Malaysian Parents’ Willingness to Vaccinate Their Children against COVID-19 Infection and Their Perception of mRNA COVID-19 Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Survey Design
2.2. Measures
2.2.1. Parents’ Demographics
2.2.2. Willingness to Vaccinate Their Children
2.2.3. Perception of Risk of Acquiring COVID-19
2.2.4. Concern about COVID-19 Vaccination for Children
2.2.5. mRNA Vaccines Acceptance
2.2.6. Knowledge of mRNA Vaccines
2.2.7. Attitudes on mRNA Vaccines
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Baseline Characteristics of the Population
3.2. Intention for Vaccination and Influencing Factors on Acceptance of COVID-19 Vaccine
3.3. mRNA Vaccines Acceptance and Attitudes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-21 December 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—21-December-2020 (accessed on 11 October 2022).
- WHO. Statement for Healthcare Professionals: How COVID-19 Vaccines Are Regulated for Safety and Effectiveness (Revised March 2022). Available online: https://www.who.int/news/item/17-05-2022-statement-for-healthcare-professionals-how-covid-19-vaccines-are-regulated-for-safety-and-effectiveness (accessed on 28 September 2022).
- Holders, J. The New York Times. Tracking Coronavirus Vaccinations around the Worlds. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 28 September 2022).
- DeRoo, S.S.; Pudalov, N.J.; Fu, L.Y. Planning for a COVID-19 vaccination program. JAMA 2020, 323, 2458–2459. [Google Scholar] [CrossRef]
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight against Pandemic. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use (accessed on 28 September 2022).
- FDA. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age (accessed on 28 September 2022).
- WHO. Interim Statement on COVID-19 Vaccination for Children and Adolescents. Available online: https://www.who.int/news/item/11-08-2022-interim-statement-on-covid-19-vaccination-for-children (accessed on 28 September 2022).
- Temsah, M.H.; Alhuzaimi, A.N.; Aljamaan, F.; Bahkali, F.; Al-Eyadhy, A.; Alrabiaah, A.; Alhaboob, A.; Bashiri, F.A.; Alshaer, A.; Temsah, O.; et al. Parental attitudes and hesitancy about COVID-19 vs. Routine childhood vaccinations: A national survey. Front. Public Health 2021, 9, 752323. [Google Scholar] [CrossRef]
- Yılmaz, M.; Sahin, M.K. Parents’ willingness and attitudes concerning the COVID-19 vaccine: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e14364. [Google Scholar] [CrossRef]
- MacDonald, N.E.; SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Limbu, Y.B.; Gautam, R.K.; Pham, L. The Health Belief Model Applied to COVID-19 Vaccine Hesitancy: A Systematic Review. Vaccines 2022, 10, 973. [Google Scholar] [CrossRef]
- Pires, C. Global Predictors of COVID-19 Vaccine Hesitancy: A Systematic Review. Vaccines 2022, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Sakamoto, H.; Abe, S.K.; Shinohara, R.; Kushima, M.; Otawa, S.; Yui, H.; Akiyama, Y.; Ooka, T.; Kojima, R.; et al. Factors of parental COVID-19 vaccine hesitancy: A cross sectional study in Japan. PLoS ONE 2021, 16, e0261121. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.B.; Bell, R.A. Parental COVID-19 vaccine hesitancy in the United States. Public Health Rep. 2022, 137, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Salazar, T.L.; Pollard, D.L.; Pina-Thomas, D.M.; Benton, M.J. Parental vaccine hesitancy and concerns regarding the COVID-19 virus. J. Pediatr. Nurs. 2022, 65, 10–15. [Google Scholar] [CrossRef]
- Scherer, A.M.; Gidengil, C.A.; Gedlinske, A.M.; Parker, A.M.; Askelson, N.M.; Woodworth, K.R.; Petersen, C.A.; Lindley, M.C. COVID-19 vaccination intentions, concerns, and facilitators among US parents of children ages 6 months through 4 years. JAMA Netw. Open 2022, 5, e2227437. [Google Scholar] [CrossRef]
- Humble, R.M.; Sell, H.; Dubé, E.; MacDonald, N.E.; Robinson, J.; Driedger, S.M.; Sadarangani, M.; Meyer, S.B.; Wilson, S.; Benzies, K.M. Canadian parents’ perceptions of COVID-19 vaccination and intention to vaccinate their children: Results from a cross-sectional national survey. Vaccine 2021, 39, 7669–7676. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.L.; Gan, G.G.; Chai, C.S.; Anuar, N.A.B.; Sindeh, W.; Chua, W.J.; Said, A.B.; Tan, S.B. The willingness of parents to vaccinate their children younger than 12 years against COVID-19: A cross-sectional study in Malaysia. BMC Public Health 2022, 22, 1265. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Vaccination hesitancy and the “myth” on mRNA-based vaccines in Italy in the COVID-19 era: Does urgency meet major safety criteria? J. Med. Virol. 2021, 93, 4049–4053. [Google Scholar] [CrossRef]
- Dror, A.A.; Daoud, A.; Morozov, N.G.; Layous, E.; Eisenbach, N.; Mizrachi, M.; Rayan, D.; Bader, A.; Francis, S.; Kaykov, E. Vaccine hesitancy due to vaccine country of origin, vaccine technology, and certification. Eur. J. Epidemiol. 2021, 36, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashid, M.; Al-Hamad, A.; Al-Hamad, A.; Yasin, Y. Myths, misconceptions, and hesitancy in people residing in Qatar toward mRNA COVID-19 vaccines: An experience exchange from Qatar University health center. Qatar Med. J. 2022, 20, 1–3. [Google Scholar] [CrossRef]
- Wong, L.P.; Alias, H.; Danaee, M.; Ahmed, J.; Lachyan, A.; Cai, C.Z.; Lin, Y.; Hu, Z.; Tan, S.Y.; Lu, Y. COVID-19 vaccination intention and vaccine characteristics influencing vaccination acceptance: A global survey of 17 countries. Infect. Dis. Poverty 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Wong, L.P.; Lin, Y.; Alias, H.; Bakar, S.A.; Zhao, Q.; Hu, Z. COVID-19 anti-vaccine sentiments: Analyses of comments from social media. Healthcare 2021, 9, 1530. [Google Scholar] [CrossRef]
- Freeman, D.; Waite, F.; Rosebrock, L.; Petit, A.; Causier, C.; East, A.; Jenner, L.; Teale, A.L.; Carr, L.; Mulhall, S. Coronavirus conspiracy beliefs, mistrust, and compliance with government guidelines in England. Psychol. Med. 2022, 52, 251–263. [Google Scholar] [CrossRef]
- MOH. COVID-19 Malaysia. Available online: https://covid-19.moh.gov.my/vaksin-covid-19/pickids (accessed on 30 September 2022).
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- CodeBlue. NPRA: No Serious Side Effects Reported from Children’s COVID-19 Vaccination. Available online: https://codeblue.galencentre.org/2022/02/16/npra-no-serious-side-effects-reported-from-childrens-covid-19-vaccination/ (accessed on 30 September 2022).
- MOH. KKMNOW: The COVID-19 vaccination. Available online: https://data.moh.gov.my/ms-MY/covid-vaccination (accessed on 11 October 2022).
- Du, M.; Tao, L.; Liu, J. The association between risk perception and COVID-19 vaccine hesitancy for children among reproductive women in China: An online survey. Front. Med. 2021, 8, 741298. [Google Scholar] [CrossRef]
- Irfan, O.; Li, J.; Tang, K.; Wang, Z.; Bhutta, Z.A. Risk of infection and transmission of SARS-CoV-2 among children and adolescents in households, communities and educational settings: A systematic review and meta-analysis. J. Glob. Health 2021, 11, 5013. [Google Scholar] [CrossRef]
- Armin, S.; Fahimzad, S.A.; Rafiei Tabatabaei, S.; Mansour Ghanaiee, R.; Marhamati, N.; Ahmadizadeh, S.N.; Behzad, A.; Hashemi, S.M.; Sadr, S.; Rajabnejad, M. COVID-19 Mortality in Children: A Referral Center Experience from Iran (Mofid Children’s Hospital, Tehran, Iran). Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 2737719. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.M.; Abbas, Q.; Chuah, S.L.; Malisie, R.F.; Pon, K.M.; Katsuta, T.; Dang, H.; Lee, P.C.; Jayashree, M.; PACCOVRA Investigators of the PACCMAN Research Group; et al. Comparative analysis of pediatric COVID-19 infection in Southeast Asia, south Asia, Japan, and China. Am. J. Trop. Med. Hyg. 2022, 105, 413. [Google Scholar] [CrossRef]
- She, J.; Liu, L.; Liu, W. Providing children with COVID-19 vaccinations is challenging due to lack of data and wide-ranging parental acceptance. Acta Paediatr. 2022, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, E. Post-vaccination COVID-19 deaths: A review of available evidence and recommendations for the global population. Clin. Exp. Vaccine Res. 2021, 10, 264. [Google Scholar] [CrossRef]
- Rosa, S.S.; Prazeres, D.M.; Azevedo, A.M.; Marques, M.P. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]

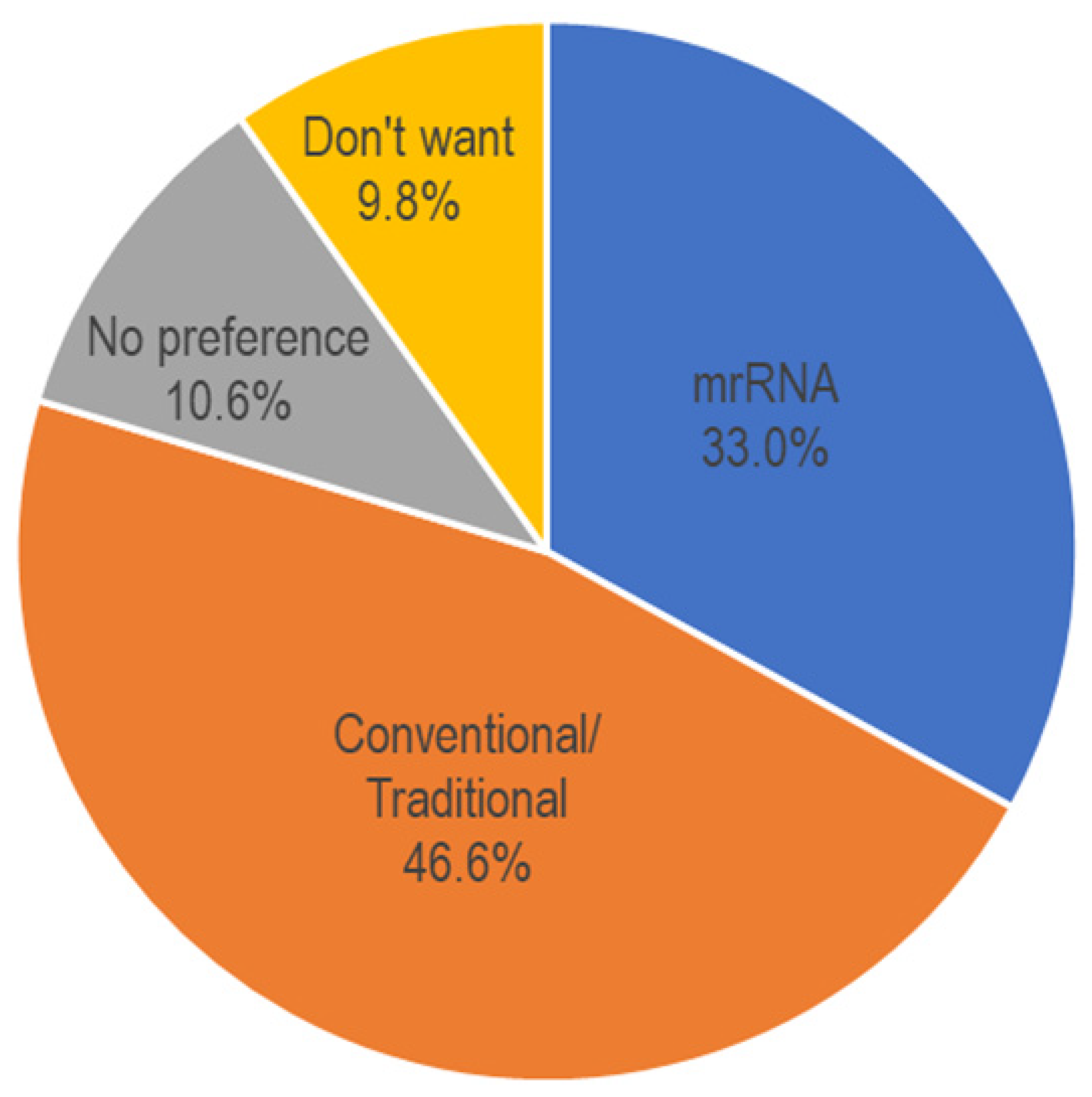
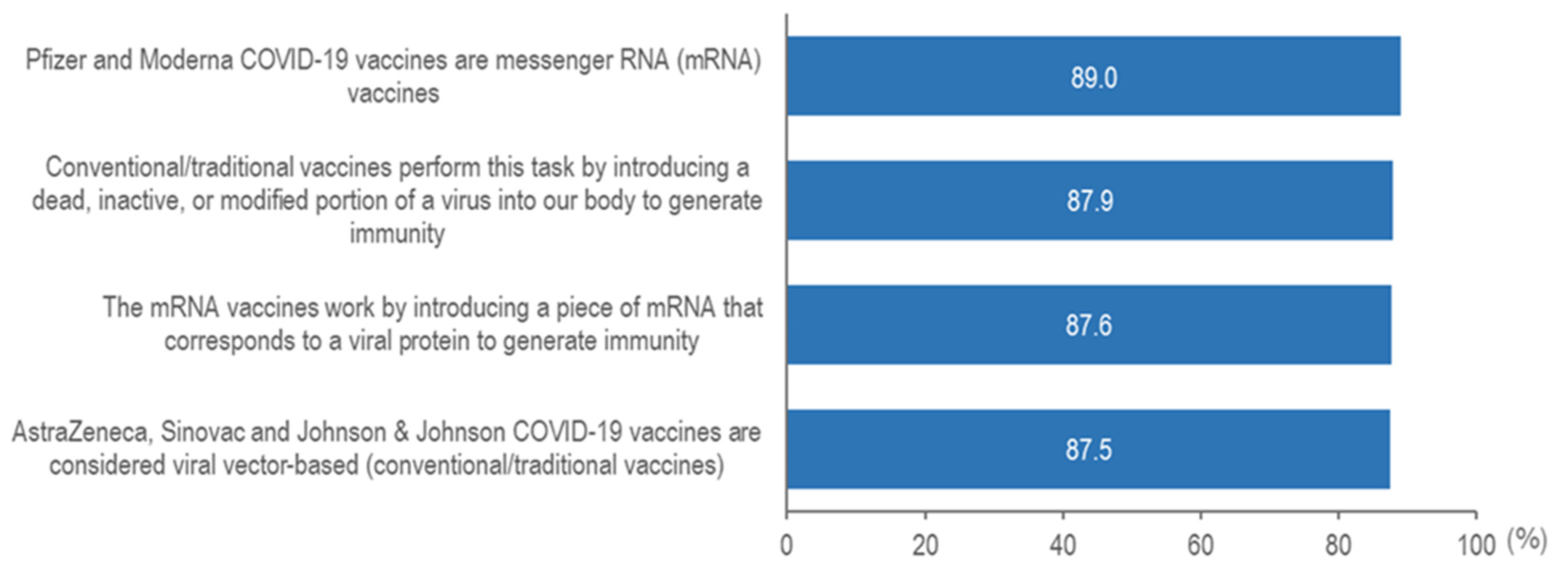
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Willingness to Vaccinate Children against COVID-19 | Willingness to Vaccinate Children against COVID-19 | ||||
| N (%) | Not Willing at all/ Somewhat Unwilling/ Undecided (n = 458) | Somewhat Willing/ Definitely Willing (n = 547) | p-Value | Somewhat Willing/ Definitely Willing vs. Not Willing at all/ Somewhat Unwilling/ Undecided | |
| Socio-demographic characteristics | |||||
| Age group (years) | |||||
| 23–34 | 295 (29.4) | 154 (51.5) | 143 (48.5) | p < 0.001 | Reference |
| 35–39 | 454 (45.3) | 217 (47.8) | 237 (52.2) | 1.36 (0.97–1.90) | |
| 40–72 | 254 (25.3) | 87 (34.3) | 167 (65.7) | 2.63 (1.66–4.16) *** | |
| Gender | |||||
| Male | 336 (33.5) | 134 (39.9) | 202 (60.1) | 0.013 | 1.32 (0.98–1.77) |
| Female | 667 (66.7) | 322 (48.3) | 345 (51.7) | Reference | |
| Ethnicity | |||||
| Malay | 198 (19.7) | 88 (44.4) | 110 (55.6) | p < 0.001 | Reference |
| Chinese | 195 (19.4) | 71 (36.4) | 124 (63.6) | 0.72 (0.44–1.17) | |
| Indian | 481 (48.0) | 250 (52.0) | 231 (48.0) | 0.39 (0.25–0.59) *** | |
| Others | 129 (12.9) | 47 (36.4) | 82 (63.6) | 0.77 (0.45–1.33) | |
| Average monthly household income (MYR) | |||||
| 5000 and below | 103 (10.3) | 63 (61.2) | 40 (38.8) | p < 0.001 | Reference |
| 5001–10,000 | 648 (64.6) | 299 (46.1) | 349 (53.9) | 0.56 (0.29–1.10) | |
| More than 10,000 | 252 (25.1) | 94 (37.3) | 158 (62.7) | 0.50 (0.23–0.59) | |
| Perceived susceptibility | |||||
| The COVID-19 vaccine for children is not needed as children are not as susceptible to COVID-19 infection co | |||||
| Strongly agree/Agree | 96 (9.6) | 87 (90.6) | 9 (9.4) | p < 0.001 | Reference |
| Disagree/ Strongly disagree | 907 (90.4) | 369 (40.7) | 538 (59.3) | 35.46 (15.26–82.40) *** | |
| Concerns over the COVID-19 vaccination for child | |||||
| Severe adverse effects after COVID-19 vaccination | |||||
| Not at all concerned/ Slightly concerned/ Moderately concerned | 288 (28.7) | 118 (41.0) | 170 (59.0) | 0.080 | |
| Extremely concern | 715 (71.3) | 338 (47.3) | 377 (52.7) | ||
| Unknown long-term side effects | |||||
| Not at all concerned/ Slightly concerned/ Moderately concerned | 252 (25.1) | 98 (38.9) | 154 (61.1) | 0.016 | 1.25 (0.90–1.75) |
| Extremely concern | 751 (74.9) | 358 (47.7) | 393 (52.3) | Reference | |
| Risk of COVID-19 infection | |||||
| Not at all concerned/ Slightly concerned/ Moderately concerned | 316 (31.5) | 136 (43.0) | 180 (57.0) | 0.306 | |
| Extremely concern | 687 (68.5) | 320 (46.6) | 367 (53.4) | ||
| Risk of death | |||||
| Not at all concerned/ Slightly concerned/ Moderately concerned | 235 (23.4) | 101 (43.0) | 134 (57.0) | 0.410 | |
| Extremely concern | 768 (76.6) | 355 (46.2) | 413 (53.8) | ||
| Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|
| Level of Concern about Vaccinating Children with a COVID-19 mRNA Vaccine | Level of Concern of Vaccinating Children with a COVID-19 mRNA Vaccine | ||||
| N (%) | Not at all Concerned (n = 366) | Slightly/ Moderately/ Extremely Concerned (n = 551) | p-Value | Slightly/ Moderately/ Extremely Concerned vs. Not at all Concerned | |
| Socio demographic characteristics | |||||
| Age group (years) | |||||
| 23–34 | 268 (29.2) | 105 (39.2) | 163 (60.8) | 0.665 | |
| 35–39 | 431 (47.0) | 176 (40.8) | 255 (59.2) | ||
| 40–72 | 218 (23.8) | 85 (39.0) | 133 (61.0) | ||
| Gender | |||||
| Male | 296 (32.3) | 116 (39.2) | 180 (60.8) | 0.773 | |
| Female | 621 (67.7) | 250 (40.3) | 371 (59.7) | ||
| Ethnicity | |||||
| Malay | 165 (18.0) | 53 (32.1) | 112 (67.9) | 0.099 | |
| Chinese | 176 (19.2) | 73 (41.5) | 103 (58.5) | ||
| Indian | 461 (50.3) | 187 (40.6) | 274 (59.4) | ||
| Others | 115 (12.5) | 53 (46.1) | 62 (53.9) | ||
| Average monthly household income (MYR) | |||||
| 5000 and below | 80 (8.7) | 7 (8.8) | 73 (91.3) | p < 0.001 | 11.65 (3.84–35.33) *** |
| 5001–10,000 | 594 (64.8) | 254 (42.8) | 340 (57.2) | 1.00 (0.52–1.93) | |
| More than 10,000 | 243 (26.5) | 105 (43.2) | 138 (56.8) | Reference | |
| Knowledge of mRNA COVID-19 vaccine | |||||
| Total knowledge score | |||||
| Score 0–3 | 136 (14.8) | 36 (26.5) | 100 (73.5) | p < 0.001 | 4.49 (2.07–9.74) *** |
| Score 4 | 781 (85.2) | 330 (42.3) | 451 (57.7) | Reference | |
| Attitudes towards COVID-19 mRNA vaccine | |||||
| I have confidence in the new and advanced technological approach used in the development of the COVID-19 mRNA vaccine | |||||
| Strongly agree/Agree | 353 (38.5) | 277 (78.5) | 76 (21.5) | p < 0.001 | Reference |
| Disagree/ Strongly disagree | 428 (46.7) | 12 (2.8) | 416 (97.2) | 3.40 (0.74–15.68) | |
| Don’t know | 136 (14.8) | 77 (56.6) | 59 (43.4) | 0.28 (0.09–0.84) * | |
| I am worried there might be unknown side effects of the mRNA vaccines that will show up months or years later | |||||
| Strongly agree/Agree | 516 (56.3) | 22 (4.3) | 494 (95.7) | p < 0.001 | 23.54 (7.87–70.35) *** |
| Disagree/ Strongly disagree | 283 (30.9) | 258 (91.2) | 25 (8.8) | 0.97 (0.29–3.25) | |
| Don’t know | 118 (12.9) | 86 (72.9) | 32 (27.1) | Reference | |
| The conventional/ traditional COVID-19 vaccines are safer than the COVID-19 mRNA vaccines | |||||
| Strongly agree/Agree | 449 (49.0) | 21 (4.7) | 428 (95.3) | p < 0.001 | 2.24 (0.69–7.31) |
| Disagree/Strongly disagree | 274 (29.9) | 238 (86.9) | 36 (13.1) | 0.54 (0.17–1.73) | |
| Don’t know | 194 (21.2) | 107 (55.2) | 87 (44.8) | Reference | |
| The COVID-19 mRNA vaccines generate a stronger immune response than the conventional/traditional vaccines | |||||
| Strongly agree/Agree | 322 (35.1) | 266 (82.6) | 56 (17.4) | p < 0.001 | Reference |
| Disagree/ Strongly disagree | 413 (45.0) | 15 (3.6) | 398 (96.4) | 1.20 (0.26–5.64) | |
| Don’t know | 182 (19.8) | 85 (46.7) | 97 (53.3) | 1.57 (0.47–5.29) | |
| The COVID-19 mRNA vaccines may contain microchip | |||||
| Strongly agree/Agree | 44 (4.8) | 1 (2.3) | 43 (97.7) | p < 0.001 | 2.31 (0.19–27.54) |
| Disagree/Strongly disagree | 437 (47.7) | 281 (64.3) | 156 (35.7) | 0.35 (0.16–0.74) ** | |
| Don’t know | 436 (47.5) | 84 (19.3) | 352 (80.7) | Reference | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, L.-P.; Lee, H.-Y.; Alias, H.; AbuBakar, S. Malaysian Parents’ Willingness to Vaccinate Their Children against COVID-19 Infection and Their Perception of mRNA COVID-19 Vaccines. Vaccines 2022, 10, 1790. https://doi.org/10.3390/vaccines10111790
Wong L-P, Lee H-Y, Alias H, AbuBakar S. Malaysian Parents’ Willingness to Vaccinate Their Children against COVID-19 Infection and Their Perception of mRNA COVID-19 Vaccines. Vaccines. 2022; 10(11):1790. https://doi.org/10.3390/vaccines10111790
Chicago/Turabian StyleWong, Li-Ping, Hai-Yen Lee, Haridah Alias, and Sazaly AbuBakar. 2022. "Malaysian Parents’ Willingness to Vaccinate Their Children against COVID-19 Infection and Their Perception of mRNA COVID-19 Vaccines" Vaccines 10, no. 11: 1790. https://doi.org/10.3390/vaccines10111790
APA StyleWong, L.-P., Lee, H.-Y., Alias, H., & AbuBakar, S. (2022). Malaysian Parents’ Willingness to Vaccinate Their Children against COVID-19 Infection and Their Perception of mRNA COVID-19 Vaccines. Vaccines, 10(11), 1790. https://doi.org/10.3390/vaccines10111790






