Effective Functional Immunogenicity of a DNA Vaccine Combination Delivered via In Vivo Electroporation Targeting Malaria Infection and Transmission
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Vaccine Plasmids
2.2. Mammalian Cell Transfection and Western Blotting
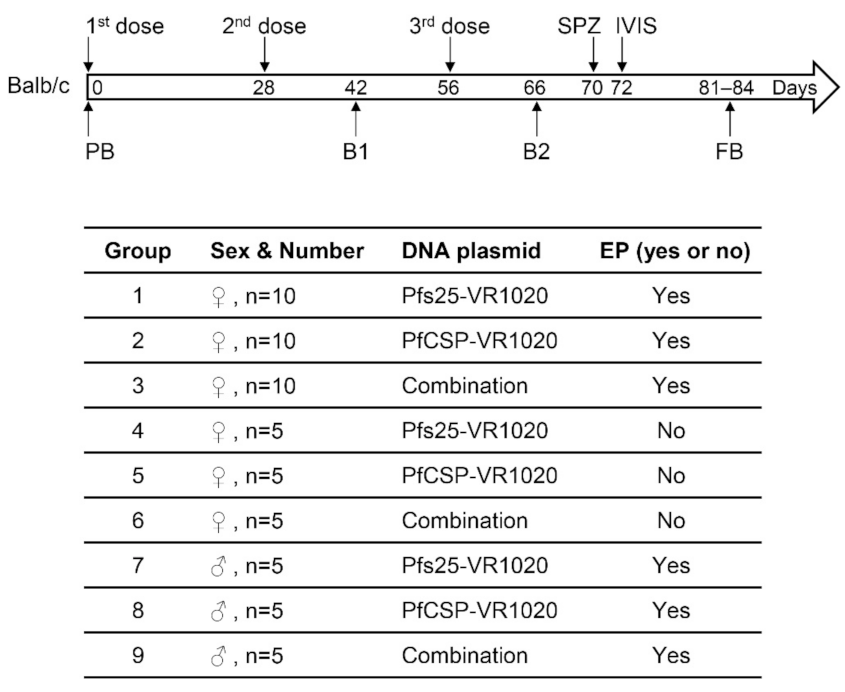
2.3. Immunization Dose and Schedule
2.4. Assessment of Antibody Titers, Isotype and Avidity by Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Standard Membrane Feeding Assays (SMFA)
2.6. Sporozoite Challenge and In Vivo Assessment of Liver-Stage Parasite Load and Blood-Stage Parasitemia
2.7. Statistical Analysis
3. Results
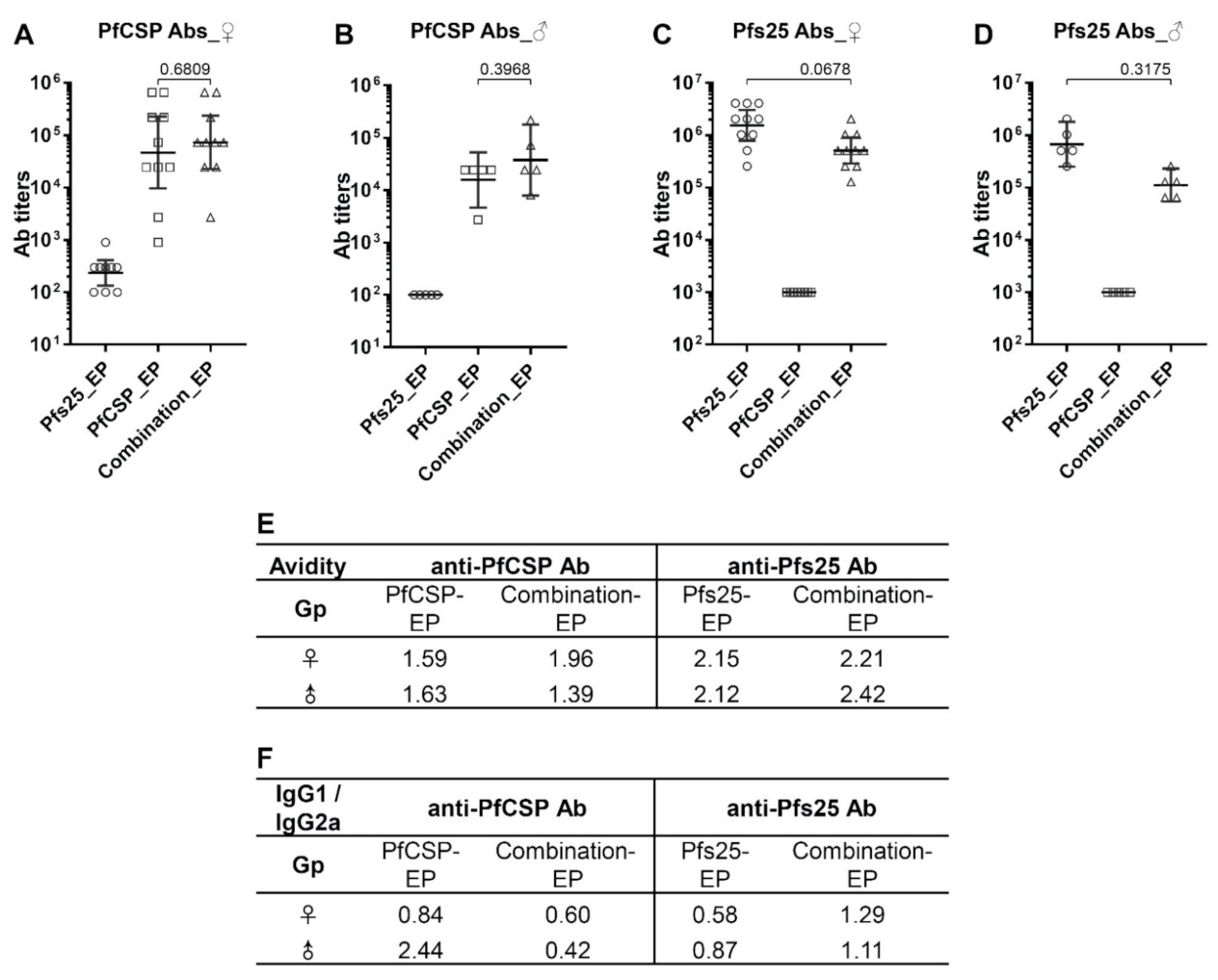
3.1. Antibody Responses in the Individual and Combined DNA Plasmid Immunization Groups
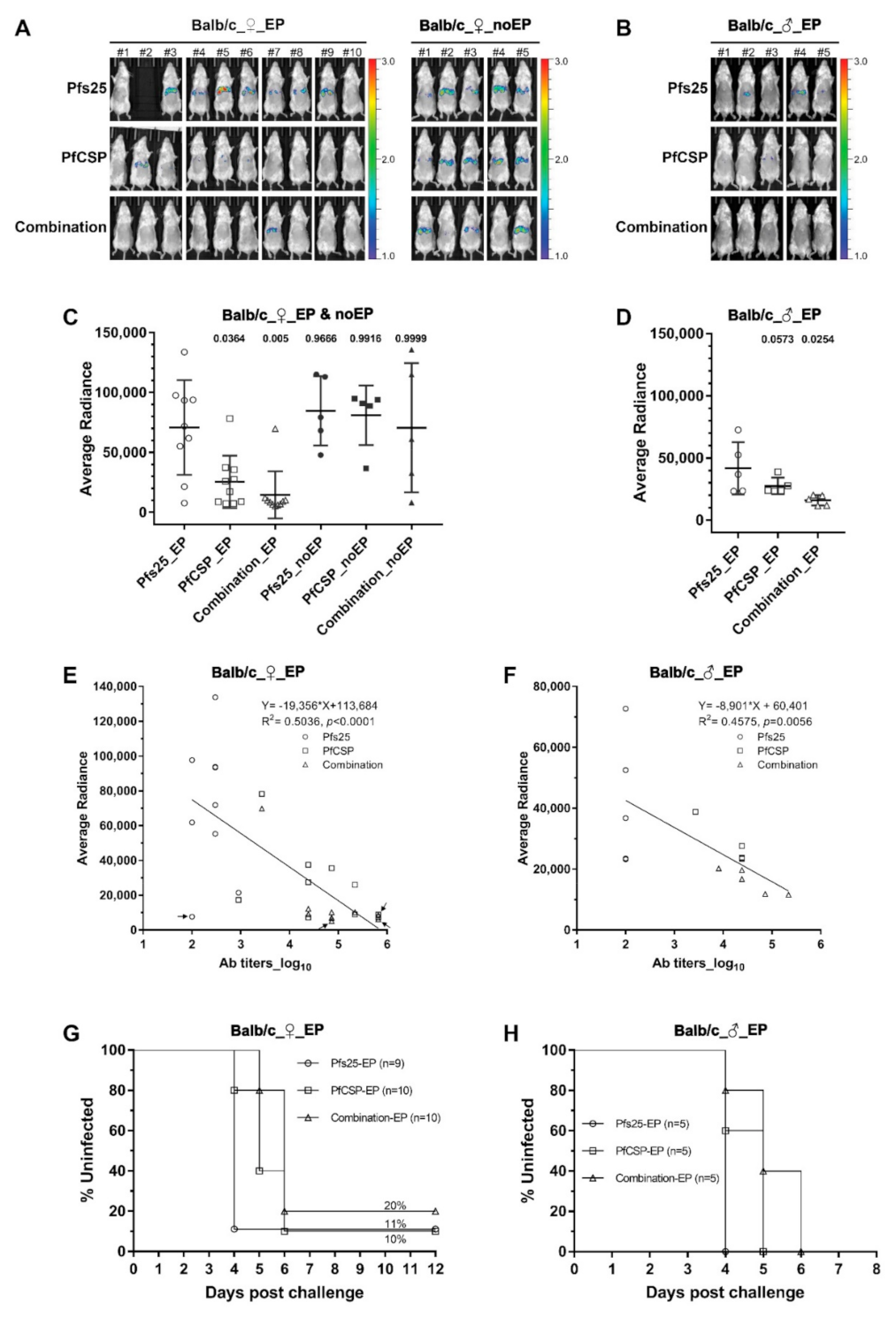
3.2. Protection against Sporozoite Challenge
3.3. Transmission Reducing Activity of Anti-Pfs25 Antibodies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- WHO. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Moorthy, V.S.; Newman, R.D.; Okwo-Bele, J.M. Malaria vaccine technology roadmap. Lancet 2013, 382, 1700–1701. [Google Scholar] [CrossRef]
- Vogel, G. WHO gives first malaria vaccine the green light. Science 2021, 374, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Laurens, M.B. RTS,S/AS01 vaccine (Mosquirix): An overview. Hum. Vaccines Immunother. 2020, 16, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Rts, S.C.T.P. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- Moris, P.; Jongert, E.; van der Most, R.G. Characterization of T-cell immune responses in clinical trials of the candidate RTS,S malaria vaccine. Hum. Vaccines Immunother. 2018, 14, 17–27. [Google Scholar] [CrossRef]
- Adepoju, P. RTS,S malaria vaccine pilots in three African countries. Lancet 2019, 393, 1685. [Google Scholar] [CrossRef]
- Duffy, P.E.; Patrick Gorres, J. Malaria vaccines since 2000: Progress, priorities, products. NPJ Vaccines 2020, 5, 48. [Google Scholar] [CrossRef]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef]
- Tachibana, M.; Takashima, E.; Morita, M.; Sattabongkot, J.; Ishino, T.; Culleton, R.; Torii, M.; Tsuboi, T. Plasmodium vivax transmission-blocking vaccines: Progress, challenges and innovation. Parasitol. Int. 2022, 87, 102525. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.E. Transmission-Blocking Vaccines: Harnessing Herd Immunity for Malaria Elimination. Expert Rev. Vaccines 2021, 20, 185–198. [Google Scholar] [CrossRef]
- Sagara, I.; Healy, S.A.; Assadou, M.H.; Gabriel, E.E.; Kone, M.; Sissoko, K.; Tembine, I.; Guindo, M.A.; Doucoure, M.; Niare, K.; et al. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: A randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect. Dis. 2018, 18, 969–982. [Google Scholar] [CrossRef]
- Shimp, R.L., Jr.; Rowe, C.; Reiter, K.; Chen, B.; Nguyen, V.; Aebig, J.; Rausch, K.M.; Kumar, K.; Wu, Y.; Jin, A.J.; et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 2013, 31, 2954–2962. [Google Scholar] [CrossRef]
- Li, Y.; Leneghan, D.B.; Miura, K.; Nikolaeva, D.; Brian, I.J.; Dicks, M.D.; Fyfe, A.J.; Zakutansky, S.E.; de Cassan, S.; Long, C.A.; et al. Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology. Sci. Rep. 2016, 6, 18848. [Google Scholar] [CrossRef] [PubMed]
- Chichester, J.A.; Green, B.J.; Jones, R.M.; Shoji, Y.; Miura, K.; Long, C.A.; Lee, C.K.; Ockenhouse, C.F.; Morin, M.J.; Streatfield, S.J.; et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A Phase 1 dose-escalation study in healthy adults. Vaccine 2018, 36, 5865–5871. [Google Scholar] [CrossRef]
- Yusuf, Y.; Yoshii, T.; Iyori, M.; Yoshida, K.; Mizukami, H.; Fukumoto, S.; Yamamoto, D.S.; Alam, A.; Emran, T.B.; Amelia, F.; et al. Adeno-Associated Virus as an Effective Malaria Booster Vaccine Following Adenovirus Priming. Front. Immunol. 2019, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Mlambo, G.; Kumar, N.; Yoshida, S. Functional immunogenicity of baculovirus expressing Pfs25, a human malaria transmission-blocking vaccine candidate antigen. Vaccine 2010, 28, 7025–7029. [Google Scholar] [CrossRef]
- Thompson, E.A.; Ols, S.; Miura, K.; Rausch, K.; Narum, D.L.; Spangberg, M.; Juraska, M.; Wille-Reece, U.; Weiner, A.; Howard, R.F.; et al. TLR-adjuvanted nanoparticle vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight 2018, 3, e120692. [Google Scholar] [CrossRef]
- Sherrard-Smith, E.; Sala, K.A.; Betancourt, M.; Upton, L.M.; Angrisano, F.; Morin, M.J.; Ghani, A.C.; Churcher, T.S.; Blagborough, A.M. Synergy in anti-malarial pre-erythrocytic and transmission-blocking antibodies is achieved by reducing parasite density. Elife 2018, 7, e35213. [Google Scholar] [CrossRef]
- Brod, F.; Miura, K.; Taylor, I.; Li, Y.; Marini, A.; Salman, A.M.; Spencer, A.J.; Long, C.A.; Biswas, S. Combination of RTS,S and Pfs25-IMX313 Induces a Functional Antibody Response Against Malaria Infection and Transmission in Mice. Front. Immunol. 2018, 9, 2780. [Google Scholar] [CrossRef]
- Yusuf, Y.; Yoshii, T.; Iyori, M.; Mizukami, H.; Fukumoto, S.; Yamamoto, D.S.; Emran, T.B.; Amelia, F.; Islam, A.; Syafira, I.; et al. A Viral-Vectored Multi-Stage Malaria Vaccine Regimen With Protective and Transmission-Blocking Efficacies. Front. Immunol. 2019, 10, 2412. [Google Scholar] [CrossRef]
- Gary, E.N.; Weiner, D.B. DNA vaccines: Prime time is now. Curr. Opin. Immunol. 2020, 65, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Doolan, D.L.; Hoffman, S.L. DNA-based vaccines against malaria: Status and promise of the Multi-Stage Malaria DNA Vaccine Operation. Int. J. Parasitol. 2001, 31, 753–762. [Google Scholar] [CrossRef]
- Lobo, C.A.; Dhar, R.; Kumar, N. Immunization of mice with DNA-based Pfs25 elicits potent malaria transmission-blocking antibodies. Infect. Immun. 1999, 67, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nyakundi, R.; Kariuki, T.; Ozwara, H.; Nyamongo, O.; Mlambo, G.; Ellefsen, B.; Hannaman, D.; Kumar, N. Functional evaluation of malaria Pfs25 DNA vaccine by in vivo electroporation in olive baboons. Vaccine 2013, 31, 3140–3147. [Google Scholar] [CrossRef]
- LeBlanc, R.; Vasquez, Y.; Hannaman, D.; Kumar, N. Markedly enhanced immunogenicity of a Pfs25 DNA-based malaria transmission-blocking vaccine by in vivo electroporation. Vaccine 2008, 26, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Bansal, G.P.; Kumar, R.; Ellefsen, B.; Hannaman, D.; Kumar, N. Evaluation of the Impact of Codon Optimization and N-Linked Glycosylation on Functional Immunogenicity of Pfs25 DNA Vaccines Delivered by In Vivo Electroporation in Preclinical Studies in Mice. Clin. Vaccine Immunol. 2015, 22, 1013–1019. [Google Scholar] [CrossRef]
- Datta, D.; Bansal, G.P.; Gerloff, D.L.; Ellefsen, B.; Hannaman, D.; Kumar, N. Immunogenicity and malaria transmission reducing potency of Pfs48/45 and Pfs25 encoded by DNA vaccines administered by intramuscular electroporation. Vaccine 2017, 35, 264–272. [Google Scholar] [CrossRef]
- Datta, D.; Bansal, G.P.; Grasperge, B.; Martin, D.S.; Philipp, M.; Gerloff, D.; Ellefsen, B.; Hannaman, D.; Kumar, N. Comparative functional potency of DNA vaccines encoding Plasmodium falciparum transmission blocking target antigens Pfs48/45 and Pfs25 administered alone or in combination by in vivo electroporation in rhesus macaques. Vaccine 2017, 35, 7049–7056. [Google Scholar] [CrossRef]
- Wang, R.; Doolan, D.L.; Le, T.P.; Hedstrom, R.C.; Coonan, K.M.; Charoenvit, Y.; Jones, T.R.; Hobart, P.; Margalith, M.; Ng, J.; et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 1998, 282, 476–480. [Google Scholar] [CrossRef]
- Sedegah, M.; Hedstrom, R.; Hobart, P.; Hoffman, S.L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc. Natl. Acad. Sci. USA 1994, 91, 9866–9870. [Google Scholar] [CrossRef]
- Le, T.P.; Coonan, K.M.; Hedstrom, R.C.; Charoenvit, Y.; Sedegah, M.; Epstein, J.E.; Kumar, S.; Wang, R.; Doolan, D.L.; Maguire, J.D.; et al. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine 2000, 18, 1893–1901. [Google Scholar] [CrossRef]
- Wang, R.; Epstein, J.; Charoenvit, Y.; Baraceros, F.M.; Rahardjo, N.; Gay, T.; Banania, J.G.; Chattopadhyay, R.; de la Vega, P.; Richie, T.L.; et al. Induction in humans of CD8+ and CD4+ T cell and antibody responses by sequential immunization with malaria DNA and recombinant protein. J. Immunol. 2004, 172, 5561–5569. [Google Scholar] [CrossRef] [PubMed]
- Chuang, I.; Sedegah, M.; Cicatelli, S.; Spring, M.; Polhemus, M.; Tamminga, C.; Patterson, N.; Guerrero, M.; Bennett, J.W.; McGrath, S.; et al. DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS ONE 2013, 8, e55571. [Google Scholar] [CrossRef] [PubMed]
- Spearman, P.; Mulligan, M.; Anderson, E.J.; Shane, A.L.; Stephens, K.; Gibson, T.; Hartwell, B.; Hannaman, D.; Watson, N.L.; Singh, K. A phase 1, randomized, controlled dose-escalation study of EP-1300 polyepitope DNA vaccine against Plasmodium falciparum malaria administered via electroporation. Vaccine 2016, 34, 5571–5578. [Google Scholar] [CrossRef]
- Tan, Z.; Zhou, T.; Zheng, H.; Ding, Y.; Xu, W. Malaria DNA vaccine gp96NTD-CSP elicits both CSP-specific antibody and CD8(+) T cell response. Parasitol. Res. 2015, 114, 2333–2339. [Google Scholar] [CrossRef]
- Hedstrom, R.C.; Doolan, D.L.; Wang, R.; Kumar, A.; Sacci, J.B., Jr.; Gardner, M.J.; Aguiar, J.C.; Charoenvit, Y.; Sedegah, M.; Tine, J.A.; et al. In vitro expression and in vivo immunogenicity of Plasmodium falciparum pre-erythrocytic stage DNA vaccines. Int. J. Mol. Med. 1998, 2, 29–38. [Google Scholar] [CrossRef]
- Deal, C.; Balazs, A.B.; Espinosa, D.A.; Zavala, F.; Baltimore, D.; Ketner, G. Vectored antibody gene delivery protects against Plasmodium falciparum sporozoite challenge in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 12528–12532. [Google Scholar] [CrossRef]
- Kumar, R.; Angov, E.; Kumar, N. Potent malaria transmission-blocking antibody responses elicited by Plasmodium falciparum Pfs25 expressed in Escherichia coli after successful protein refolding. Infect. Immun. 2014, 82, 1453–1459. [Google Scholar] [CrossRef]
- Birkholtz, L.M.; Blatch, G.; Coetzer, T.L.; Hoppe, H.C.; Human, E.; Morris, E.J.; Ngcete, Z.; Oldfield, L.; Roth, R.; Shonhai, A.; et al. Heterologous expression of plasmodial proteins for structural studies and functional annotation. Malar. J. 2008, 7, 197. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Mlambo, G.; Kanatani, S.; Sinnis, P.; Dimopoulos, G. Plasmodium falciparum Gametocyte Culture and Mosquito Infection Through Artificial Membrane Feeding. J. Vis. Exp. 2020, 161, e61426. [Google Scholar] [CrossRef]
- Flores-Garcia, Y.; Herrera, S.M.; Jhun, H.; Perez-Ramos, D.W.; King, C.R.; Locke, E.; Raghunandan, R.; Zavala, F. Optimization of an in vivo model to study immunity to Plasmodium falciparum pre-erythrocytic stages. Malar. J. 2019, 18, 426. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.A.; Christensen, D.; Munoz, C.; Singh, S.; Locke, E.; Andersen, P.; Zavala, F. Robust antibody and CD8(+) T-cell responses induced by P. falciparum CSP adsorbed to cationic liposomal adjuvant CAF09 confer sterilizing immunity against experimental rodent malaria infection. NPJ Vaccines 2017, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Kisalu, N.K.; Idris, A.H.; Weidle, C.; Flores-Garcia, Y.; Flynn, B.J.; Sack, B.K.; Murphy, S.; Schon, A.; Freire, E.; Francica, J.R.; et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med. 2018, 24, 408–416. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.M.; Bah, M.A.; Tursi, N.J.; Brooks, R.C.; Patel, A.; Esquivel, R.; Eaton, A.; Jhun, H.; Chu, J.; Kim, K.; et al. Strategic Variants of CSP Delivered as SynDNA Vaccines Demonstrate Heterogeneity of Immunogenicity and Protection from Plasmodium Infection in a Murine Model. Infect. Immun. 2021, 89, e0072820. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Epstein, J.; Baraceros, F.M.; Gorak, E.J.; Charoenvit, Y.; Carucci, D.J.; Hedstrom, R.C.; Rahardjo, N.; Gay, T.; Hobart, P.; et al. Induction of CD4(+) T cell-dependent CD8(+) type 1 responses in humans by a malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 2001, 98, 10817–10822. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Wang, R.; Doolan, D.L.; Charoenvit, Y.; Hedstrom, R.C.; Gardner, M.J.; Hobart, P.; Tine, J.; Sedegah, M.; Fallarme, V.; Sacci, J.B., Jr.; et al. Simultaneous induction of multiple antigen-specific cytotoxic T lymphocytes in nonhuman primates by immunization with a mixture of four Plasmodium falciparum DNA plasmids. Infect. Immun. 1998, 66, 4193–4202. [Google Scholar] [CrossRef]
- Dobano, C.; Widera, G.; Rabussay, D.; Doolan, D.L. Enhancement of antibody and cellular immune responses to malaria DNA vaccines by in vivo electroporation. Vaccine 2007, 25, 6635–6645. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Hayashi, C.T.H.; Zavala, F.; Tripathi, A.K.; Simonyan, H.; Young, C.N.; Clark, L.C.; Usuda, Y.; Van Parys, J.M.; Kumar, N. Effective Functional Immunogenicity of a DNA Vaccine Combination Delivered via In Vivo Electroporation Targeting Malaria Infection and Transmission. Vaccines 2022, 10, 1134. https://doi.org/10.3390/vaccines10071134
Cao Y, Hayashi CTH, Zavala F, Tripathi AK, Simonyan H, Young CN, Clark LC, Usuda Y, Van Parys JM, Kumar N. Effective Functional Immunogenicity of a DNA Vaccine Combination Delivered via In Vivo Electroporation Targeting Malaria Infection and Transmission. Vaccines. 2022; 10(7):1134. https://doi.org/10.3390/vaccines10071134
Chicago/Turabian StyleCao, Yi, Clifford T. H. Hayashi, Fidel Zavala, Abhai K. Tripathi, Hayk Simonyan, Colin N. Young, Leor C. Clark, Yukari Usuda, Jacob M. Van Parys, and Nirbhay Kumar. 2022. "Effective Functional Immunogenicity of a DNA Vaccine Combination Delivered via In Vivo Electroporation Targeting Malaria Infection and Transmission" Vaccines 10, no. 7: 1134. https://doi.org/10.3390/vaccines10071134
APA StyleCao, Y., Hayashi, C. T. H., Zavala, F., Tripathi, A. K., Simonyan, H., Young, C. N., Clark, L. C., Usuda, Y., Van Parys, J. M., & Kumar, N. (2022). Effective Functional Immunogenicity of a DNA Vaccine Combination Delivered via In Vivo Electroporation Targeting Malaria Infection and Transmission. Vaccines, 10(7), 1134. https://doi.org/10.3390/vaccines10071134






