift Valley fever vaccine: Safety and immunogenicity trials. Vaccine 1986, 4, 179–184. [Google Scholar] [CrossRef]
- Kark, J.D.; Aynor, Y.; Peters, C.J. A rift Valley fever vaccine trial. I. Side effects and serologic response over a six-month follow-up. Am. J. Epidemiol. 1982, 116, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Kark, J.D.; Aynor, Y.; Peters, C.J. A Rift Valley fever vaccine trial: 2. Serological response to booster doses with a comparison of intradermal versus subcutaneous injection. Vaccine 1985, 3, 117–122. [Google Scholar] [CrossRef]
- Pittman, P.R.; Liu, C.T.; Cannon, T.L.; Makuch, R.S.; Mangiafico, J.A.; Gibbs, P.H.; Peters, C.J. Immunogenicity of an inactivated Rift Valley fever vaccine in humans: A 12-year experience. Vaccine 1999, 18, 181–189. [Google Scholar] [CrossRef]
- Muller, R.; Saluzzo, J.-F.; Lopez, N.; Dreier, T.; Turell, M.; Smith, J.; Bouloy, M. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am. J. Trop. Med. Hyg. 1995, 53, 405–411. [Google Scholar] [CrossRef]
- Dungu, B.; Louw, I.; Lubisi, A.; Hunter, P.; von Teichman, B.F.; Bouloy, M. Evaluation of the efficacy and safety of the Rift Valley Fever Clone 13 vaccine in sheep. Vaccine 2010, 28, 4581–4587. [Google Scholar] [CrossRef]
- Lo, M.M.; Mbao, V.; Sierra, P.; Thiongane, Y.; Diop, M.; Donadeu, M.; Dungu, B. Safety and immunogenicity of Onderstepoort Biological Products’ Rift Valley fever Clone 13 vaccine in sheep and goats under field conditions in Senegal. Onderstepoort J. Vet. Res. 2015, 82, 857. [Google Scholar] [CrossRef]
- Njenga, M.K.; Njagi, L.; Thumbi, S.M.; Kahariri, S.; Githinji, J.; Omondi, E.; Baden, A.; Murithi, M.; Paweska, J.; Ithondeka, P.M.; et al. Randomized controlled field trial to assess the immunogenicity and safety of rift valley fever clone 13 vaccine in livestock. PLoS Negl. Trop. Dis. 2015, 9, e0003550. [Google Scholar] [CrossRef]
- Sindato, C.; Karimuribo, E.D.; Swai, E.S.; Mboera, L.E.G.; Rweyemamu, M.M.; Paweska, J.T.; Salt, J. Safety, Immunogenicity and Antibody Persistence of Rift Valley Fever Virus Clone 13 Vaccine in Sheep, Goats and Cattle in Tanzania. Front. Vet. Sci. 2021, 8, 779858. [Google Scholar] [CrossRef]
- Daouam, S.; Ghzal, F.; Naouli, Y.; Tadlaoui, K.O.; Ennaji, M.M.; Oura, C.; El Harrak, M. Safety and immunogenecity of a live attenuated Rift Valley fever vaccine (CL13T) in camels. BMC Vet. Res. 2016, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Daouam, S.; Boumart, Z.; Elarkam, A.; Hamdi, J.; Tadlaoui, K.O.; Ennaji, M.M.; Harraka, M. Comparative thermo-stability of two Rift Valley fever virus vaccine candidate CL13T with a recombinant arMP-12ΔNSm21/384. Bioinformation 2020, 16, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Makoschey, B.; van Kilsdonk, E.; Hubers, W.R.; Vrijenhoek, M.P.; Smit, M.; Wichgers Schreur, P.J.; Kortekaas, J.; Moulin, V. Rift Valley Fever Vaccine Virus Clone 13 Is Able to Cross the Ovine Placental Barrier Associated with Foetal Infections, Malformations, and Stillbirths. PLoS Negl. Trop. Dis. 2016, 10, e0004550. [Google Scholar] [CrossRef] [PubMed]
- Boumart, Z.; Bamouh, Z.; Hamdi, J.; Safini, N.; Tadlaoui, K.O.; Bettinger, G.; Watts, D.M.; Elharrak, M. Safety and immunogenicity of the Rift Valley fever arMP-12 ΔNSm21/384 candidate vaccine in pregnant ewes. Vaccine X 2020, 6, 100070. [Google Scholar] [CrossRef]
- Morrill, J.C.; Laughlin, R.C.; Lokugamage, N.; Wu, J.; Pugh, R.; Kanani, P.; Adams, L.G.; Makino, S.; Peters, C.J. Immunogenicity of a recombinant Rift Valley fever MP-12-NSm deletion vaccine candidate in calves. Vaccine 2013, 31, 4988–4994. [Google Scholar] [CrossRef]
- Morrill, J.C.; Laughlin, R.C.; Lokugamage, N.; Pugh, R.; Sbrana, E.; Weise, W.J.; Adams, L.G.; Makino, S.; Peters, C.J. Safety and immunogenicity of recombinant Rift Valley fever MP-12 vaccine candidates in sheep. Vaccine 2013, 31, 559–565. [Google Scholar] [CrossRef]
- Weingartl, H.M.; Nfon, C.K.; Zhang, S.; Marszal, P.; Wilson, W.C.; Morrill, J.C.; Bettinger, G.E.; Peters, C.J. Efficacy of a recombinant Rift Valley fever virus MP-12 with NSm deletion as a vaccine candidate in sheep. Vaccine 2014, 32, 2345–2349. [Google Scholar] [CrossRef]
- Nyundo, S.; Adamson, E.; Rowland, J.; Palermo, P.M.; Matiko, M.; Bettinger, G.E.; Wambura, P.; Morrill, J.C.; Watts, D.M. Safety and immunogenicity of Rift Valley fever MP-12 and arMP-12ΔNSm21/384 vaccine candidates in goats (Capra aegagrus hircus) from Tanzania. Onderstepoort J. Vet. Res. 2019, 86, e1–e8. [Google Scholar] [CrossRef]
- Boumart, Z.; Daouam, S.; Bamouh, Z.; Jazouli, M.; Tadlaoui, K.O.; Dungu, B.; Bettinger, G.; Watts, D.M.; Elharrak, M. Safety and immunogenicity of a live attenuated Rift Valley Fever recombinant arMP-12ΔNSm21/384 vaccine candidate for sheep, goats and calves. Vaccine 2019, 37, 1642–1650. [Google Scholar] [CrossRef]
- Gowen, B.B.; Westover, J.B.; Sefing, E.J.; Bailey, K.W.; Nishiyama, S.; Wandersee, L.; Scharton, D.; Jung, K.H.; Ikegami, T. MP-12 virus containing the clone 13 deletion in the NSs gene prevents lethal disease when administered after Rift Valley fever virus infection in hamsters. Front. Microbiol. 2015, 6, 651. [Google Scholar] [CrossRef][Green Version]
- Ly, H.J.; Nishiyama, S.; Lokugamage, N.; Smith, J.K.; Zhang, L.; Perez, D.; Juelich, T.L.; Freiberg, A.N.; Ikegami, T. Attenuation and protective efficacy of Rift Valley fever phlebovirus rMP12-GM50 strain. Vaccine 2017, 35, 6634–6642. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, J.; Oreshkova, N.; van Keulen, L.; Kant, J.; Bosch, B.J.; Bouloy, M.; Moulin, V.; Goovaerts, D.; Moormann, R.J. Comparative efficacy of two next-generation Rift Valley fever vaccines. Vaccine 2014, 32, 4901–4908. [Google Scholar] [CrossRef] [PubMed]
- Bird, B.H.; Albariño, C.G.; Hartman, A.L.; Erickson, B.R.; Ksiazek, T.G.; Nichol, S.T. Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J. Virol. 2008, 82, 2681–2691. [Google Scholar] [CrossRef]
- Bird, B.H.; Maartens, L.H.; Campbell, S.; Erasmus, B.J.; Erickson, B.R.; Dodd, K.A.; Spiropoulou, C.F.; Cannon, D.; Drew, C.P.; Knust, B.; et al. Rift Valley fever virus vaccine lacking the NSs and NSm genes is safe, nonteratogenic, and confers protection from viremia, pyrexia, and abortion following challenge in adult and pregnant sheep. J. Virol. 2011, 85, 12901–12909. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Johnston, S.C.; Piper, A.; Botto, M.; Donnelly, G.; Shamblin, J.; Albariño, C.G.; Hensley, L.E.; Schmaljohn, C.; Nichol, S.T.; et al. Attenuation and efficacy of live-attenuated Rift Valley fever virus vaccine candidates in non-human primates. PLoS Negl. Trop. Dis. 2018, 12, e0006474. [Google Scholar] [CrossRef]
- Campbell, C.L.; Snell, T.K.; Bennett, S.; Wyckoff, J.H., 3rd; Heaslip, D.; Flatt, J.; Harris, E.K.; Hartman, D.A.; Lian, E.; Bird, B.H.; et al. Safety study of Rift Valley Fever human vaccine candidate (DDVax) in mosquitoes. Transbound. Emerg. Dis. 2021, 69, 2621–2633. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Oreshkova, N.; Moormann, R.J.; Kortekaas, J. Creation of Rift Valley fever viruses with four-segmented genomes reveals flexibility in bunyavirus genome packaging. J. Virol. 2014, 88, 10883–10893. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Kant, J.; van Keulen, L.; Moormann, R.J.; Kortekaas, J. Four-segmented Rift Valley fever virus induces sterile immunity in sheep after a single vaccination. Vaccine 2015, 33, 1459–1464. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; van Keulen, L.; Kant, J.; Kortekaas, J. Four-segmented Rift Valley fever virus-based vaccines can be applied safely in ewes during pregnancy. Vaccine 2017, 35, 3123–3128. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Oreshkova, N.; van Keulen, L.; Kant, J.; van de Water, S.; Soós, P.; Dehon, Y.; Kollár, A.; Pénzes, Z.; Kortekaas, J. Safety and efficacy of four-segmented Rift Valley fever virus in young sheep, goats and cattle. NPJ Vaccines 2020, 5, 65. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Oymans, J.; Kant, J.; van de Water, S.; Kollár, A.; Dehon, Y.; Soós, P.; Pénzes, Z.; van Keulen, L.; Kortekaas, J. A single vaccination with four-segmented rift valley fever virus prevents vertical transmission of the wild-type virus in pregnant ewes. NPJ Vaccines 2021, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Spik, K.; Shurtleff, A.; McElroy, A.K.; Guttieri, M.C.; Hooper, J.W.; SchmalJohn, C. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine 2006, 24, 4657–4666. [Google Scholar] [CrossRef] [PubMed]
- Lagerqvist, N.; Näslund, J.; Lundkvist, A.; Bouloy, M.; Ahlm, C.; Bucht, G. Characterisation of immune responses and protective efficacy in mice after immunisation with Rift Valley Fever virus cDNA constructs. Virol. J. 2009, 6, 6. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Heise, M.T.; Ross, T.M. Vaccination with DNA plasmids expressing Gn coupled to C3d or alphavirus replicons expressing Gn protects mice against Rift Valley fever virus. PLoS Negl. Trop. Dis. 2010, 4, e725. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, G.; Martín-Folgar, R.; Hevia, E.; Boshra, H.; Brun, A. Protection against lethal Rift Valley fever virus (RVFV) infection in transgenic IFNAR(−/−) mice induced by different DNA vaccination regimens. Vaccine 2010, 28, 2937–2944. [Google Scholar] [CrossRef]
- Lorenzo, G.; López-Gil, E.; Ortego, J.; Brun, A. Efficacy of different DNA and MVA prime-boost vaccination regimens against a Rift Valley fever virus (RVFV) challenge in sheep 12 weeks following vaccination. Vet. Res. 2018, 49, 21. [Google Scholar] [CrossRef]
- Chrun, T.; Lacôte, S.; Urien, C.; Jouneau, L.; Barc, C.; Bouguyon, E.; Contreras, V.; Ferrier-Rembert, A.; Peyrefitte, C.N.; Busquets, N.; et al. A Rift Valley fever virus Gn ectodomain-based DNA vaccine induces a partial protection not improved by APC targeting. NPJ Vaccines 2018, 3, 14. [Google Scholar] [CrossRef]
- Gonzalez-Valdivieso, J.; Borrego, B.; Girotti, A.; Moreno, S.; Brun, A.; Bermejo-Martin, J.F.; Arias, F.J. A DNA Vaccine Delivery Platform Based on Elastin-Like Recombinamer Nanosystems for Rift Valley Fever Virus. Mol. Pharm. 2020, 17, 1608–1620. [Google Scholar] [CrossRef]
- Selina, O.; Imatdinov, I.; Balysheva, V.; Akasov, R.; Kryukov, A.; Balyshev, V.; Markvicheva, E. Microencapsulated plasmids expressing Gn and Gc glycoproteins of Rift Valley Fever virus enhance humoral immune response in mice. Biotechnol. Lett. 2020, 42, 529–536. [Google Scholar] [CrossRef]
- Wallace, D.B.; Ellis, C.E.; Espach, A.; Smith, S.J.; Greyling, R.R.; Viljoen, G.J. Protective immune responses induced by different recombinant vaccine regimes to Rift Valley fever. Vaccine 2006, 24, 7181–7189. [Google Scholar] [CrossRef]
- Wallace, D.B.; Mather, A.; Kara, P.D.; Naicker, L.; Mokoena, N.B.; Pretorius, A.; Nefefe, T.; Thema, N.; Babiuk, S. Protection of Cattle Elicited Using a Bivalent Lumpy Skin Disease Virus-Vectored Recombinant Rift Valley Fever Vaccine. Front. Vet. Sci. 2020, 7, 256. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.H.; Penn-Nicholson, A.; Wang, D.; Woraratanadharm, J.; Harr, M.K.; Luo, M.; Maher, E.M.; Holbrook, M.R.; Dong, J.Y. A complex adenovirus-vectored vaccine against Rift Valley fever virus protects mice against lethal infection in the presence of preexisting vector immunity. Clin. Vaccine Immunol. 2009, 16, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, J.; de Boer, S.M.; Kant, J.; Vloet, R.P.; Antonis, A.F.; Moormann, R.J. Rift Valley fever virus immunity provided by a paramyxovirus vaccine vector. Vaccine 2010, 28, 4394–4401. [Google Scholar] [CrossRef] [PubMed]
- Papin, J.F.; Verardi, P.H.; Jones, L.A.; Monge-Navarro, F.; Brault, A.C.; Holbrook, M.R.; Worthy, M.N.; Freiberg, A.N.; Yilma, T.D. Recombinant Rift Valley fever vaccines induce protective levels of antibody in baboons and resistance to lethal challenge in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 14926–14931. [Google Scholar] [CrossRef]
- Warimwe, G.M.; Lorenzo, G.; Lopez-Gil, E.; Reyes-Sandoval, A.; Cottingham, M.G.; Spencer, A.J.; Collins, K.A.; Dicks, M.D.; Milicic, A.; Lall, A.; et al. Immunogenicity and efficacy of a chimpanzee adenovirus-vectored Rift Valley fever vaccine in mice. Virol. J. 2013, 10, 349. [Google Scholar] [CrossRef]
- Warimwe, G.M.; Gesharisha, J.; Carr, B.V.; Otieno, S.; Otingah, K.; Wright, D.; Charleston, B.; Okoth, E.; Elena, L.G.; Lorenzo, G.; et al. Chimpanzee Adenovirus Vaccine Provides Multispecies Protection against Rift Valley Fever. Sci. Rep. 2016, 6, 20617. [Google Scholar] [CrossRef]
- Stedman, A.; Wright, D.; Wichgers Schreur, P.J.; Clark, M.H.A.; Hill, A.V.S.; Gilbert, S.C.; Francis, M.J.; van Keulen, L.; Kortekaas, J.; Charleston, B.; et al. Safety and efficacy of ChAdOx1 RVF vaccine against Rift Valley fever in pregnant sheep and goats. NPJ Vaccines 2019, 4, 44. [Google Scholar] [CrossRef]
- Dulal, P.; Wright, D.; Ashfield, R.; Hill, A.V.; Charleston, B.; Warimwe, G.M. Potency of a thermostabilised chimpanzee adenovirus Rift Valley Fever vaccine in cattle. Vaccine 2016, 34, 2296–2298. [Google Scholar] [CrossRef]
- López-Gil, E.; Lorenzo, G.; Hevia, E.; Borrego, B.; Eiden, M.; Groschup, M.; Gilbert, S.C.; Brun, A. A single immunization with MVA expressing GnGc glycoproteins promotes epitope-specific CD8+-T cell activation and protects immune-competent mice against a lethal RVFV infection. PLoS Negl. Trop. Dis. 2013, 7, e2309. [Google Scholar] [CrossRef]
- Busquets, N.; Lorenzo, G.; López-Gil, E.; Rivas, R.; Solanes, D.; Galindo-Cardiel, I.; Abad, F.X.; Rodríguez, F.; Bensaid, A.; Warimwe, G.; et al. Efficacy assessment of an MVA vectored Rift Valley Fever vaccine in lambs. Antivir. Res. 2014, 108, 165–172. [Google Scholar] [CrossRef]
- López-Gil, E.; Moreno, S.; Ortego, J.; Borrego, B.; Lorenzo, G.; Brun, A. MVA Vectored Vaccines Encoding Rift Valley Fever Virus Glycoproteins Protect Mice against Lethal Challenge in the Absence of Neutralizing Antibody Responses. Vaccines 2020, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Pinilla, E.; Marín-López, A.; Moreno, S.; Lorenzo, G.; Utrilla-Trigo, S.; Jiménez-Cabello, L.; Benavides, J.; Nogales, A.; Blasco, R.; Brun, A.; et al. A protective bivalent vaccine against Rift Valley fever and bluetongue. NPJ Vaccines 2020, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Said, A.; Elmanzalawy, M.; Ma, G.; Damiani, A.M.; Osterrieder, N. An equine herpesvirus type 1 (EHV-1) vector expressing Rift Valley fever virus (RVFV) Gn and Gc induces neutralizing antibodies in sheep. Virol. J. 2017, 14, 154. [Google Scholar] [CrossRef]
- Soi, R.K.; Rurangirwa, F.R.; McGuire, T.C.; Rwambo, P.M.; DeMartini, J.C.; Crawford, T.B. Protection of sheep against Rift Valley fever virus and sheep poxvirus with a recombinant capripoxvirus vaccine. Clin. Vaccine Immunol. 2010, 17, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hao, M.; Feng, N.; Jin, H.; Yan, F.; Chi, H.; Wang, H.; Han, Q.; Wang, J.; Wong, G.; et al. Genetically Modified Rabies Virus Vector-Based Rift Valley Fever Virus Vaccine is Safe and Induces Efficacious Immune Responses in Mice. Viruses 2019, 11, 919. [Google Scholar] [CrossRef]
- Kortekaas, J.; Antonis, A.F.; Kant, J.; Vloet, R.P.; Vogel, A.; Oreshkova, N.; de Boer, S.M.; Bosch, B.J.; Moormann, R.J. Efficacy of three candidate Rift Valley fever vaccines in sheep. Vaccine 2012, 30, 3423–3429. [Google Scholar] [CrossRef]
- Faburay, B.; Lebedev, M.; McVey, D.S.; Wilson, W.; Morozov, I.; Young, A.; Richt, J.A. A glycoprotein subunit vaccine elicits a strong Rift Valley fever virus neutralizing antibody response in sheep. Vector-BorneZoonotic Dis. 2014, 14, 746–756. [Google Scholar] [CrossRef]
- Faburay, B.; Wilson, W.C.; Gaudreault, N.N.; Davis, A.S.; Shivanna, V.; Bawa, B.; Sunwoo, S.Y.; Ma, W.; Drolet, B.S.; Morozov, I.; et al. A Recombinant Rift Valley Fever Virus Glycoprotein Subunit Vaccine Confers Full Protection against Rift Valley Fever Challenge in Sheep. Sci. Rep. 2016, 6, 27719. [Google Scholar] [CrossRef]
- Wilson, W.C.; Faburay, B.; Trujillo, J.D.; Ragan, I.; Sunwoo, S.Y.; Morozov, I.; Shivanna, V.; Balogh, A.; Urbaniak, K.; McVey, D.S.; et al. Preliminary Evaluation of a Recombinant Rift Valley Fever Virus Glycoprotein Subunit Vaccine Providing Full Protection against Heterologous Virulent Challenge in Cattle. Vaccines 2021, 9, 748. [Google Scholar] [CrossRef]
- Wichgers Schreur, P.J.; Tacken, M.; Gutjahr, B.; Keller, M.; van Keulen, L.; Kant, J.; van de Water, S.; Lin, Y.; Eiden, M.; Rissmann, M.; et al. Vaccine Efficacy of Self-Assembled Multimeric Protein Scaffold Particles Displaying the Glycoprotein Gn Head Domain of Rift Valley Fever Virus. Vaccines 2021, 9, 301. [Google Scholar] [CrossRef]
- Heise, M.T.; Whitmore, A.; Thompson, J.; Parsons, M.; Grobbelaar, A.A.; Kemp, A.; Paweska, J.T.; Madric, K.; White, L.J.; Swanepoel, R.; et al. An alphavirus replicon-derived candidate vaccine against Rift Valley fever virus. Epidemiol. Infect. 2009, 137, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Gorchakov, R.; Volkova, E.; Yun, N.; Petrakova, O.; Linde, N.S.; Paessler, S.; Frolova, E.; Frolov, I. Comparative analysis of the alphavirus-based vectors expressing Rift Valley fever virus glycoproteins. Virology 2007, 366, 212–225. [Google Scholar] [CrossRef][Green Version]
- Oreshkova, N.; van Keulen, L.; Kant, J.; Moormann, R.J.; Kortekaas, J. A single vaccination with an improved nonspreading Rift Valley fever virus vaccine provides sterile immunity in lambs. PLoS ONE 2013, 8, e77461. [Google Scholar] [CrossRef]
- Dodd, K.A.; Bird, B.H.; Metcalfe, M.G.; Nichol, S.T.; Albariño, C.G. Single-dose immunization with virus replicon particles confers rapid robust protection against Rift Valley fever virus challenge. J. Virol. 2012, 86, 4204–4212. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Terasaki, K.; Ramirez, S.I.; Morrill, J.C.; Makino, S. Development of a novel, single-cycle replicable rift valley Fever vaccine. PLoS Negl. Trop. Dis. 2014, 8, e2746. [Google Scholar] [CrossRef]
- Terasaki, K.; Juelich, T.L.; Smith, J.K.; Kalveram, B.; Perez, D.D.; Freiberg, A.N.; Makino, S. A single-cycle replicable Rift Valley fever phlebovirus vaccine carrying a mutated NSs confers full protection from lethal challenge in mice. Sci. Rep. 2018, 8, 17097. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, J.; Oreshkova, N.; Cobos-Jiménez, V.; Vloet, R.P.; Potgieter, C.A.; Moormann, R.J. Creation of a nonspreading Rift Valley fever virus. J. Virol. 2011, 85, 12622–12630. [Google Scholar] [CrossRef] [PubMed]
- Näslund, J.; Lagerqvist, N.; Habjan, M.; Lundkvist, A.; Evander, M.; Ahlm, C.; Weber, F.; Bucht, G. Vaccination with virus-like particles protects mice from lethal infection of Rift Valley Fever Virus. Virology 2009, 385, 409–415. [Google Scholar] [CrossRef]
- Mandell, R.B.; Koukuntla, R.; Mogler, L.J.; Carzoli, A.K.; Freiberg, A.N.; Holbrook, M.R.; Martin, B.K.; Staplin, W.R.; Vahanian, N.N.; Link, C.J.; et al. A replication-incompetent Rift Valley fever vaccine: Chimeric virus-like particles protect mice and rats against lethal challenge. Virology 2010, 397, 187–198. [Google Scholar] [CrossRef]
- Li, Y.; Han, L.; Zhao, Y.; Zheng, X.; Wang, H.; Gai, W.; Jin, H.; Li, G.; Wang, Q.; Feng, N.; et al. Immunogenicity Assessment of Rift Valley Fever Virus Virus-Like Particles in BALB/c Mice. Front. Vet. Sci. 2020, 7, 62. [Google Scholar] [CrossRef]
- Pittman, P.R.; McClain, D.; Quinn, X.; Coonan, K.M.; Mangiafico, J.; Makuch, R.S.; Morrill, J.; Peters, C.J. Safety and immunogenicity of a mutagenized, live attenuated Rift Valley fever vaccine, MP-12, in a Phase 1 dose escalation and route comparison study in humans. Vaccine 2016, 34, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Gowen, B.B.; Bailey, K.W.; Scharton, D.; Vest, Z.; Westover, J.B.; Skirpstunas, R.; Ikegami, T. Post-exposure vaccination with MP-12 lacking NSs protects mice against lethal Rift Valley fever virus challenge. Antivir. Res. 2013, 98, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, W.N.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Walker, V.; Denholm, R.; Akbari, A.; Omigie, E.; Hollings, S.; et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med. 2022, 19, e1003926. [Google Scholar] [CrossRef]
- Makris, M.; Pavord, S. Most cases of Thrombosis and Thrombocytopenia Syndrome (TTS) post ChAdOx-1 nCov-19 are Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT). Lancet Reg. Health Eur. 2022, 12, 100274. [Google Scholar] [CrossRef] [PubMed]
- Frolov, I.; Hoffman, T.A.; Prágai, B.M.; Dryga, S.A.; Huang, H.V.; Schlesinger, S.; Rice, C.M. Alphavirus-based expression vectors: Strategies and applications. Proc. Natl. Acad. Sci. USA 1996, 93, 11371–11377. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Wang, J.G.; Davis, N.L.; Johnston, R.E. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: Effect of an attenuating mutation in the 5′ untranslated region. J. Virol. 2001, 75, 3706–3718. [Google Scholar] [CrossRef]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- de Boer, S.M.; Kortekaas, J.; Antonis, A.F.; Kant, J.; van Oploo, J.L.; Rottier, P.J.; Moormann, R.J.; Bosch, B.J. Rift Valley fever virus subunit vaccines confer complete protection against a lethal virus challenge. Vaccine 2010, 28, 2330–2339. [Google Scholar] [CrossRef]
- Kortekaas, J. One Health approach to Rift Valley fever vaccine development. Antivir. Res. 2014, 106, 24–32. [Google Scholar] [CrossRef]
- Petrova, V.; Kristiansen, P.; Norheim, G.; Yimer, S.A. Rift valley fever: Diagnostic challenges and investment needs for vaccine development. BMJ Glob. Health 2020, 5, e002694. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Moderna applies for US and EU approval as vaccine trial reports 94.1% efficacy. BMJ 2020, 371, m4709. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene 2021, 28, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kaseke, N.; Hosking, S.G. Sub-Saharan Africa electricity supply inadequacy: Implications. East. Afr. Soc. Sci. Res. Rev. 2013, 29, 113–132. [Google Scholar] [CrossRef]
- Stitz, L.; Vogel, A.; Schnee, M.; Voss, D.; Rauch, S.; Mutzke, T.; Ketterer, T.; Kramps, T.; Petsch, B. A thermostable messenger RNA based vaccine against rabies. PLoS Negl. Trop. Dis. 2017, 11, e0006108. [Google Scholar] [CrossRef]
| Vaccine Type | Vaccine Name | Vaccine Design | References |
|---|---|---|---|
| Conventional live attenuated | Smithburn | Neurotropic Smithburn Rift Valley fever virus (RVFV) strain attenuated through 102 serial passages in mouse brain | [44,45,46,47,48,49,50] |
| MP-12 | RVFV ZH548 strain plaque passaged 12 times in human fetal lung fibroblast cells (MRC-5) in the presence of the mutagen 5 fluorouracil | [51,52,53,54,55,56,57,58,59,60,61] | |
| Inactivated | NDBR103 | Formalin-inactivated RVFV Entebbe strain cultured in monkey kidney cells | [62,63,64] |
| TSI-GSD-200 | Formalin-inactivated RVFV Entebbe strain cultured in diploid fetal rhesus lung cells | [34,65,66,67,68] | |
| Genetically modified live attenuated | Clone 13 | Plaque purified naturally mutated RVFV 74HB59 strain having a 69% deletion in the nonstructural protein S (NSs) gene | [69,70,71,72,73,74,75,76] |
| arMP-12ΔNSm21/384 | Recombinant MP-12 virus with deletions in the NSm gene | [77,78,79,80,81,82,83] | |
| rMP-12-GM50 | Recombinant MP-12 virus with a total of 584 silent mutations in all three RVFV genome segments | [84] | |
| R566 | A recombinant RVFV containing the small (S) segment of Clone 13 and the Large (L) and medium (M) segments of MP-12 | [85] | |
| rRVF-ΔNSs:GFP-ΔNSm, ΔNSs-ΔNSm rRVFV | A recombinant RVFV ZH501 strain lacking nonstructural protein M (NSm) and NSs genes with and without the enhanced green fluorescent protein (eGFP) marker | [86,87,88,89] | |
| RVFV 4S | A recombinant RVFV having four segments, i.e., L, S without its NSs, and M split into a glycoprotein n (Gn) and a glycoprotein c (Gc) segment | [90,91,92,93,94] | |
| DNA | RVFV + NSm DNA and RVFV-NSm DNA | DNA plasmid pWRG7077 encoding the RVFV M segment with or without the NSm gene | [95] |
| RVFV cDNA N and RVFV cDNA GnGc | pcDNA3.1/V5-His® TOPO (Invitrogen) encoding nucleoprotein N or Gn and Gc genes of RVFV | [96] | |
| Gn-cd3 DNA | DNA plasmid PTR600 expressing RVFV Gn coupled to 3 copies of the complement protein C3d as a molecular adjuvant | [97] | |
| pCMV-M4 and pCMV-N | pCMV vector (Clontech) encoding RVFV M segment (Nsm, Gn, and Gc) and N open reading frames (ORFs) | [98] | |
| pCMV-GnGc | pCMV vector (Clontech) encoding the MP-12 GnGc open reading frame starting from the fourth in-frame start codon | [99] | |
| peGn, pscDEC-eGn, and pscCD11c-eGn | pcDNA3.1 vector (Invitrogen) encoding the extracellular portion of RVFV Gn targeted to dendritic cells through fusion with a single-chain variable fragment (scFV) anti-ovine DEC205 (pscDEC-eGn) or scFV anti-ovine CD11c (pscCD11c-eGn) or untargeted (peGn) | [100] | |
| Gn-ELRs | A plasmid pCMVNSmGn encoding MP-12 NSm/Gn and various elastin-like recombinamers (ELRs) | [101] | |
| phRVF/Gn and phRVF/Gc | Biodegradable alginate (ALG)/poly-L-lysine (PLL) microcapsules entrapped with phMGFP (Promega) plasmids expressing Gn and Gc sequences of RVFV strain 1974-VNIIVViM and fusion protein F sequences of human parainfluenza virus 1 (HPIV-1) | [102] | |
| Viral vectored | rLSDV–RVFV | Recombinant lumpy skin disease virus vaccine expressing RVFV Gn and Gc | [103,104] |
| CAdVax-RVF | A nonreplicating complex adenovirus vector encoding RVFV ZH548M12 strain Gn and Gc sequences that were fused upstream with the human CD4 signal sequence | [105] | |
| NDFL-GnGC | A recombinant Newcastle disease virus LaSota strain containing a codon optimised RVFV Gn and Gc gene sequences from RVFV M35/74 strain | [106] | |
| vCOGnGc and vCOGnGcγ | Recombinant vaccinia virus attenuated by the deletion of IFN-γ binding protein gene and insertional inactivation of the thymidine kinase gene, expressing RVFV Gn and Gc from RVFV ZH548 strain with or without the human IFN-γ gene | [107] | |
| ChAdOx1-GnGC | A replication-deficient chimpanzee adenovirus vector ChAdOx1 encoding MP-12 Gn and Gc sequences | [108,109,110,111] | |
| rMVA-Gn/Gc and rMVA-N | Recombinant Modified Vaccinia Ankara (MVA) viruses encoding MP-12 protein N or Gn and Gc genes fused in-frame with human tissue plasminogen activator leader sequences at the N terminus | [99,112,113,114] | |
| MVA-GnGc-VP2, MVA-GnGc-NS1 and MVA-GnGc-NS1-Nt | Recombinant MVA expressing RVFV Gn and Gc sequences of MP-12 in addition to the Bluetongue virus (BTV) proteins VP2, NS1, or a truncated form of NS1 (NS1-Nt) | [115] | |
| rH_Gn-Gc | Equine herpesvirus type 1 strain RaCH expressing codon optimised RVFV Gn and Gc genes from the RVFV ZH501 strain | [116,117] | |
| rKS1/RVFV | A recombinant capripox virus expressing RVFV Gn and Gc glycoproteins | [117] | |
| rSRV9-eGn | An inactivated recombinant rabies virus vector rSRV9 cloned with a codon-optimised RVFV Gn ectodomain gene from MP-12 | [118] | |
| Subunit | Gne-S3 | Gn ectodomain produced using the Drosophila expression system (Invitrogen, Carlsbad, CA, USA) and formulated in Stimune water-in-oil adjuvant (Prionics, Lelystad, The Netherlands) | [119] |
| RVFV Gne and RVFV Gc | Baculovirus-expressed Gn ectodomain and Gc proteins formulated with a water-in-oil adjuvant montanide ISA25 (Seppic, France) | [120,121,122] | |
| Gn-head MPSPs | RVFV Gn head attached to multimeric protein scaffold particles (MPSP) through spontaneous isopeptide bond formation between spytag and spycatcher “bacterial superglue” to yield antigen-decorated nanoparticles | [123] | |
| Viral replicons | REP91-RVF(M) and Rgird-RVFV(M) | Alphavirus replicon vectors based on mosquito (AR86) and human (Girdwood) isolates of Sindbus virus expressing RVFV Gn, Gc, and NSm protein | [124] |
| SINRepspGn and VEEVRepspGn | Sinbus and Venezuelan equine encephalitis virus TC-83 replicon particles expressing RVFV Gn | [125] | |
| NSR-Gn | A non-spreading RVFV Gn replicon containing RVFV L segment and an S segment encoding Gn in place of the NSs gene | [126] | |
| RVF-VRP | Single-cycle RVFV replicon particles rescued from BSR-T7/5 cells transfected with RVFV L, RVFS-ΔNSm/ΔNSs:GFP, and pC-GnGc plasmids | [127] | |
| scMP12 and scMP-12-mutNS | Single-cycle replicons rescued from BSR-T7/5 cells transfected with plasmids expressing MP-12 L, N, and Gn/Gc proteins, as well as L RNA, S RNA encoding N and GFP and M RNA encoding a mutant envelope protein lacking an endoplasmic reticulum retrieval signal. For scMP-12mutNs, the S RNA encoded N and NSsR16H/M250K | [128,129] | |
| RVFV RRP | Replicons made by transfecting baby hamster kidney cell lines maintaining L and S segments (whose NSs had been replaced with GFP) with the RVFV strain 35/74 M genome segment starting at the fourth in-frame start codon | [130] | |
| Virus-like particles | Ren-VLPs | VLPs made by transfecting 293T cells with the expression plasmids p18 subcloned with L, N and M segments and a reporter plasmid containing Renilla luciferase gene (Ren-Luc) | [131] |
| RVFV chimVLPs | Chimeric VLPs containing RVFV Gn and Gc, nucleoprotein N, and the gag protein of Moloney murine leukemia virus | [132] | |
| RVFV GnGc VLP | VLPs generated through the expression of Gn and Gc in the Drosophila insect cell expression system | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitandwe, P.K.; McKay, P.F.; Kaleebu, P.; Shattock, R.J. An Overview of Rift Valley Fever Vaccine Development Strategies. Vaccines 2022, 10, 1794. https://doi.org/10.3390/vaccines10111794
Kitandwe PK, McKay PF, Kaleebu P, Shattock RJ. An Overview of Rift Valley Fever Vaccine Development Strategies. Vaccines. 2022; 10(11):1794. https://doi.org/10.3390/vaccines10111794
Chicago/Turabian StyleKitandwe, Paul Kato, Paul F. McKay, Pontiano Kaleebu, and Robin J. Shattock. 2022. "An Overview of Rift Valley Fever Vaccine Development Strategies" Vaccines 10, no. 11: 1794. https://doi.org/10.3390/vaccines10111794
APA StyleKitandwe, P. K., McKay, P. F., Kaleebu, P., & Shattock, R. J. (2022). An Overview of Rift Valley Fever Vaccine Development Strategies. Vaccines, 10(11), 1794. https://doi.org/10.3390/vaccines10111794






