Immunotherapies for the Treatment of Drug Addiction
Abstract
1. Introduction
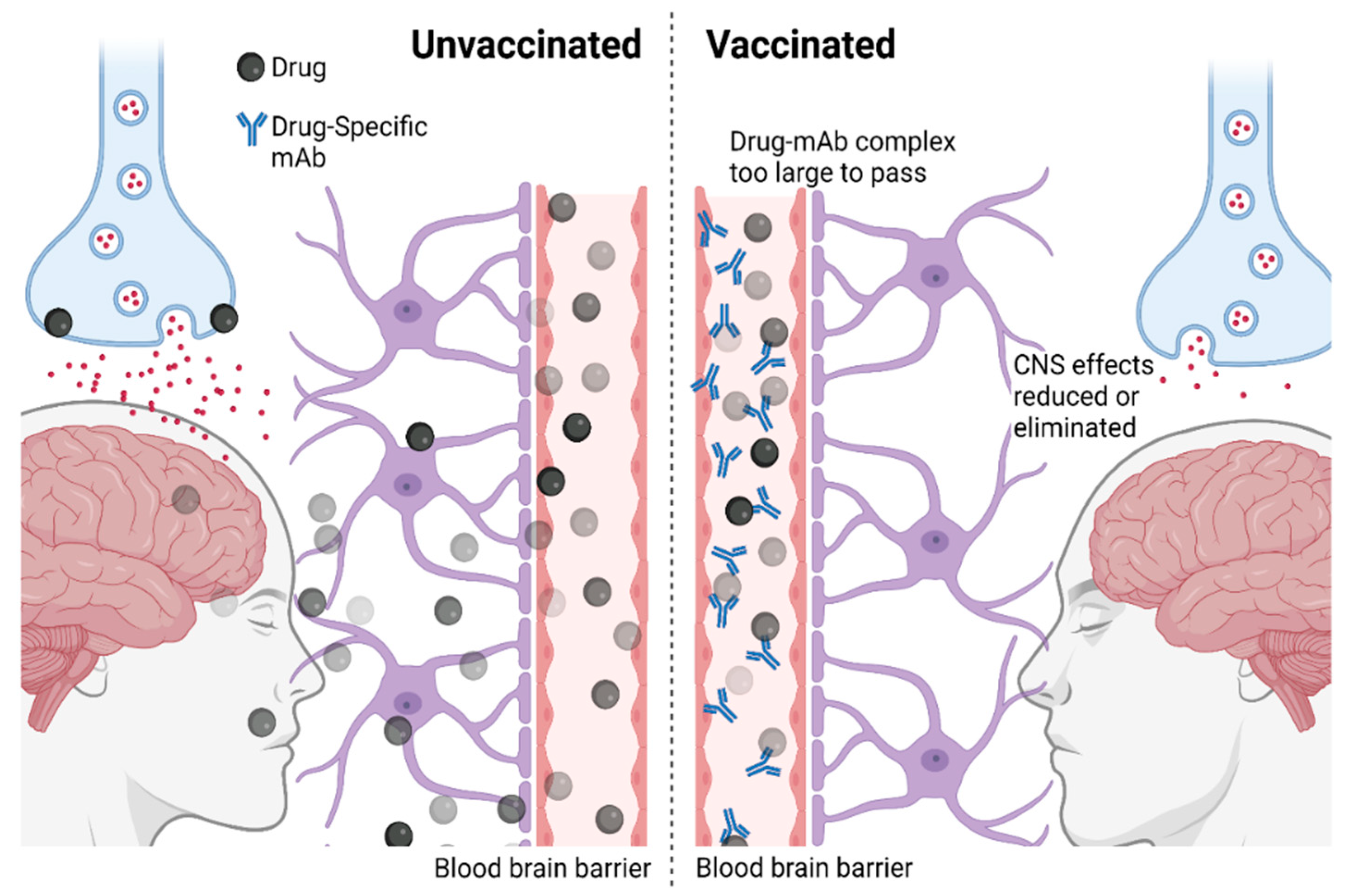
2. Immunotherapies against Addiction: Mechanisms
3. Platforms for Vaccine Development against Drug Abuse
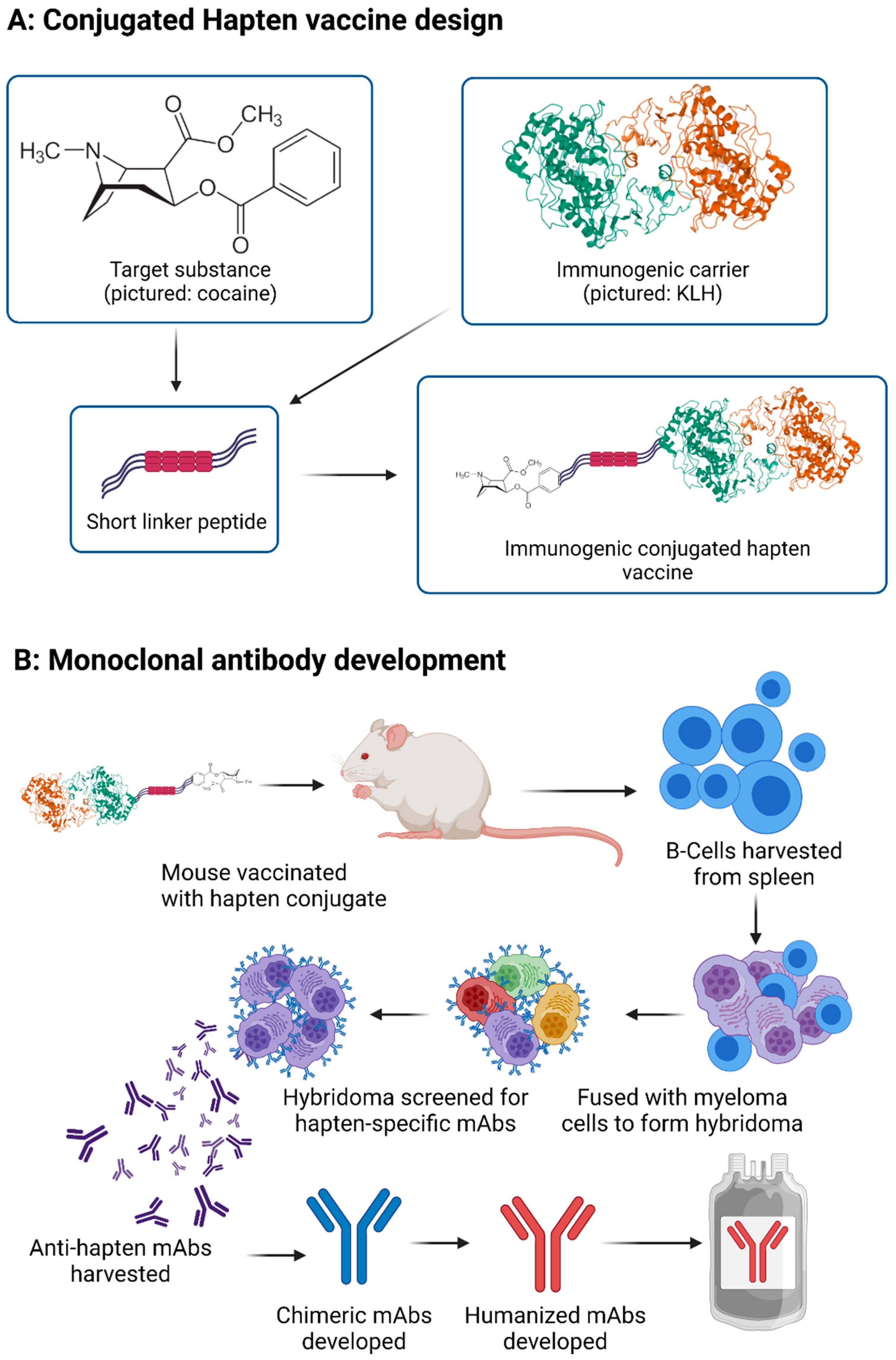
3.1. Vaccine through Hapten-Carrier Design
3.2. Monoclonal Antibody Development
4. Anti-METH Immunotherapies
4.1. Active Immunizations
4.2. Passive Immunization with METH mAbs
5. Anti-Cocaine Immunotherapies
6. Anti-Nicotine Immunotherapies
7. Anti-Opioid Immunotherapies
8. Biomarkers and Vaccine Efficacy
9. Expert Opinion
10. Novel Drugs of Abuse
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Merz, F. United Nations Office on Drugs and Crime: World Drug Report 2017. SIRIUS—Z. Strateg. Anal. 2018, 2, 85–86. [Google Scholar]
- United Nations. World Drug Report 2019; United Nations: San Francisco, CA, USA, 2019. [Google Scholar]
- Ritchie, H.; Roser, M. Drug Use. Our World in Data. 2019. Available online: https://ourworldindata.org/drug-use (accessed on 1 December 2019).
- National Institute on Drug Abuse (NIDA). Trends and Statistics. Available online: https://www.drugabuse.gov/drug-topics/trends-statistics (accessed on 1 June 2020).
- Shorter, D.; Kosten, T.R. Vaccines in the treatment of substance abuse. Focus 2011, 9, 25–30. [Google Scholar] [CrossRef]
- Pratt, K.P. Anti-drug antibodies: Emerging approaches to predict, reduce or reverse biotherapeutic immunogenicity. Antibodies 2018, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Hassanzadeganroudsari, M.; Nurgali, K.; Apostolopoulos, V. Vaccine development against methamphetamine drug addiction. Expert Rev. Vaccines 2020, 19, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Xiaoshan, T.; Junjie, Y.; Wenqing, W.; Yunong, Z.; Jiaping, L.; Shanshan, L.; Selva, N.K.; Kui, C. Immunotherapy for treating methamphetamine, heroin and cocaine use disorders. Drug Discov. Today 2020, 25, 610–619. [Google Scholar] [CrossRef]
- Zhao, Z.; Powers, K.; Hu, Y.; Raleigh, M.; Pentel, P.; Zhang, C. Engineering of a hybrid nanoparticle-based nicotine nanovaccine as a next-generation immunotherapeutic strategy against nicotine addiction: A focus on hapten density. Biomaterials 2017, 123, 107–117. [Google Scholar] [CrossRef]
- Davidson, M.; Mayer, M.; Habib, A.; Rashidi, N.; Filippone, R.T.; Fraser, S.; Prakash, M.D.; Sinnayah, P.; Tangalakis, K.; Mathai, M.L.; et al. Methamphetamine Induces Systemic Inflammation and Anxiety: The Role of the Gut–Immune–Brain Axis. Int. J. Mol. Sci. 2022, 23, 11224. [Google Scholar] [CrossRef]
- Davidson, M.; Rashidi, N.; Nurgali, K.; Apostolopoulos, V. The Role of Tryptophan Metabolites in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 9968. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hassanzadeganroudsari, M.; Kypreos, E.; Feehan, J.; Apostolopoulos, V. Immune to addiction: How immunotherapies can be used to combat methamphetamine addiction. Expert Rev. Vaccines 2021, 20, 707–715. [Google Scholar] [CrossRef]
- Shen, X.; Orson, F.M.; Kosten, T.R. Vaccines against drug abuse. Clin. Pharmacol. Ther. 2012, 91, 60–70. [Google Scholar] [CrossRef]
- Heekin, R.D.; Shorter, D.; Kosten, T.R. Current status and future prospects for the development of substance abuse vaccines. Expert Rev. Vaccines 2017, 16, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Wu, K.-L.; Tsai, H.-M.; Chen, C.-H. Treatment of methamphetamine abuse: An antibody-based immunotherapy approach. J. Food Drug Anal. 2013, 21, S82–S86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Y.-H.; Chen, C.-H. The development of antibody-based immunotherapy for methamphetamine abuse: Immunization, and virus-mediated gene transfer approaches. Curr. Gene Ther. 2013, 13, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Bonese, K.; Wainer, B.; Fitch, F.; Rothberg, R.; Schuster, C. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 1974, 252, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.C.; Gunnell, M.; Che, Y.; Goforth, R.L.; Carroll, F.I.; Henry, R.; Liu, H.; Owens, S.M. Using hapten design to discover therapeutic monoclonal antibodies for treating methamphetamine abuse. J. Pharmacol. Exp. Ther. 2007, 322, 30–39. [Google Scholar] [CrossRef]
- Byrnes-Blake, K.A.; Laurenzana, E.M.; Landes, R.D.; Gentry, W.B.; Owens, S.M. Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. Eur. J. Pharmacol. 2005, 521, 86–94. [Google Scholar] [CrossRef]
- Bremer, P.T.; Janda, K.D. Conjugate vaccine immunotherapy for substance use disorder. Pharmacol. Rev. 2017, 69, 298–315. [Google Scholar] [CrossRef]
- Collins, K.C.; Schlosburg, J.E.; Bremer, P.T.; Janda, K.D. Methamphetamine vaccines: Improvement through hapten design. J. Med. Chem. 2016, 59, 3878–3885. [Google Scholar] [CrossRef]
- Shen, X.Y.; Kosten, T.A.; Lopez, A.Y.; Kinsey, B.M.; Kosten, T.R.; Orson, F.M. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013, 129, 41–48. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hassanzadeganroudsari, M.; Apostolopoulos, V. Why METH users are at high risk of fatality due to COVID-19 infection? Expert Rev. Vaccines 2020, 19, 1101–1103. [Google Scholar] [CrossRef]
- Moreno, A.Y.; Azar, M.R.; Warren, N.A.; Dickerson, T.J.; Koob, G.F.; Janda, K.D. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Mol. Pharm. 2010, 7, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Janda, K.D. Immunopharmacotherapeutic advancements in addressing methamphetamine abuse. RSC Chem. Biol. 2021, 2, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Hassanzadeganroudsari, M.; Feehan, J.; Deraos, G.; Nurgali, K.; Matsoukas, J.; Apostolopoulos, V. Development and characterization of a novel conjugated methamphetamine vaccine. Vaccine 2022, 40, 5882–5891. [Google Scholar] [CrossRef]
- Alving, C.R.; Matyas, G.R.; Torres, O.; Jalah, R.; Beck, Z. Adjuvants for vaccines to drugs of abuse and addiction. Vaccine 2014, 32, 5382–5389. [Google Scholar] [CrossRef]
- Michael Owens, S.; Atchley, W.T.; Hambuchen, M.D.; Peterson, E.C.; Brooks Gentry, W. Monoclonal antibodies as pharmacokinetic antagonists for the treatment of (+)-methamphetamine addiction. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2011, 10, 892–898. [Google Scholar] [CrossRef]
- Gentry, W.B.; Rüedi-Bettschen, D.; Owens, S.M. Anti-(+)-Methamphetamine Monoclonal Antibody Antagonists Designed to Prevent the Progression of Human Diseases of Addiction. Clin. Pharmacol. Ther. 2010, 88, 390–393. [Google Scholar] [CrossRef]
- Little, M.; Kipriyanov, S.; Le Gall, F.; Moldenhauer, G. Of mice and men: Hybridoma and recombinant antibodies. Immunol. Today 2000, 21, 364–370. [Google Scholar] [CrossRef]
- Prakash, M.D.; Tangalakis, K.; Antonipillai, J.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. Methamphetamine: Effects on the brain, gut and immune system. Pharmacol. Res. 2017, 120, 60–67. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Raza, A.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Methamphetamine and its immune-modulating effects. Maturitas 2019, 121, 13–21. [Google Scholar] [CrossRef]
- Cheng, L.; Kim, S.; Chung, A.; Castro, A. Amphetamines: New radioimmunoassay. FEBS Lett. 1973, 36, 339–342. [Google Scholar] [CrossRef]
- Byrnes-Blake, K.A.; Carroll, F.I.; Abraham, P.; Owens, S.M. Generation of anti-(+) methamphetamine antibodies is not impeded by (+) methamphetamine administration during active immunization of rats. Int. Immunopharmacol. 2001, 1, 329–338. [Google Scholar] [CrossRef]
- Kinsey, B. Vaccines against drugs of abuse: Where are we now? Ther. Adv. Vaccines 2014, 2, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.Y.; Mayorov, A.V.; Janda, K.D. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J. Am. Chem. Soc. 2011, 133, 6587–6595. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.L.; Moreno, A.Y.; Aarde, S.M.; Creehan, K.M.; Vandewater, S.A.; Vaillancourt, B.D.; Wright Jr, M.J.; Janda, K.D.; Taffe, M.A. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol. Psychiatry 2013, 73, 721–728. [Google Scholar] [CrossRef]
- Rüedi-Bettschen, D.; Wood, S.L.; Gunnell, M.G.; West, C.M.; Pidaparthi, R.R.; Carroll, F.I.; Blough, B.E.; Owens, S.M. Vaccination protects rats from methamphetamine-induced impairment of behavioral responding for food. Vaccine 2013, 31, 4596–4602. [Google Scholar] [CrossRef]
- Haile, C.N.; Kosten, T.A.; Shen, X.Y.; O’Malley, P.W.; Winoske, K.J.; Kinsey, B.M.; Wu, Y.; Huang, Z.; Lykissa, E.D.; Naidu, N. Altered methamphetamine place conditioning in mice vaccinated with a succinyl-methamphetamine-tetanus-toxoid vaccine. Am. J. Addict. 2015, 24, 748–755. [Google Scholar] [CrossRef]
- Nguyen, J.; Bremer, P.; Hwang, C.; Vandewater, S.; Collins, K.; Creehan, K.; Janda, K.; Taffe, M. Effective active vaccination against methamphetamine in female rats. Drug Alcohol Depend. 2017, 175, 179–186. [Google Scholar] [CrossRef]
- McMillan, D.; Hardwick, W.; Li, M.; Gunnell, M.; Carroll, F.I.; Abraham, P.; Owens, S.M. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J. Pharmacol. Exp. Ther. 2004, 309, 1248–1255. [Google Scholar] [CrossRef]
- Daniels, J.; Wessinger, W.; Hardwick, W.; Li, M.; Gunnell, M.; Hall, C.; Owens, S.; McMillan, D. Effects of anti-phencyclidine and anti-(+)-methamphetamine monoclonal antibodies alone and in combination on the discrimination of phencyclidine and (+)-methamphetamine by pigeons. Psychopharmacology 2006, 185, 36–44. [Google Scholar] [CrossRef]
- Stevens, M.W.; Stevens, M.W.; Stevens, M.W.; Stevens, M.W.; Tawney, R.L.; Tawney, R.L.; Tawney, R.L.; Tawney, R.L.; West, C.M.; West, C.M. Preclinical characterization of an anti-methamphetamine monoclonal antibody for human use. In Proceedings of the MAbs; Taylor & Francis: Abingdon, UK, 2014; pp. 547–555. [Google Scholar]
- Harris, A.C.; LeSage, M.G.; Shelley, D.; Perry, J.L.; Pentel, P.R.; Owens, S.M. The anti-(+)-methamphetamine monoclonal antibody mAb7F9 attenuates acute (+)-methamphetamine effects on intracranial self-stimulation in rats. PLoS ONE 2015, 10, e0118787. [Google Scholar] [CrossRef]
- Lobo, E.D.; Hansen, R.J.; Balthasar, J.P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004, 93, 2645–2668. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. World Drug Report 2018 (Set of 5 Booklets); United Nations: San Francisco, CA, USA, 2018. [Google Scholar]
- Domingo, C.B.; Shorter, D.; Kosten, T.R. Vaccines for treating cocaine use disorders. In Biologics to Treat Substance Use Disorders; Springer: Berlin/Heidelberg, Germany, 2016; pp. 25–36. [Google Scholar]
- Landry, D.W.; Zhao, K.; Yang, G.; Glickman, M.; Georgiadis, T.M. Antibody-catalyzed degradation of cocaine. Science 1993, 259, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Basmadjian, G.P.; Singh, S.; Sastrodjojo, B.; Smith, B.T.; Avor, K.; Chang, F.; Mills, S.L.; Seale, T.W. Generation of polyclonal catalytic antibodies against cocaine using transition state analogs of cocaine conjugated to diphtheria toxoid. Chem. Pharm. Bull. 1995, 43, 1902–1911. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berkman, C.E.; Underiner, G.E.; Cashman, J.R. Synthesis of an immunogenic template for the generation of catalytic antibodies for (−)-cocaine hydrolysis. J. Org. Chem. 1996, 61, 5686–5689. [Google Scholar] [CrossRef]
- Deng, S.X.; de Prada, P.; Landry, D.W. Anticocaine catalytic antibodies. J. Immunol. Methods 2002, 269, 299–310. [Google Scholar] [CrossRef]
- Mets, B.; Winger, G.; Cabrera, C.; Seo, S.; Jamdar, S.; Yang, G.; Zhao, K.; Briscoe, R.J.; Almonte, R.; Woods, J.H. A catalytic antibody against cocaine prevents cocaine’s reinforcing and toxic effects in rats. Proc. Natl. Acad. Sci. USA 1998, 95, 10176–10181. [Google Scholar] [CrossRef]
- McKenzie, K.M.; Mee, J.M.; Rogers, C.J.; Hixon, M.S.; Kaufmann, G.F.; Janda, K.D. Identification and Characterization of Single Chain Anti-cocaine Catalytic Antibodies. J. Mol. Biol. 2007, 365, 722–731. [Google Scholar] [CrossRef]
- Cai, X.; Tsuchikama, K.; Janda, K.D. Modulating cocaine vaccine potency through hapten fluorination. J. Am. Chem. Soc. 2013, 135, 2971–2974. [Google Scholar] [CrossRef]
- Cai, X.; Whitfield, T.; Hixon, M.S.; Grant, Y.; Koob, G.F.; Janda, K.D. Probing active cocaine vaccination performance through catalytic and noncatalytic hapten design. J. Med. Chem. 2013, 56, 3701–3709. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Xue, L.; Hou, S.; Jin, Z.; Zhang, T.; Zheng, F.; Zhan, C.-G. Long-acting cocaine hydrolase for addiction therapy. Proc. Natl. Acad. Sci. USA 2016, 113, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.; Geng, L.; Gao, Y.; Zhang, B.; Miller, J.D.; Reyes, S.; Brimijoin, S. Reward and toxicity of cocaine metabolites generated by cocaine hydrolase. Cell. Mol. Neurobiol. 2015, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.; Gao, Y.; Geng, L.; LeBrasseur, N.; White, T.; Brimijoin, S. Preclinical studies on neurobehavioral and neuromuscular effects of cocaine hydrolase gene therapy in mice. J. Mol. Neurosci. 2014, 53, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.T.; Carey, K.A.; Narasimhan, D.; Nichols, J.; Berlin, A.A.; Lukacs, N.W.; Sunahara, R.K.; Woods, J.H.; Ko, M.-C. Amelioration of the cardiovascular effects of cocaine in rhesus monkeys by a long-acting mutant form of cocaine esterase. Neuropsychopharmacology 2011, 36, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.T.; Brim, R.L.; Noon, K.R.; Narasimhan, D.; Lukacs, N.W.; Sunahara, R.K.; Woods, J.H.; Ko, M.-C. Repeated administration of a mutant cocaine esterase: Effects on plasma cocaine levels, cocaine-induced cardiovascular activity, and immune responses in rhesus monkeys. J. Pharmacol. Exp. Ther. 2012, 342, 205–213. [Google Scholar] [CrossRef]
- Gao, Y.; Brimijoin, S. Lasting reduction of cocaine action in neostriatum—A hydrolase gene therapy approach. J. Pharmacol. Exp. Ther. 2009, 330, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Brimijoin, S.; Orson, F.; Kosten, T.R.; Kinsey, B.; Shen, X.Y.; White, S.J.; Gao, Y. Anti-cocaine antibody and butyrylcholinesterase-derived cocaine hydrolase exert cooperative effects on cocaine pharmacokinetics and cocaine-induced locomotor activity in mice. Chem.-Biol. Interact. 2013, 203, 212–216. [Google Scholar] [CrossRef][Green Version]
- Carrera, M.R.A.; Ashley, J.A.; Zhou, B.; Wirsching, P.; Koob, G.F.; Janda, K.D. Cocaine vaccines: Antibody protection against relapse in a rat model. Proc. Natl. Acad. Sci. USA 2000, 97, 6202–6206. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.R.A.; Trigo, J.M.; Wirsching, P.; Roberts, A.J.; Janda, K.D. Evaluation of the anticocaine monoclonal antibody GNC92H2 as an immunotherapy for cocaine overdose. Pharmacol. Biochem. Behav. 2005, 81, 709–714. [Google Scholar] [CrossRef]
- Collins, G.T.; Zaks, M.E.; Cunningham, A.R.; Clair, C.S.; Nichols, J.; Narasimhan, D.; Ko, M.-C.; Sunahara, R.K.; Woods, J.H. Effects of a long-acting mutant bacterial cocaine esterase on acute cocaine toxicity in rats. Drug Alcohol Depend. 2011, 118, 158–165. [Google Scholar] [CrossRef]
- Kosten, T.R.; Domingo, C.B.; Shorter, D.; Orson, F.; Green, C.; Somoza, E.; Sekerka, R.; Levin, F.R.; Mariani, J.J.; Stitzer, M. Vaccine for cocaine dependence: A randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014, 140, 42–47. [Google Scholar] [CrossRef]
- Haney, M.; Gunderson, E.W.; Jiang, H.; Collins, E.D.; Foltin, R.W. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol. Psychiatry 2010, 67, 59–65. [Google Scholar] [CrossRef] [PubMed]
- West, R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 1018–1036. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L. Nicotine addiction. N. Engl. J. Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef]
- Cahill, K.; Stevens, S.; Lancaster, T. Pharmacological treatments for smoking cessation. JAMA 2014, 311, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, N.A. Strategies to help a smoker who is struggling to quit. JAMA 2012, 308, 1573–1580. [Google Scholar] [CrossRef]
- Anthenelli, R.M.; Benowitz, N.L.; West, R.; St Aubin, L.; McRae, T.; Lawrence, D.; Ascher, J.; Russ, C.; Krishen, A.; Evins, A.E. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet 2016, 387, 2507–2520. [Google Scholar] [CrossRef]
- Collins, S.E.; Witkiewitz, K.; Kirouac, M.; Marlatt, G.A. Preventing relapse following smoking cessation. Curr. Cardiovasc. Risk Rep. 2010, 4, 421–428. [Google Scholar] [CrossRef]
- García-Gómez, L.; Hernández-Pérez, A.; Noé-Díaz, V.; Riesco-Miranda, J.A.; Jiménez-Ruiz, C. Smoking cessation treatments: Current psychological and pharmacological options. Rev. Investig. Clin. 2019, 71, 7–16. [Google Scholar] [CrossRef]
- Pentel, P.R.; Raleigh, M.D.; LeSage, M.G.; Thisted, T.; Horrigan, S.; Biesova, Z.; Kalnik, M.W. The nicotine-degrading enzyme NicA2 reduces nicotine levels in blood, nicotine distribution to brain, and nicotine discrimination and reinforcement in rats. BMC Biotechnol. 2018, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Kallupi, M.; Xue, S.; Zhou, B.; Janda, K.D.; George, O. An enzymatic approach reverses nicotine dependence, decreases compulsive-like intake, and prevents relapse. Sci. Adv. 2018, 4, eaat4751. [Google Scholar] [CrossRef]
- Thisted, T.; Biesova, Z.; Walmacq, C.; Stone, E.; Rodnick-Smith, M.; Ahmed, S.S.; Horrigan, S.K.; Van Engelen, B.; Reed, C.; Kalnik, M.W. Optimization of a nicotine degrading enzyme for potential use in treatment of nicotine addiction. BMC Biotechnol. 2019, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Langone, J.J.; Gjika, H.B.; Van Vunakis, H. Nicotine and its metabolites. Radioimmunoassays for nicotine and cotinine. Biochemistry 1973, 12, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Prieto, I. Nicotine antibody production: Comparison of two nicotine conjugates in different animal species. Biochem. Biophys. Res. Commun. 1975, 67, 583–589. [Google Scholar] [CrossRef]
- Matsushita, H.; Noguchi, M.; Tamaki, E. Conjugate of bovine serum albumin with nicotine. Biochem. Biophys. Res. Commun. 1974, 57, 1006–1010. [Google Scholar] [CrossRef]
- Matsukura, S.; Sakamoto, N.; Imura, H.; Matsuyama, H.; Tamada, T.; Ishiguro, T.; Muranaka, H. Radioimmunoassay of nicotine. Biochem. Biophys. Res. Commun. 1975, 64, 574–580. [Google Scholar] [CrossRef]
- Castro, A.; Monji, N.; Ali, H.; Yi, J.M.; Bowman, E.R.; McKennis, H., Jr. Nicotine antibodies: Comparison of ligand specificities of antibodies produced against two nicotine conjugates. Eur. J. Biochem. 1980, 104, 331–340. [Google Scholar] [CrossRef]
- Hieda, Y.; Keyler, D.E.; Vandevoort, J.T.; Kane, J.K.; Ross, C.A.; Raphael, D.E.; Niedbalas, R.S.; Pentel, P.R. Active Immunization Alters the Plasma Nicotine Concentration in Rats. J. Pharmacol. Exp. Ther. 1997, 283, 1076–1081. [Google Scholar]
- Isomura, S.; Wirsching, P.; Janda, K.D. An immunotherapeutic program for the treatment of nicotine addiction: Hapten design and synthesis. J. Org. Chem. 2001, 66, 4115–4121. [Google Scholar] [CrossRef]
- Carrera, M.R.o.A.; Ashley, J.A.; Hoffman, T.Z.; Isomura, S.; Wirsching, P.; Koob, G.F.; Janda, K.D. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorganic Med. Chem. 2004, 12, 563–570. [Google Scholar] [CrossRef]
- Meijler, M.M.; Matsushita, M.; Altobell, L.J.; Wirsching, P.; Janda, K.D. A new strategy for improved nicotine vaccines using conformationally constrained haptens. J. Am. Chem. Soc. 2003, 125, 7164–7165. [Google Scholar] [CrossRef]
- Moreno, A.Y.; Azar, M.R.; Koob, G.F.; Janda, K.D. Probing the protective effects of a conformationally constrained nicotine vaccine. Vaccine 2012, 30, 6665–6670. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.B.; De, B.P.; Hicks, M.J.; Janda, K.D.; Kaminsky, S.M.; Worgall, S.; Crystal, R.G. Suppression of nicotine-induced pathophysiology by an adenovirus hexon-based antinicotine vaccine. Hum. Gene Ther. 2013, 24, 595–603. [Google Scholar] [CrossRef]
- de Villiers, S.H.L.; Lindblom, N.; Kalayanov, G.; Gordon, S.; Baraznenok, I.; Malmerfelt, A.; Marcus, M.M.; Johansson, A.M.; Svensson, T.H. Nicotine hapten structure, antibody selectivity and effect relationships: Results from a nicotine vaccine screening procedure. Vaccine 2010, 28, 2161–2168. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, H.; Huang, W.; Zhang, C. A novel and efficient nicotine vaccine using nano-lipoplex as a delivery vehicle. Hum. Vaccines Immunother. 2014, 10, 64–72. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Pryde, D.C.; Gervais, D.P.; Stead, D.R.; Zhang, N.; Benoit, M.; Robertson, K.; Kim, I.J.; Tharmanathan, T.; Merson, J.R.; et al. Enhancing immunogenicity of a 3′aminomethylnicotine-DT-conjugate anti-nicotine vaccine with CpG adjuvant in mice and non-human primates. Int. Immunopharmacol. 2013, 16, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Smith, D.; Frazier, E.; Hoerle, R.; Ehrich, M.; Zhang, C. The next-generation nicotine vaccine: A novel and potent hybrid nanoparticle-based nicotine vaccine. Biomaterials 2016, 106, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Harris, B.; Hu, Y.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Rational incorporation of molecular adjuvants into a hybrid nanoparticle-based nicotine vaccine for immunotherapy against nicotine addiction. Biomaterials 2018, 155, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Hu, Y.; Huang, W.; de Villiers, S.; Pentel, P.; Zhang, J.; Dorn, H.; Ehrich, M.; Zhang, C. Negatively Charged Carbon Nanohorn Supported Cationic Liposome Nanoparticles: A Novel Delivery Vehicle for Anti-Nicotine Vaccine. J. Biomed. Nanotechnol. 2015, 11, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Hatsukami, D.K.; Rennard, S.; Jorenby, D.; Fiore, M.; Koopmeiners, J.; de Vos, A.; Horwith, G.; Pentel, P.R. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin. Pharmacol. Ther. 2005, 78, 456–467. [Google Scholar] [CrossRef]
- Hatsukami, D.K.; Jorenby, D.E.; Gonzales, D.; Rigotti, N.A.; Glover, E.D.; Oncken, C.A.; Tashkin, D.P.; Reus, V.I.; Akhavain, R.C.; Fahim, R.E.; et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin. Pharmacol. Ther. 2011, 89, 392–399. [Google Scholar] [CrossRef]
- Hoogsteder, P.H.; Kotz, D.; van Spiegel, P.I.; Viechtbauer, W.; van Schayck, O.C. Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: A randomized placebo-controlled trial. Addiction 2014, 109, 1252–1259. [Google Scholar] [CrossRef]
- Tonstad, S.; Heggen, E.; Giljam, H.; Lagerbäck, P.; Tønnesen, P.; Wikingsson, L.D.; Lindblom, N.; de Villiers, S.; Svensson, T.H.; Fagerström, K.O. Niccine®, a nicotine vaccine, for relapse prevention: A phase II, randomized, placebo-controlled, multicenter clinical trial. Nicotine Tob. Res. 2013, 15, 1492–1501. [Google Scholar] [CrossRef]
- Cornuz, J.; Zwahlen, S.; Jungi, W.F.; Osterwalder, J.; Klingler, K.; van Melle, G.; Bangala, Y.; Guessous, I.; Müller, P.; Willers, J. A vaccine against nicotine for smoking cessation: A randomized controlled trial. PLoS ONE 2008, 3, e2547. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.I.; Bergman, J. Effects of the Nanoparticle-Based Vaccine, SEL-068, on Nicotine Discrimination in Squirrel Monkeys. Neuropsychopharmacology 2015, 40, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Thorn, J.; Gervais, D.P.; Stead, D.R.; Zhang, N.; Benoit, M.; Cartier, J.; Kim, I.-J.; Bhattacharya, K.; Finneman, J.I. Anti-nicotine vaccines: Comparison of adjuvanted CRM197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int. Immunopharmacol. 2015, 29, 663–671. [Google Scholar] [CrossRef]
- Nekhayeva, I.A.; Nanovskaya, T.N.; Pentel, P.R.; Keyler, D.E.; Hankins, G.D.; Ahmed, M.S. Effects of nicotine-specific antibodies, Nic311 and Nic-IgG, on the transfer of nicotine across the human placenta. Biochem. Pharmacol. 2005, 70, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Pentel, P.R.; Dufek, M.B.; Roiko, S.; LeSage, M.G.; Keyler, D.E. Differential effects of passive immunization with nicotine-specific antibodies on the acute and chronic distribution of nicotine to brain in rats. J. Pharmacol. Exp. Ther. 2006, 317, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Roiko, S.A.; Harris, A.C.; LeSage, M.G.; Keyler, D.E.; Pentel, P.R. Passive immunization with a nicotine-specific monoclonal antibody decreases brain nicotine levels but does not precipitate withdrawal in nicotine-dependent rats. Pharmacol. Biochem. Behav. 2009, 93, 105–111. [Google Scholar] [CrossRef]
- Raleigh, M.D.; Beltraminelli, N.; Fallot, S.; LeSage, M.G.; Saykao, A.; Pentel, P.R.; Fuller, S.; Thisted, T.; Biesova, Z.; Horrigan, S. Attenuating nicotine’s effects with high affinity human anti-nicotine monoclonal antibodies. PLoS ONE 2021, 16, e0254247. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Baron, D.; Hauser, M.; Henriksen, S.; Thanos, P.; Black, C.; Siwicki, D.; Modestino, E.; Downs, B.; Badgaiyan, S. Americas’ opioid/psychostimulant epidemic would benefit from general population early identification of genetic addiction risk especially in children of alcoholics (COAs). J. Syst. Integr. Neurosci. 2019, 5, 1. [Google Scholar]
- Spector, S.; Parker, C.W. Morphine: Radioimmunoassay. Science 1970, 168, 1347–1348. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.J.; Parker, C.W.; Williams, R.C., Jr. γ-Globulin binding of morphine in heroin addicts. J. Lab. Clin. Med. 1972, 80, 155–164. [Google Scholar] [PubMed]
- Hill, J.H.; Wainer, B.H.; Fitch, F.W.; Rothberg, R.M. Delayed clearance of morphine from the circulation of rabbits immunized with morphine-6-hemisuccinate bovine serum albumin. J. Immunol. 1975, 114, 1363–1368. [Google Scholar]
- Kantak, K.M. Vaccines against drugs of abuse: A viable treatment option? Drugs 2003, 63, 341–352. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Mehraby, M.; Zarbakhsh, M.; Farzaneh, H. Design and synthesis of a morphine-6-succinyl-bovine serum albumin hapten for vaccine development. Biotechnol. Appl. Biochem. 1999, 30, 139–146. [Google Scholar] [PubMed]
- Ma, L.X.; Zhou, Q.; Zheng, H.B.; Li, S.B. Preparation and characterization of anti-morphine vaccine antibody. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2006, 22, 368–370. [Google Scholar]
- Akbarzadeh, A.; Norouzian, D.; Farhangi, A.; Mehrabi, M.; Chiani, M.; Zare, D.; Saffari, Z.; Mortazavi, M.; Nikdel, A. Immunotherapy of 347 volunteer outpatient morphine addicts by human therapeutic morphine vaccine in Kermanshah province of Iran. J. Pharmacol. Toxicol. 2009, 4, 30–35. [Google Scholar] [CrossRef]
- Anton, B.; Leff, P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine 2006, 24, 3232–3240. [Google Scholar] [CrossRef]
- Pravetoni, M.; Comer, S.D. Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 2019, 158, 107662. [Google Scholar] [CrossRef]
- Torres, O.B.; Matyas, G.R.; Rao, M.; Peachman, K.K.; Jalah, R.; Beck, Z.; Michael, N.L.; Rice, K.C.; Jacobson, A.E.; Alving, C.R. Heroin-HIV-1 (H2) vaccine: Induction of dual immunologic effects with a heroin hapten-conjugate and an HIV-1 envelope V2 peptide with liposomal lipid A as an adjuvant. NPJ Vaccines 2017, 2, 13. [Google Scholar] [CrossRef]
- Sulima, A.; Jalah, R.; Antoline, J.F.; Torres, O.B.; Imler, G.H.; Deschamps, J.R.; Beck, Z.; Alving, C.R.; Jacobson, A.E.; Rice, K.C. A stable heroin analogue that can serve as a vaccine hapten to induce antibodies that block the effects of heroin and its metabolites in rodents and that cross-react immunologically with related drugs of abuse. J. Med. Chem. 2018, 61, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Jalah, R.; Torres, O.B.; Mayorov, A.V.; Li, F.; Antoline, J.F.; Jacobson, A.E.; Rice, K.C.; Deschamps, J.R.; Beck, Z.; Alving, C.R. Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on CRM197 carriers. Bioconjugate Chem. 2015, 26, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Beck, Z.; Torres, O.B.; Matyas, G.R.; Lanar, D.E.; Alving, C.R. Immune response to antigen adsorbed to aluminum hydroxide particles: Effects of co-adsorption of ALF or ALFQ adjuvant to the aluminum-antigen complex. J. Control. Release 2018, 275, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Luo, Y.X.; Sun, C.Y.; Xue, Y.X.; Zhu, W.L.; Shi, H.S.; Zhai, H.F.; Shi, J.; Lu, L. A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J. Neurochem. 2011, 119, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Stowe, G.N.; Vendruscolo, L.F.; Edwards, S.; Schlosburg, J.E.; Misra, K.K.; Schulteis, G.; Mayorov, A.V.; Zakhari, J.S.; Koob, G.F.; Janda, K.D. A vaccine strategy that induces protective immunity against heroin. J. Med. Chem. 2011, 54, 5195–5204. [Google Scholar] [CrossRef]
- Kosten, T.A.; Shen, X.Y.; O’Malley, P.W.; Kinsey, B.M.; Lykissa, E.D.; Orson, F.M.; Kosten, T.R. A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 223–229. [Google Scholar] [CrossRef]
- Bremer, P.T.; Schlosburg, J.E.; Lively, J.M.; Janda, K.D. Injection route and TLR9 agonist addition significantly impact heroin vaccine efficacy. Mol. Pharm. 2014, 11, 1075–1080. [Google Scholar] [CrossRef]
- Bremer, P.T.; Schlosburg, J.E.; Banks, M.L.; Steele, F.F.; Zhou, B.; Poklis, J.L.; Janda, K.D. Development of a Clinically Viable Heroin Vaccine. J. Am. Chem. Soc. 2017, 139, 8601–8611. [Google Scholar] [CrossRef]
- Hwang, C.S.; Bremer, P.T.; Wenthur, C.J.; Ho, S.O.; Chiang, S.; Ellis, B.; Zhou, B.; Fujii, G.; Janda, K.D. Enhancing Efficacy and Stability of an Antiheroin Vaccine: Examination of Antinociception, Opioid Binding Profile, and Lethality. Mol. Pharm. 2018, 15, 1062–1072. [Google Scholar] [CrossRef]
- Blake, S.; Bremer, P.T.; Zhou, B.; Petrovsky, N.; Smith, L.C.; Hwang, C.S.; Janda, K.D. Developing Translational Vaccines against Heroin and Fentanyl through Investigation of Adjuvants and Stability. Mol. Pharm. 2021, 18, 228–235. [Google Scholar] [CrossRef]
- Stone, A.E.; Scheuermann, S.E.; Haile, C.N.; Cuny, G.D.; Velasquez, M.L.; Linhuber, J.P.; Duddupudi, A.L.; Vigliaturo, J.R.; Pravetoni, M.; Kosten, T.A. Fentanyl conjugate vaccine by injected or mucosal delivery with dmLT or LTA1 adjuvants implicates IgA in protection from drug challenge. NPJ Vaccines 2021, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Schuchat, A.; Houry, D.; Guy, G.P. New data on opioid use and prescribing in the United States. JAMA 2017, 318, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Baehr, C.; Kelcher, A.H.; Khaimraj, A.; Reed, D.E.; Pandit, S.G.; AuCoin, D.; Averick, S.; Pravetoni, M. Monoclonal antibodies counteract opioid-induced behavioral and toxic effects in mice and rats. J. Pharmacol. Exp. Ther. 2020, 375, 469–477. [Google Scholar] [CrossRef]
- Raleigh, M.D.; Baruffaldi, F.; Peterson, S.J.; Le Naour, M.; Harmon, T.M.; Vigliaturo, J.R.; Pentel, P.R.; Pravetoni, M. A fentanyl vaccine alters fentanyl distribution and protects against fentanyl-induced effects in mice and rats. J. Pharmacol. Exp. Ther. 2019, 368, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.A.; Blake, S.; Faunce, K.E.; Hwang, C.S.; Natori, Y.; Zhou, B.; Bremer, P.T.; Janda, K.D.; Banks, M.L. Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacology 2019, 44, 1681–1689. [Google Scholar] [CrossRef]
- Barrientos, R.C.; Bow, E.W.; Whalen, C.; Torres, O.B.; Sulima, A.; Beck, Z.; Jacobson, A.E.; Rice, K.C.; Matyas, G.R. Novel vaccine that blunts fentanyl effects and sequesters ultrapotent fentanyl analogues. Mol. Pharm. 2020, 17, 3447–3460. [Google Scholar] [CrossRef]
- Pravetoni, M.; Pentel, P.R.; Potter, D.N.; Chartoff, E.H.; Tally, L.; LeSage, M.G. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS ONE 2014, 9, e101807. [Google Scholar] [CrossRef]
- Pravetoni, M.; Le Naour, M.; Tucker, A.M.; Harmon, T.M.; Hawley, T.M.; Portoghese, P.S.; Pentel, P.R. Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J. Med. Chem. 2013, 56, 915–923. [Google Scholar] [CrossRef]
- Raleigh, M.; Peterson, S.; Laudenbach, M.; Baruffaldi, F.; Carroll, F.; Comer, S.D.; Navarro, H.; Langston, T.; Runyon, S.; Winston, S. Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLoS ONE 2017, 12, e0184876. [Google Scholar] [CrossRef]
- Harandi, A.M. Vaccine Biomarkers: In Search of a Goldilocks Approach. EBioMedicine 2018, 29, 1–2. [Google Scholar] [CrossRef]
- Taylor, J.; Laudenbach, M.; Tucker, A.; Jenkins, M.; Pravetoni, M. Hapten-specific naive B cells are biomarkers of vaccine efficacy against drugs of abuse. J. Immunol. Methods 2014, 405, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Laudenbach, M.; Baruffaldi, F.; Robinson, C.; Carter, P.; Seelig, D.; Baehr, C.; Pravetoni, M. Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Sci. Rep. 2018, 8, 5508. [Google Scholar] [CrossRef]
- Crouse, B.; Robinson, C.; Kelcher, A.H.; Laudenbach, M.; Abrahante, J.E.; Pravetoni, M. Mechanisms of interleukin 4 mediated increase in efficacy of vaccines against opioid use disorders. NPJ Vaccines 2020, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Koob, G.; Baler, R. Biomarkers in substance use disorders. ACS Chem. Neurosci. 2015, 6, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Habelt, B.; Arvaneh, M.; Bernhardt, N.; Minev, I. Biomarkers and neuromodulation techniques in substance use disorders. Bioelectron. Med. 2020, 6, 4. [Google Scholar] [CrossRef]
- Wang, G.S.; Hoyte, C. Novel drugs of abuse. Pediatr. Rev. 2019, 40, 71–78. [Google Scholar] [CrossRef]
- Vandrey, R.; Johnson, M.W.; Johnson, P.S.; Khalil, M.A. Novel drugs of abuse: A snapshot of an evolving marketplace. Adolesc. Psychiatry 2013, 3, 123–134. [Google Scholar] [CrossRef]
- Rech, M.A.; Donahey, E.; Cappiello Dziedzic, J.M.; Oh, L.; Greenhalgh, E. New drugs of abuse. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 189–197. [Google Scholar] [CrossRef]
- Vunakis, H.V.; Farrow, J.T.; Gjika, H.B.; Levine, L. Specificity of the antibody receptor site to D-lysergamide: Model of a physiological receptor for lysergic acid diethylamide. Proc. Natl. Acad. Sci. USA 1971, 68, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
| Preclinical and Clinical Studies of Anti-METH Vaccines | ||||
|---|---|---|---|---|
| Hapten System | Carrier | Animal | Study Outcome | Ref |
| N-(4-aminobutyl) methamphetamine | BSA | Mice | Development and validation of a radioimmunoassay | [33] |
| (+) METH with a six-carbon spacer group at the para position of the ring structure | KLH | Sprague–Dawley rats | Rats injected with METH hapten-KLH conjugated vaccine developed METH antibody titers | [34] |
| MH1-MH7 | KLH | GIX Mice | METH haptens MH2, MH6 and MH7 conjugated with carrier proteins demonstrated very promising anti-METH antibody titer and METH affinity in mice model | [36] |
| MH6 | KLH | Rats | METH haptens MH6 conjugated with KLH reduced METH-induced thermoregulatory and locomotor effects | [37] |
| SMO9 | KLH | Rats | SM09 conjugated with KLH prevented rats from METH-induced impairment of food responses | [38] |
| N-succinylmethamphetamine (SMA) | KLH and TT | Mice | SMA-KLH/TT conjugated vaccine reduced the METH-induced hyperlocomotion successfully in the mice model | [39] |
| Nine (9) METH haptens with alkylating and peptide linkers | TT and DT | Webster mice | Hapten 12 conjugated with TT & alum/CpG ODN 1826 as adjuvant demonstrated excellent antibody titer (300,000) and reduced METH-induced locomotor activity compared to controls | [21] |
| MH6 | KLH | Wistar rats | MH6-KLH conjugated vaccine reduced the locomotor activity caused by 0.25 mg/kg METH administration by IP injection. | [40] |
| Preclinical and Clinical Studies Using Anti-METH mAbs | ||||
| mAbs Description | Model Tested | Study Results | Ref | |
| Assessment of mAbs with different affinity | Rats | Two anti-METH mAbs with different affinities were assessed and mAb with higher affinity were more effective to reduce the locomotor activity | [34] | |
| Anti-(+)METH mAbs (mAb; KD = 11 nM) | Sprague–Dawley rats | A significant reduction in METH concentration in brain (>60%) and increase in METH serum concentration (>6600%) | [19] | |
| Murine derived anti-METH mAbs (mAbH4 & mAbH8) | Rats | Both of the mAbs were effective in retaining METH in the circulation and prevented METH entry in the CNS and its subsequent effects. | [41] | |
| Assessment of anti-METH and anti-phencyclidine (PCP) mAbs combination | Pigeons | This combination demonstrated significant selectivity and prevented the drug induced behavioral effects. | [42] | |
| Human-mouse chimeric mAb for (+) METH with high selectivity and affinity | Rats | ch-mAb7F9 mAb demonstrated excellent binding potential with METH and altered the distribution (leass METH in brain and more in blood) of METH and clearance from the body. | [43] | |
| Chimeric -mAb7F9 | Rats | ch-mAb7F9 demonstarted attenuation in addiction related effect after METH acute dose | [44] | |
| ch-mAb7F9 | Humans | A Phase 1 clinical trial including 42 volunteers. A dose of 0.2 to 20 mg/kg bodyweight was administered to assess the safety of ch-mAb7F9 in humans. No adverse effect were reported | NCT01603147 | |
| IXT-m200 (ch-mAb7F9) | Humans | A phase IIa study was conducted to investigate the effects of anti-METH mAb and their effectiveness to retain the METH in the bloodstream. This is a randomized interventional clinical trial of 126 participants. The study demonstrated that IXT-m200 (ch-mAb7F9) altered METH AUC and Cmax significantly which was accounted to 30 fold and 8 fold respectively | NCT03336866 | |
| IXT-m200 (ch-mAb7F9) | Humans | This phase II study is currently recruiting volunteers to investigate the effectiveness of IXT-m200 (ch-mAb7F9) in people with acute METH toxicity. | NCT04715230 | |
| Platform Description | Study Details | Model Tested | Ref |
|---|---|---|---|
| Catalytic antibodies | Three active catalytic mAbs identified by high-throughput assay procedure and evaluated for their ability to hydrolyze cocaine | In vitro | [50] |
| Catalytic antibodies | Six cocaine and one non-cocaine novel transition state analogs were synthesized, characterized and evaluated in animal models. 6 out 7 analogues demonstrated high anti-cocaine titers in mice | Mice | [49] |
| Catalytic antibodies | Catalytic antibody mAb 15A10 was produced using a transition-state analog for the hydrolysis of cocaine. mAb 15A10 was effective in protecting the rats in cocaine overdose model. | Rats | [52] |
| Viral gene transfer of cocaine hydrolage (CocH) | CocH at 0.3 or 1 mg/kg was effective in reducing drug levels in plasma and brain of mice given cocaine (10 mg/kg, subcutaneously or 20 mg/kg intraperitoneally). MAb at 8 mg/kg had little effect on cocaine distribution. CocH and mAb alone were not effective to suppress locomotor activity induced by high dose cocaine (100 mg/kg body weight) but these two candidates completely suppressed the locomotor activity when given in combination. | Mice | [62] |
| Hapten design and conjugation with carrier protein | Three fluorine-containing cocaine haptens (GNF, GNCF and GN5F) and one chlorine-containing cocaine hapten (GNCl) were synthesized, based on a chemical scaffold of succinyl norcocaine (SNC). These haptens were conjugated with KLH and evaluated in a mice model. GNF-KLH demonstrated higher affinity and antibodies compared to parent compound SNC | Swiss Webster mice | [54] |
| Hapten design and conjugation with carrier protein | Rats vaccinated with GNC-KLH did not restore cocaine self-administration behavior when given a non-contingent cocaine infusion for 2 days. Active immunization with GNC-KLH produced an 8-fold rightward shift of the dose-effect function for cocaine. | Rats | [63] |
| Catalytic and non-catalytic hapten design | The effectiveness of noncatalytic and catalytic anti-cocaine vaccine was evaluated in mice. A cocaine-like hapten GNE and a cocaine transition-state analogue GNT were conjugated with KLH and both vaccines demonstrated high levels of cocaine-specific antibodies and suppressed cocaine-induced locomotor behavior. However, with repeated cocaine administration antibodies and protecting effects of catalytic vaccine waned. | Mice | [55] |
| Anti-cocaine mAb | The effectiveness of the anti-cocaine mAb GNC92H2 was examined in a cocaine overdose model. 93 mg/kg (LD50) of cocaine was administered to Swiss albino mice. GNC92H2 mAb was administered at dose levels ranging from 30 to 190 mg/kg. Significant blockade of cocaine toxicity was observed with the higher dose of GNC92H2 (190 mg/kg). | Swiss albino mice | [64] |
| CocH | This study reported that CocH gene transfer therapy was effective to prevent the negative impact on the heart, brain reward system, and locomotor activity caused by cocaine metabolites. | Rats | [57] |
| CocH-Fc | A novel cocaine hydrolase catalyzing enzyme accelerated the metabolism of cocaine in rat blood even after 20 days of a single dose of CocH-Fc. This new construct has extended biological half-life (107 h) compared to the original enzyme (8 h) | Rats | [57] |
| Double mutant cocaine esterage (DM CocE) | A single dose of DM CocE was effective in catalyzing the cocaine in plasma. DM CocE effectively prevented the cocaine-induced increase in blood pressure, heart rate and locomotor activity. | rhesus monkeys | [59] |
| Double mutant cocaine esterage (DM CocE) | The repeated administration of DM CocH was effective in hydrolyzing the cocaine in the plasma within 5 to 8 min. The repeat administration of DM CocH produced anti-CocE antibodies, but it did not alter the effectiveness of DM CocE in metabolizing the cocaine in plasma, cardiovascular effects | rhesus monkeys | [60] |
| DM CocE | This study investigated the effectiveness of DM CocE against cocaine toxicity and reverse the cardiovascular toxicities. This study concluded that DM CocE was effective in protecting cocaine induced convulsion, cardiovascular changes and shifted the cocaine induced lethality to 10-fold right. | Rats | [65] |
| Active cocaine vaccine (TA–CD) | This was a double blind, randomized multicenter trial to evaluate the effectiveness of anti-cocaine vaccine (TA-CD) in 300 participants. In this study an IgG levels ≥ 42 μg/mL (high IgG) was satisfactory, and this level was achieved by 67% of the vaccinated participants receiving five vaccinations. Although for the full 16 weeks cocaine positive urine rates showed no significant difference among the three groups (placebo, high, low IgG), after week 8, more vaccinated than placebo subjects attained abstinence for at least two weeks of the trial (24% vs. 18%), and the high IgG group had the most cocaine-free urines for the last 2 weeks of treatment. However, neither was significant. | Clinical trial, phase III | [66] |
| Active cocaine vaccine (TA–CD) | This is a randomized, double blind phase II study conducted with 15 participants for a period of 13 weeks. TA-CD was administered at two dose levels (82 µg, n = 4; 360 µg, n = 6) at weeks 1, 3, 5, and 9. The level of antibody varied among the subjects and individuals with higher antibodies had immediate (within 4 min of cocaine smoking) and robust (55–81%) reduction in ratings of good drug effect and cocaine quality, while those in the lower half showed only a non-significant attenuation (6–26%). | Clinical trial, Phase II | NCT00965263 [67] |
| Clinical Studies | ||||
|---|---|---|---|---|
| Candidate | Hapten System | Carrier | Study Outcomes | Clinical Trial Identifier |
| NicVax | 3′aminomethylnicotine | Pseudomonas aeruginosa rEPA | Phase III efficacy not demonstrated; increased abstinence related to antibody titer in phase II proof-of-concept | NCT01304810 NCT00598325 NCT00218413 NCT00318383 NCT00836199 NCT01102114 |
| Nic002 | O-succinyl-3′- hydroxymethylnicotine | VLP from bacteriophage Qβ | Primary end point not met in interim analysis of phase Iib study; per-protocol analysis of phase II study showed continuous abstinence rate at month 6 was 56% in high antibody group vs. 32.1% for placebo | NCT01280968 NCT00736047 NCT00369616 |
| Niccine | IP18 | Tetanus toxoid | Non-relapse rate at 1 year 43.3% for Niccine group versus 51.1% for placebo (95% CI = −20.6% to 4.9%)a | EudraCT 2007– 003250-29 |
| TA-NIC | Nicotine N1-butryic acid | rCTB | Failed to demonstrate efficacy in phase II proof of- concept | NCT00633321 |
| NIC7-001 | 5-aminoethoxy-nicotine | CRM | Study results not reported | NCT01672645 |
| SEL-068 | Nicotine | Proprietary polymer-based nanoparticle technology containing TLR agonist. | This study was designed to assess the safety and tolerability of subcutaneous injection of vaccine SEL-068. Study result not reported | NCT01478893 |
| NicA2 | Nicotine catalytic enzyme | Isolated from Pseudomonas putida S16, | This is study investigated the effect of NicA2 catalyzing enzymes. A NicA2 dose of 5 mg/kg reduced the brain Nic concentration 55% after 1 min and 92 % after Nic dose. The blood Nic concentration was below detection limit after the 1st or 5th Nic dose. | [75] |
| NicA-J1 | Nicotine catalytic enzyme (reengineered) | Originally isolated from Pseudomonas putida S16 and then genetically engineered | This candidate completely prevented nicotine entry into rats’ brain and nicotine like compulsive behavior | [76] |
| NicA2 variants | Catalytic enzyme | NA | The investigators isolated and identified several NicA2 variants with improved efficacy. Among all the variants characterized, A107R reduced the nicotine entry to brain 3-fold higher than wild type and the PEGylation of the enzymes improved it shelf life in the circulation | [77] |
| Candidate Name | Composition | Adjuvants | Study Outcomes | Ref. |
|---|---|---|---|---|
| Morphine-BSA | morphine-6-hemisuccinate conjugated with bovine serum albumin (BSA) | Not reported | The rabbits immunised with the Morphine-BSA vaccine significantly alter the morphine clearance during the first four hours of the morphine injection (6 mg/kg BW). | [109] |
| M-6-S-BSA | A morphine-6-succinyl conjugated to immunogenic protein carrier BSA | Freund’s complete adjuvant | In this study, the developed vaccine was given to goats, rabbits, mice, and rats at the dose of 2 mg/kg BW. The vaccine was given weekly up to 7 weeks and on week 8, each animal was injected with 2 mg Morphine sulfate/kg BW to assess the efficacy. The vaccinated animal demonstrated reduced locomotor activity compared to the control group | [111] |
| M-6-S-BSA | A morphine-6-succinyl conjugated to immunogenic protein carrier BSA | Not reported | BALB/c mice and SD rats were treated with the vaccine and demonstrated strong (up to 1:200,000 and over 1:20,000) and morphine-specific antibody titers. Radiant heat tail-flick reflex test also demonstrated that this vaccine can reduce the antinociceptive against morphine | [112] |
| M-6-S-BSA | A morphine-6-succinyl conjugated to immunogenic protein carrier BSA | Not reported | 347 morphine addicted people were vaccinated with M-6-S-BSA. Antibody response and safety was monitored one year. The antibody titre was at peak after the 3 months of the first injection and vaccine was well tolerated by the addicts. | [113] |
| M-TT | Morphine sulfate was conjugated with TT. A long spacer linker was used to connect the Morphine and TT | Not reported | This vaccine generated strong antibody response and prevented self-administration of heroin in immunized rats. | [114] |
| MorHap-TT Furthermore, combination of MorHap-TT+ palm-CV2 (HIV vaccine) | Morphine hapten was conjugated to carrier protein TT | MPL A ALF | In this study heroin and HIV vaccine was combined to assess the dual immunogenic profile. Immunised mice with both injections demonstrated satisfactory results. Palm-CV2induced anti-cyclic peptide titers at the degree of >106 and antibodies also prevented the binding of V2 peptide to the HIV-1 α4β7 integrin receptor. The anti-MorHap antibody was effective to prevent hyperlocomotion and antinociception induced by heroin. | [116] |
| 6-AmHap-TT | A novel hapten 6-AmHap was synthesized and conjugated with protein carrier TT | Liposomal MPLA (ALFA) | This study reported that the novel vaccine generated strong antibody response against heroin in mice model and demonstrated cross reactivity with codeine, oxycodone, hydrocodone, hydromorphone, and oxymorphone | [117] |
| MorHap-TT and cross-reactive material 197 (CRM197). | Heroin/morphine hapten (MorHap) conjugated with TT and CRM197 | L(MPLA) | Immunization of mice with these vaccines produced strong antibody titers (400–1500 ug/mL) against heroin and its metabolites 6-acetylmorphien and morphine. TT based vaccine demonstrated better inhibition of heroin induced antinociception which correlates with its hapten density. | [118] |
| M-KLH | Morphine is conjugated with KLH | Not reported | The study reported the development of M-KLH conjugate and assessment of of efficacy in rat models. The conjugated vaccine demonstrated strong antibody response and was able to attenuate heroin induces locomotor activity. The dopamine concentration in the brain was significantly lower in vaccinated mice compared to KLH group (126.08 ± 22.05 ng/mL vs. 45.58 ± 8.36 ng/mL) | [120] |
| Heroin/Morphine-KLH | Two heroin and morphine like haptens were synthesized and conjugated with KLH | Not reported | Heroin like vaccine system was effective to block self-administered of Heroin and antinociception induced by heroin. | [121] |
| KLH-6-SM | 6-SM hapten was conjugated with KLH | Not reported | This study was designed to investigate the efficacy of a morphine like vaccine against morphine and other heroin like metabolites. This study reported that antibody binding was prevented by free morphine and heroin like metabolites, reduced antinociception caused by morphine and reduced the morphine concentration in the rat’s brain. | [122] |
| Heroin-KLH | Heroin in conjugated with immunogenic protein carrier KLH | TLR9 agonist CpG ODN 1826 | The routes of immunization have been investigated by vaccinating the mice via SC and IP. Mice vaccinated via SC demonstrated inferior antibody responses compared to IP. CpG ODN 1826 increased the antibody response significanatly compared to control. | [123] |
| Various | Various hapten system has been conjugated with various carrier protein such as TT, DT, KLH | Al(OH)3 CpG ODN 1826 | The study inbvestigated a series of hapten system and carrier protein along with adjuvants. A combination of hapten (HerCOOH), adjuvant (CpG ODN + alum), carrier protein (TT) was found to be efficacious. | [124] |
| Various | 20 vaccine formulations have been investigated | TLR9 & TLR3 agonist | This study investigated the 20-vaccine formulation varying the carrier protein and adjuvants. TLR3 and TLR9 based vaccine formulation alone demonstrated strong antibody titer but combination of these two did not improve the antibody titre. Stability study revealed that TLR3 based formulation was more stable than TLR9. TLR9 + alum heroin vaccine formulation was effective to prevent the heroin lethal dose toxicities. | [125] |
| Heroin-TT/CRM and fentanyl-TT/CRM vaccine | Heroin and fentanyl is conjugated with TT/CRM along with adjuvants alum and CpG ODN | Advax/CpG ODN/δ-inulin | This study reported that inulin-based Heroin vaccine along with CpG ODN provided superior efficacy compared to other combination. Freeze dried vaccine formulation demonstrated stability up to one year at room temperature. | [126] |
| FEN-TT | FEN conjugated to TT | Liposome with MPLA adsorb on Alum | The investigators developed a liposomal conjugated vaccine system using MPL A and alum and reported that this vaccine demonstrated a strong antibody response in order of greater than 106 and the antinociceptive dose–response curve was shifted to the right | [132] |
| FEN-TT | FEN conjugated to TT | Not reported | This study investigated the effectiveness of the FEN-TT conjugated vaccine to alter the FEN self-administration in an experimental model called “fentanyl vs. food choice model”. This vaccine was effective to reduce the FEN reinforcement significantly and increased the food reinforcement. The study also demonstrated that this conjugated vaccine prevented the FEN withdrawal following 12 h FEN session. | [131] |
| FEN-sKLH and FEN-KLH | Fentanyl (FEN) conjugated to subunit KLH (sKLH) or KLH | Not reported | The study demonstrated that both FEN-KLH and FEN-sKLH reduced the hot plate-induced antinociception and distribution of Fentanyl to the brain. However, FEN-sKLH was more effective in reducing respiratory depression and overdose toxicity after cumulative administration of 50 µg/kg fentanyl dose. | [130] |
| FEN-BSA or FEN-TT | Fentanyl conjugated with BSA or TT | alum, dmLT, or LTA1 | This study investigated the effect of various routes of administration and the adjuvant system. This is study demonstrated that FEN-TT conjugate with dmLT, or LTA1 adjuvant demonstrated superior efficacy when administered sublingually. | [127] |
| OXY-dKLH | Oxycodon conjugated with KLH dimer and adsorbed into | Alhydrogel | This study conducted in animal model confirmed that vaccinated mice demonstrated reduced effect on two dangerous cause of Oxycodone overdose fatality which are respiratory degression and heart rate. | [135] |
| (6OXY(Gly)4–KLH) | Oxycodone conjugated with tetra glycine linker and KLH immunogenic carrier | Not reported | The study reported that (6OXY(Gly)4–KLH) vaccine increased drug serum binding and reduced the distribution drug to the brain. | [134] |
| OXY-KLH | Oxycodone conjugated with KLH | Not reported | The study confirmed that OXY-KLH vaccine demonstrated increased amount of adenylate cyclase 5 (Adcy5), decreased amount of early growth response protein 2 (Egr2) and the early immediate gene c-Fos in the striatum. These findings further confirmed that this vaccine has the capability of reducing the reinforcing effects of oxycodone. | [133] |
| Oxy (Gly)4-sKLH | Oxycodone was linked with tetra glycine peptide linker and KLH immunogenic carrier | Not reported | A phase 1 & 2 study has been registered recently with clinicalTrial.gov (accessed on 11 October 2022) and currently recruiting participants. This trial is going to investigate the effect of vaccine Oxy (Gly)4-sKLH against oxycodone. This is a multisite study aiming to assess the safety, degree of antibody and efficacy. | NCT04458545 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.K.; Davidson, M.; Kypreos, E.; Feehan, J.; Muir, J.A.; Nurgali, K.; Apostolopoulos, V. Immunotherapies for the Treatment of Drug Addiction. Vaccines 2022, 10, 1778. https://doi.org/10.3390/vaccines10111778
Hossain MK, Davidson M, Kypreos E, Feehan J, Muir JA, Nurgali K, Apostolopoulos V. Immunotherapies for the Treatment of Drug Addiction. Vaccines. 2022; 10(11):1778. https://doi.org/10.3390/vaccines10111778
Chicago/Turabian StyleHossain, Md Kamal, Majid Davidson, Erica Kypreos, Jack Feehan, Joshua Alexander Muir, Kulmira Nurgali, and Vasso Apostolopoulos. 2022. "Immunotherapies for the Treatment of Drug Addiction" Vaccines 10, no. 11: 1778. https://doi.org/10.3390/vaccines10111778
APA StyleHossain, M. K., Davidson, M., Kypreos, E., Feehan, J., Muir, J. A., Nurgali, K., & Apostolopoulos, V. (2022). Immunotherapies for the Treatment of Drug Addiction. Vaccines, 10(11), 1778. https://doi.org/10.3390/vaccines10111778









