Abstract
It has been demonstrated that noncoding RNAs have significant physiological and pathological roles. Modulation of noncoding RNAs may offer therapeutic approaches as per recent findings. Small RNAs, mostly long noncoding RNAs, siRNA, and microRNAs make up noncoding RNAs. Inhibiting or promoting protein breakdown by binding to 3’ untranslated regions of target mRNA, microRNAs post-transcriptionally control the pattern of gene expression. Contrarily, long non-coding RNAs perform a wider range of tasks, including serving as molecular scaffolding, decoys, and epigenetic regulators. This article provides instances of long noncoding RNAs and microRNAs that may be a biomarker of CVD (cardiovascular disease). In this paper we highlight various RNA-based vaccine formulation strategies designed to target these biomarkers—that are either currently in the research pipeline or are in the global pharmaceutical market—along with the physiological hurdles that need to be overcome.
1. Introduction
Even · though most of the genome is transcribed, only around 1–2% of exons actually code for proteins. The remaining noncoding segments consist of short and long-noncoding RNAs (lncRNAs). The vast majority of short noncoding RNAs are microRNAs (miRNAs). MiRNAs, the vast majority of which range in length from 21 to 23 nucleotides, play crucial roles in many aspects of biology, including cell development, proliferation, and survival [1]. The most recent version of miRBase reports that 1881 human miRNA precursors and 2588 human miRNAs have been uncovered. By binding to complementary 3′-untranslated regions of target mRNAs, they prevent translation or trigger mRNA disintegration. The precursor pri-miRNAs are the starting point from which miRNAs are produced. A 70-nucleotide-long premiRNA is produced when a pri-miRNA is synthesised within the nucleus with the help of the drosha-DGCR8 DGCR (DiGeorge syndrome chromosomal region) complex. Following further processing in the cytoplasm, the mature miRNA, which is close to 22 nucleotides in length, is produced [2]. Since a single miRNA may modify the expression level of so many mRNAs and hence influence so many biological processes in numerous tissues, it may not be surprising that expression levels of miRNAs have been discovered in a number of pathological states, such as cardiovascular or metabolic disorders [3,4]. They consequently emerged as viable therapeutic targets. Existing techniques for altering miRNA performance fall into two categories. Overexpression of a target miRNA can be achieved with the use of miRNA mimics or viral vectors, whereas its inhibition can be achieved with antisense oligonucleotides (ASOs) or genetic knockout mice models. Small noncoding RNAs are less diverse than lncRNAs, which are >200 nucleotides long. So far, investigations have led to the identification of over 60,000 lncRNAs in the human genome [5]. Intergenic areas, intronic segments of protein-coding genes, and even antisense strands belonging to a particular gene are responsible for the formation of lncRNAs. Eventually, lncRNAs are also produced via the reverse splicing of exons, which creates circular RNAs.
They can serve as a scaffolding for protein complexes, a decoy for molecules under target so as to dampen their activity, or an epigenetic regulator of gene transcription [6,7,8]. As an added bonus, lncRNAs can influence transcription and post-transcriptional gene regulation by interacting with DNA, RNA, or miRNAs through splicing or sponging. Like miRNAs, numerous lncRNAs are dysregulated in a wide variety of diseases. Understanding if lncRNAs can be therapeutically targeted like miRNAs will be beneficial. To induce lncRNA degradation and inhibit their function, gapmers or small interfering RNAs (siRNAs) are effective agents [9]. Table 1 discuss the important miRNAsin various CVD along with their mechanism of action.

Table 1.
Illustrations of Some of the Most Important microRNAs in the CVD.
This review article investigated the benefits and probable drawbacks of employing noncoding RNA vaccines as therapeutic targets in the management of CVD amidst a lot of clinical controversies along with various physiological hurdles that need to be considered while developing such formulations. A summary of key miRNAs and lncRNAs that have been linked to cardiovascular diseases are discussed. This article also covers the various delivery strategies and challenges associated with the delivery of non-coding RNAs. Last but not the least, the article presents six principles based on FDA/EUA-approved RNA-based therapy for vaccine development to be clinically relevant in the management of cardiovascular diseases. Clinicians and experimental researchers have started focusing on this particular field of therapy alongside the conventional allopathy system of medicine. Accordingly, this review article on this new field of therapy provides insight and contributes to developing future management of cardiovascular diseases.
2. Therapeutics
2.1. Addressing Pressing Issues of CVD Using miRNA Inhibitors
As was mentioned above, miRNAs are known to control post-infarction remodelling and angiogenesis, cardiac hypertrophy and fibrosis, arrhythmias, atherosclerosis, and metabolic disorders [3,4,9,33,34]. In order to block or overexpress certain miRNAs, investigators have mostly used genetically altered mice, RNA therapies, or viral vectors to study the function of miRNAs. The major purpose of ASOs or siRNAs is to block a specific miRNA. For improved stability against RNases, both compounds have undergone chemical modification, primarily employing phosphorothioate backbones. In addition, RNAs were altered to lessen the potential for triggering an innate immune response, improve cellular absorption by improving cholesterol conjugation, and reduce off-target effects leading to improvement in the drug’s pharmacodynamics. Antisense molecules are perfect complements to their intended microRNA targets, which prevents them from coupling with their designated mRNA targets and therefore limits miRNA expression. Chemical modifications allow us to classify miRNA inhibitors, known as antimiRs, into distinct groups. The cellular uptake of certain RNAs, for example, was enhanced by increasing cholesterol conjugation, and the risk of activating an innate immune response decreased so as to improve pharmacodynamic properties. MiRNA inhibitory effect is reduced by antisense molecules that perfectly complement the target miRNA and prevent base pairing with the designated mRNA targets. MiRNA inhibitors or antimiRs, are classified according to the specific chemical modifications used to reduce off-target effects; they reduce the possibility of eliciting an innate immune response and improve cellular uptake via cholesterol conjugation so as to improve the pharmacodynamics. MiRNA inhibitory effect is reduced by antisense molecules that perfectly complement the target miRNA and prevent base pairing with the designated mRNA targets. AntimiRs are generally distinguished by the chemical modifications they cause.
2.2. Antisense Oligonucleotides (ASO)
In this group, antagonists and locked nucleic acid (LNA) antimiRs are well-known participants. The oligonucleotides that make up antisense miRNAs (antagomiRs) have a structure of 2′-O-methyl, 2′-fluoro, or 2′-O-methoxyethyl accompanied by a second phosphorothioate backbone linked with the sulphur atom in place of the non-bridging oxygen atoms present in the phosphate group. The phosphorothioate backbone’s capacity to bind to plasma proteins (particularly albumin) so as to augment stability through nuclease resistance results in decreased renal clearance and better pharmacokinetic qualities. By adding a 2′-O-Me, 2′-fluoro, or 2′-methoxyethyl group, it is possible to increase the target miRNA’s ability to bind to the compound and minimise off-target effects. Cholesterol, meanwhile, increases the ability of antagomiRs to enter cells and inhibit them. The discipline of oligonucleotide chemistry has advanced significantly owing to the emergence of LNA-modified antimiRs. Linking the 2′-oxygen and 4′-carbon to form a bridge that resembles the C3′ end of a ribonucleotide is necessary for chemically locking LNAs. In a variety of in vivo studies, including those involving nonhuman primates, and clinical investigations, deoxyribonucleotide with a locked ribonucleotide sequence formed mixmers which have shown great potential [35,36]. LNA-based antimiRs are 15–16 nucleotides in length and show great specificity for the miRNA they are designed to inhibit. The 8-mer LNA-based antimiR (small LNAs) is a different LNA-based antimiR variant [37]. Small LNAs can target many miRNA families at once despite their similar functions because they bind only the miRNA seed domain. Therefore, it may be possible to increase therapeutic potential in particular disease states by simultaneously targeting each and every member of an miRNA family. One such example is a small LNA that specifically targets the miR-15 family’s seed region (miR-15a, 15b, 16-1, 16-2, 495, and 497).
In comparison with the classic LNA-based antimiR form, which only targeted a single miRNA family member, this LNA was far more efficient in derepressing downstream targets. Different short and long LNAs were all taken up by heart tissue, demonstrating that antimiR length is not necessarily a determining factor in cellular uptake. However, it appears that short LNAs are not always as efficient as the longer type of oligonucleotides [10]. In contrast to small LNAs targeted against miR-21, antagomir therapy improves cardiac fibrosis and hypertrophy while inhibiting miR-21. To enhance the performance of LNA antimiRs, two additional methods were created. Selenomethylene LNAs were distinct from the family of bicyclic RNA analogues because they had higher activity, stable metabolism, and affinities for inhibiting miR-21 in cancerous cells [38]. Another tactic is known as the “small RNA zip,” and it attempts to address related issues. Based on LNAs, short RNA zippers are designed to bridge the gap between two miRNA sequences that are only half complete due to a nucleotide gap. Using short RNA zippers, miR-17 and miR-221 were repressed in cell lines of breast cancer. However, there are currently no published reports on the potential use of either of the innovative inhibitory approaches in the treatment of CVD [39]. At last, peptide nucleic acids (a type of ASO) are an alternative to phosphate-sugar polynucleotides in which the nucleobases are connected to a flexible pseudopeptide polymer. Methyl carbonyl groups replace the phosphodiester backbone and connect the purine and pyrimidine bases to the monomer units of N-(2-aminoethyl) glycine. Peptide nucleic acids are highly resistant to DNases and proteinases and have a high binding affinity for their intended sequence. According to in vivo studies, peptide nucleic acids are effective against miRNAs, as demonstrated by the suppression of miR-155 in mouse B cells [40,41].
2.3. siRNAs and other Inhibitory Techniques
siRNAs are an alternative to ASOs, which are employed to silence miRNAs. These are chemically modified RNA duplexes used to enhance the nuclease stability and cellular uptake, much like antimiRs. The target miRNA is silenced when an siRNA binds to its loop region. For instance, transplanting skeletal myoblasts in mice with myocardial infarction was found to have fewer arrhythmogenic effects when miR-181a expression was reduced by employing siRNAs. MiRNA target site blockers are another targeting tactic. Due to their complementary binding, they prevent miRNAs from reaching their mRNA target region. By employing this technique, individual miRNA targets might be protected as opposed to all targets being affected simultaneously. Target site blockers demonstrated a particular reduction in miR-155’s capacity to bind to the Cebpb (CCAAT/enhancer-binding protein) gene in the hypothalamus of neonates.
Last but not least, miRNA sponges, which are generated within cells from transgenes, may be utilised to alter or reduce miRNA function. Inserted into the 3’ untranslated domain, a target miRNA can link with 4–10 complementary sites on sponge RNAs. It is feasible to select a binding site that interferes with the binding of a given miRNA to its targets by either attaching to the miRNA seed portions or basing itself on a specific mRNA target sequence. Since miRNA sponges require transgenic construct, they may be administered to tissue in living animals through viral vectors. Adenoviral eGFP (enhanced green fluorescence protein) sponges were used to downregulate miR-133 in cardiac myocytes in a mouse model depicting ventricular hypertrophy. MiRNA sponges require a large quantity to bind a large amount of endogenous miRNA in a cell [42,43,44,45]. Results from a wide range of cardiovascular studies in mice and occasionally in larger animal models, such as pigs, show that the aforementioned antimiRs effectively reduce miRNA activity in the artery wall and cardiomyoytes.
2.4. Antagomirs as Therapeutic Agents
Antagomirs, or anti-miRs, are a type of synthetically created oligonucleotide that aim to inhibit endogenous microRNAs (also known as miRNAs or miRs) [46]. MiR-92a antagomirs increase neovascularization following hindlimb ischemia and cardiac function recovery in mice [16,47]. Single injection (300 µg/mice; diluted in in vivo jet PEI solution) of microsphere antagomiR-92a prevented unfavourable infarct remodelling in a percutaneous pig model of reperfused AMI [18]. Furthermore, atherosclerosis and endothelial dysfunction were decreased even more by miR-92a antagonists in mice and rats [29]. In addition, AntagomiRs suppressed miR-25, which is elevated in a failing heart and shares a seed sequence with miR-92a. This treatment enhanced cardiac function, lengthened life, and slowed down the progression of heart failure in mice [27]. Later experiments with strong decoys confirmed the positive advantages of miR-25 suppression. However, a separate study found that antagomiR-25 delivered through i.p. injection of 80 mg/kg dose, spontaneously produced irregularities in cardiac function [48,49].
The notion that differences in antagomiR-25 formulation and concentration are responsible for the observed effects is still debatable. Heart function was found to improve in ischemia/reperfusion-induced injury when miR-320 is inhibited by antagomirs. Eventually, miR-212/132 antagonism reverses TAC-induced ventricular hypertrophy and failing heart in mice [50,51].
The use of LNA-based antimiRs, which suppress miRNAs, has been demonstrated and used in various disease models. Protecting against aneurysms, LNAs that target miR-29 do so by boosting matrix synthesis and preserving the arterial wall integrity [52,53]. Increased cardiac function and survival in failing hearts are additional benefits of therapeutic miR-208a inhibition with LNA-based antimiRs [54]. Suppressing the miR-34 family or inhibiting miR-34a improves myocardial infarct healing by reducing cell death and fibrosis [13,14,15]. Two types of miR-34a inhibitors, antagomiRs and LNAs, were equally efficacious in chronic myocardial infarction in mice. However, as a dose-response curve was not presented, the results should be interpreted with caution due to the fact that varying amounts of antimiR were utilised. It was also speculated that LNA-based antimiRs targeting miR-34a might increase cardiomyocyte proliferation and extend the window of opportunity for regeneration in postnatal animals. The favourable benefits of antagomiR-based miR-92a reduction were validated using LNA-based antimiRs in a pig ischemia/reperfusion model [13,15,17].
2.5. CVD Treatment with lncRNA Inhibition
ShRNAs, which are produced from siRNAs, modified ASOs, and gapmers, can silence lncRNAs in the same way as anti-miRs silence miRNAs. Gapmers can target nucleus rich with lncRNAs despite nuclear barriers, while small interfering RNAs (siRNAs) and ASOs preferentially target lncRNAs in the cytoplasm. To carry out something like this, ribonuclease H-dependent RNA cleavage must be introduced [6,55,56]. Since lncRNAs are mostly nuclear, antisense LNA gapmers have become the standard. These gapmers are hybrid ASOs with a deoxynucleotide monomer core long enough to initiate the RNase H cleavage critical phase. The 2′-O-modified ribonucleotides on both sides of the central block provide further defence against nuclease degradation. Gapmers are often stabilised for in vivo treatment approaches employing a phosphorothioate backbone, in the same way as other ASOs. Gapmers have been used to specifically target PCSK9 (proprotein convertase subtilisin/kexin type 9) present in the livers of nonhuman primates in in vivo studies. Nonetheless, hepatotoxicity from off-target effects halted a phase I clinical study. In contrast, no adverse events were reported over the whole year of observation of gapmers against hypoxia-inducing factor 1 [57]. Numerous studies show a potential for in vivo silencing of cardiovascular-related lncRNAs; for example, injection of gapmer, which pharmacologically suppresses the hypoxia-induced lncRNA Malat-1, decreases endothelial cell proliferation and ischemia-induced re-vascularization [58]. Inhibition of Meg3 after TAC reduced myocardial fibrosis and enhanced diastolic function, whereas gapmers against Chast dramatically reduced myocardial hypertrophy [59]. Thus, both therapy methods provide potential therapeutic options for halting heart remodelling. LincRNA-p21 and APF were both effectively used to demonstrate how lncRNAs may be inhibited by siRNAs. As was previously reported, lincRNA-p21 is a critical modulator of proliferation and cellular death in the case of atherosclerosis by suppressing p53 transcriptional activity.
Suppressing it using lentivirus-mediated siRNA release targeting lincRNA-p21 causes neointima hyperplasia in a carotid artery damage model. Damage produced by ischemia/reperfusion was mitigated in mice when the lncRNA APF, which controls the death of autophagic cells in in vitro studies, was inhibited by siRNAs [60,61]. The adequate suppression of lncRNAs in vivo requires large and frequent dosages of siRNAs and gapmers (4–20 mg/kg, several recurrent injections). Potential dose-dependent toxicities may be taken into consideration throughout preclinical and clinical testing depending on the chemistry of the inhibitor being employed. Gapmers may cause hepatotoxicity in a way that is dependent on RNase H1 in addition to the normal toxicities of RNAs [62].
Reducing RNase H1 levels before administering LNA-modified gapmers greatly reduced their hepatotoxicity, suggesting that off-target RNase-dependent RNA breakdown is responsible for the toxicity. Another challenge in developing lncRNA therapeutics is that, unlike miRNAs, lncRNAs are not necessarily conserved across species.
Therefore, it is crucial to carefully organise and execute animal experiments that focus on the action mechanism of a specific lncRNA, as well as studies on toxicity, before adopting the findings in the clinic. One option is to employ the human-specific gene instead of the endogenously expressed sequence in animal models of toxicology. However, this has the downside of limiting research into the harmful effects of a gapmer to only those that do not depend on hybridization. Humanized models or organoid cultures may be useful for elucidating the role of lncRNAs in humans. This is an important step since lncRNAs’ effects on chromatin structures or epigenetic regulatory processes may be nuanced, and the development of lncRNA therapeutics may be hampered if the underlying mechanism is not understood. Most mRNAs and miRNAs are found in the cytoplasm, whereas lncRNAs are primarily expressed in the nucleus, where they may be a part of inaccessible complex structures. Small compounds intended to selectively interfere with conserved RNA structures, such as those that disrupt RNA protein complexes, may be advantageous. Table 2 summarises some crucial lncRNAs in CVD.

Table 2.
Long non-coding RNA.
3. Pharmaceutics of RNA-Based Vaccine Delivery
3.1. Hurdles in the Systemic Delivery of siRNA
Whatever may be the biochemical and molecular mechanism, all RNA payloads must avoid off-target organ clearance, enter the target tissue, interact with the appropriate cell type in a dynamic microenvironment, be taken up via endocytosis, and then escape the endosome without triggering a detrimental immune response. Unlike ASOs, siRNAs, and other short RNA therapies, mRNA and DNA-mediated therapies need a vehicle for cellular entry. Numerous polymer- and LNP-based RNA delivery technologies have been developed by researchers. We have discussed the problems that come with using siRNA.
3.2. Stability in the Circulatory System
When injecting siRNA into a vein, stability in the circulatory system should be the primary concern. Naked siRNA is easily broken down by several endogenous enzymes and can become aggregated together by serum proteins in the circulation, thus it is important that the siRNA delivery mechanism is "cohesive" enough to prevent this. Surface qualities that facilitate engagement with the intended cellular targets must be presented by the delivery method while simultaneously limiting nonspecific opsonization, phagocytosis, and eventually immune triggering. Since naked siRNA gets cleared from the bloodstream within 5 min of intravenous delivery, its circulation must be maintained for it to be effective [73,74,75,76,77].
3.3. Vascular Endothelium as the Semiselective Barrier
The endothelium controls the diffusion of substances into tissues by serving as a selective barrier between the arterial lumen and surrounding tissue. Basically, there are three types of normal capillary endothelium: continuous endothelium, fenestrated capillaries, and discontinuous capillaries [78]. To achieve a high capacity for crossing the barrier posed by endothelium, the siRNA delivery size should be less than 150 nm, since this is the size limit imposed by the normal endothelial structure. Retention and enhanced permeability are the two factors on which the efficacy of a drug largely depends. Modifying the localised shape of blood arteries can also affect the efficacy of drug delivery. Nanoparticles (NPs) with a size of 500 nm or less, and often less than 150 nm, showed dramatically augmented penetration and retention properties in "leaky" tumour blood arteries [79].
3.4. Extracellular Matrix Diffusion
The extracellular matrix (ECM) is a complex network of polysaccharides and fibrous protein gels that surround and support cells. The siRNA-loaded vehicle must first cross the vascular endothelium before entering the ECM. Larger NPs have trouble penetrating tumour tissue due to the tight nature of the ECM. Since nanocarriers are so tiny, they are better able to transport siRNA into tissues that have low permeability. Furthermore, NPs’ charges may potentially have an impact on particle diffusion. It has been shown that the diffusion of neutral particles is quicker than the charged ones [80].
3.5. Cytoplasmic Delivery
For siRNA to be effective, the delivery mechanism must first cross the cell membrane, then enter the cytoplasm, and finally discharge its cargo. Due to its negative charge, however, siRNA has a hard time crossing the negatively charged cell membrane. Endocytosis mechanisms such clathrin-mediated endocytosis (CME), macropinocytosis, and caveolae-/lipid raft-mediated endocytosis are typically used by nanocarriers to deliver siRNAs cargo into cells [81,82]. After macropinocytosis, siRNA is typically delivered to the endosome where it is degraded enzymatically. Once in place, the siRNA transporter quickly fuses with lysosomal vesicles. Consequently, successful siRNA dispersion also needs endosome egress.
Compared with macropinocytosis and CME, caveolae-/lipid raft-mediated endocytosis is a less well-understood process [83,84]. Direct cytosolic administration of the therapeutic agent, via the lipid raft mode, has been proven to be possible and may provide a novel method for improved cytosolic siRNA delivery [85,86,87].
A variety of non-viral siRNA delivery vehicles have been fabricated to avoid systemic delivery problems. These include polymers or lipid polymer hybrid NPs, lipid-based NPs, hydrogels, microbubbles, inorganic NPs such as gold, quantum dots, silica, carbon nanotubes, iron oxides, exosomes, and oligonucleotide NPs [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104]. When compared with inorganic carriers and viral vectors, lipid-based NPs stand out as having superior biocompatibility and biodegradable qualities.
4. Delivery Strategies to Improve the Targeting and Therapeutic Efficacy of Non-Coding RNA-Based Vaccines
There are still obstacles to overcome despite the extensive study of oligonucleotide-based therapeutics targeting shRNA (short hairpin RNA) and lncRNAs in the cardiovascular domain. There are two major aims. The goal is to lessen the amount of drug needed to block noncoding RNAs in cardiovascular tissues and reduce hybridization-independent toxicity. The toxicity and sequence-specific adverse effects of using antisense therapy can be mitigated through targeted delivery to specific cell types. Dose-dependent toxicities and off-target effects can be mitigated by using more efficient delivery techniques which increase cellular uptake or cell-type selectivity. A ubiquitously expressed miRNA may perform beneficial and detrimental effects in different cell types. Therapeutic overexpression or inhibition of an miRNA requires targeting the proper cell type. MiRNA inhibitors, such as those belonging to miR-15 or miR-34 families, increase cardiomyocyte proliferation and heart regeneration [11,13]. Such a strong miRNA inhibitor will likely enhance division in other cell types, thereby promoting tumour growth, while stimulating cardiomyocyte proliferation may have therapeutic effects and allow for prolonged heart regeneration. MiR-34 and miR-15 limit tumour development, and miR-34 overexpression are looked into for possible cancer treatment [105,106,107]. Below we have discussed some drug delivery options for noncoding RNAs.
4.1. Lipid and Lipid-Based Nanoparticle Vaccines
The Food and Drug Administration (FDA) has approved the use of LNPs (lipid nanoparticles) for the transport of siRNA to the liver [108] and mRNA vaccines [109,110]. Micelles, liposomes, and LNPs (Figure 1) are all different forms of lipids differentiated by the overall size of the hydrophilic head group and hydrophobic tail or tails [111]. Each of the four major components (cationic or ionizable lipid, helper lipid, cholesterol, and poly(ethylene glycol) (PEG)-lipid) (Figure 2) constitute FDA-approved LNPs. Studies using lipid-based delivery systems complexed with nucleic acid [112,113] indicate that lipid structure modifies LNPs’ interactions with cells [114]. Hundreds to thousands of chemically distinct lipid delivery systems [115,116] have been developed as a result of the fact that lipid structure influences the delivery of nucleic acids and that such systems can be easily produced using Michael addition-based, epoxide-based, and alcohol-based chemical reactions. Many of these studies aimed to enhance the delivery of siRNA to hepatocytes or the liver cells of mice [117]. In conjunction with a more rational approach to lipid design [114], these investigations reduced the dosage required for robust in vivo gene silencing in the hepatocytes of mice from around 1.0 mg/kg [118] to 0.002 mg/kg. DLin-KC2-DMA is an ionizable lipid [114]; cKK-E12 is a peptide-like lipid compound [119]; DLin-MC3-DMA [120] was used in patisiran to treat hATTR [108]; and C12-200 was synthesised using an epoxide–amine reaction [117], LNPs have been used to transport messenger RNA (mRNA) to the livers of mice, non-human primates, and humans. Some LNPs made use of lipids that have been optimised for siRNA transport. For instance, mRNA was transported to the liver in mice using LNPs in conjugation withcKK-E12 [121,122], C12-200 [123], and DLin-MC3-DMA [124]. mRNA has also been transported to the mice hepatocytes by more recently reported lipids such as LP01 [125] (Intellia Therapeutics), Lipid H [126] (Moderna), and FTT5 [127] (Ohio State and Beam Therapeutics) (Figure 3).
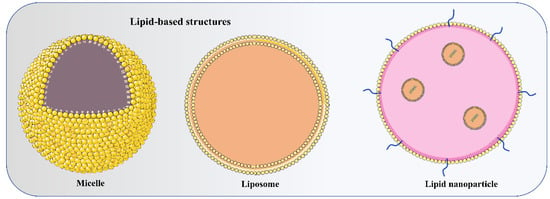
Figure 1.
Depicts the basic structure of micelle (monolayer), liposome (bilayer), and lipid nanoparticle (LNP) (multiple lipid layers with microdomain) used to deliver non-coding RNAs to target cells.
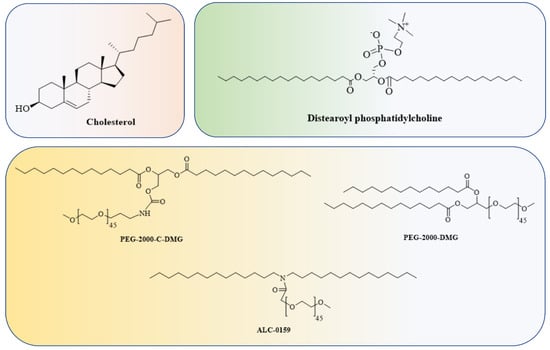
Figure 2.
Cholesterol, a helper lipid, a PEG-lipid, and the RNA payload are common components of LNPs.
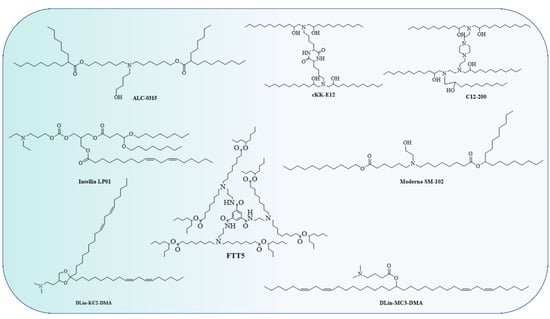
Figure 3.
Though the lipids have different chemical structures and chain lengths, the presence of a tertiary amine at acidic pH takes a proton and turns it into a cation, i.e., a cationic lipid. This cationic lipid binds to the anionic part of RNA, forming a stable LNP.
Two LNPs made using an undisclosed cationic or ionizable lipid, PEG-lipid, cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) were recently used to transport mRNA expressing a base-editing Cas9 and sgRNA targeting PCSK9 to the liver in non-human primates [128,129]. After a single injection of LNP, the PCSK9 gene was silenced permanently. Long-term PCSK9 inhibition with antibodies [130] or siRNA [131] has been shown to improve CVD in humans. Beam Therapeutics independently reported long-lasting effects in the livers of non-human primates after LNP-mediated base editor delivery [132].
Complementing prior preclinical studies, Intellia has also disclosed data in patients dosed with NTLA-2001 to inactivate the TTR gene. There is evidence that TTR inactivation is effective in people, as therapy with siRNA or ASOs targeting TTR decreased the development of hATTR amyloidosis with polyneuropathy [133,134]. Alnylam, Moderna, and Pfizer/BioNTech/Acuitas LNPs each include four extra components in addition to their respective RNA payloads: cholesterol; the PEG-lipids PEG-2000-C-DMG (Alnylam), PEG-2000-DMG (Moderna), or ALC-0159 (Pfizer/BioNTech/Acuitas); and DSPC. While the majority of preclinical research has focused on how the structure of cationic or ionizable lipids influences transport, the other three components may also have an effect [135,136]. An LNP that was initially designed to deliver small interfering RNA (siRNA) to endothelial cells in the lungs and blood vessels of mice [137] and non-human primates [138] has been retargeted to deliver siRNA [139], small guide RNA (sgRNA), or messenger RNA (mRNA) [140] to endothelial cells in the bone marrow, liver, and spleen following intravenous administration and lung epithelial cells after nebulization. Other examples include how altering the PEG-lipid structure or its molar fraction influences LNP pharmacokinetics and hepatic siRNA delivery in mice [141,142,143,144]. The lipid “anchors” the PEG-lipid inside the LNP, whilst the hydrophilic PEG interacts with blood plasma water to produce an aqueous barrier [145].
In a similar way, oxidised cholesterols [121], esterified cholesterols [140], and cholesterol analogues such as phytosterols [113] have all been reported to enhance LNP dispersion in cell cultures and in mice, despite the fact that the majority of LNPs are formed from unmodified cholesterols. The addition of modified cholesterol to LNPs may affect their structure [146], but the mechanism by which cholesterol mediates delivery enhancements remains unknown. It has been found that substituting a different lipid for DSPC improves LNP transport to the spleen and lungs [122,147]. Similarly, LNPs were targeted to the lung and spleen by selective organ targeting [148] by adding another lipid to the LNP and therefore changing it from a four-component to a five-component system. Beam Therapeutics [132] and Intellia [149] have published data demonstrating that LNPs can be manufactured to transport mRNA to haematopoietic stem and progenitor cells in mice, a discovery that speaks well for the development of in vivo haematopoietic stem-cell-targeting therapeutics.
MiR-153-3p is protective against ischemia/reperfusion injury, although its role in myocardial infarction (MI) is uncertain. Liposome nanoparticles and HA-cationic liposomes (CLPs) for miR-153-3p transport and mechanistic activities of miR-153-3p were modified by nHA-CLPs in MI-induced cardiomyocyte injury. miR-153-3p protected cardiomyocytes against apoptosis and damage generated by MI. It was observed that miR-153-3p binds to the 3’ untranslated region of KLF5 (Kruppel-like factor 5) and suppresses its production. The inhibition of NF-κB by miR-153-3p reduced inflammation [150].
4.2. Polymer-Based Nanoparticle Vaccines
Furthermore, polymers and polymeric nanoparticles are used in several non-viral RNA delivery techniques, especially dendrimers (Figure 4) [151]. In order to modify how polymers transport RNA into cells, chemists can adjust properties including charge, degradation rate, and molecular weight [152,153]. Poly(lactic-co-glycolic acid) is a conventional polymer (PLGA). PLGA drug delivery systems have been approved by the FDA for the delivery of small-molecule therapeutics but not for nucleic acids [154]. PLGA (Figure 5) does not possess a positive charge which is important to form a complex with the anionic RNA phosphodiester backbone at neutral pH. Therefore, researchers have incorporated cationic groups into the PLGA moiety, such as chitosan, to facilitate the transport of siRNA in mice [155]. Preclinical investigations of several CVD have shown promise for the use of siRNA-based nanoparticle platforms, such as liposome-RNA complexes and polymer-based RNA complexes [156]. Possible strategies for selectively targeting active endothelium include liposomal nanoparticles (cationic amphiphiles) loaded with antibodies against VCAM-1 or E-selectin for siRNA delivery to activated primary ECs in vitro [157]. Conjugation of siRNA has seen extensive use of polymeric forms, knocking down the advanced glycation end products (RAGE) receptors through direct injection into infarcted regions in myocardial infarction (rat model) successfully. A conjugation of deoxycholic-acid-modified polyethylenimine and siRNA was used against the RAGE mRNA efficiently silencing its expression, eventually resulting in decreased levels of inflammatory cytokines, in vivo apoptosis, and ventricular remodelling [158]. A silica nanoparticle method was formulated to deliver miR-24 in AMI (acute myocardial infarction). Dithiobis(succinimidyl propionate) (DSP) was coupled with polyethylenimine (PEI), followed by functionalized silica nanoparticles (F-silica), which noncovalently changed DSP–PEI on the surface of the nanoparticles; this was achieved using the reverse microemulsion technique. Finally, an F-silica-miR-24 gene carrier complex was produced. Nanoparticles had substantial cellular uptake and retained miR-24’s function. F-silica-miR-24 was shown to be a successful miR-24 replacement therapy in primary cardiomyocytes from rat models of AMI, with favourable biocompatibility. These results suggest that regulating miRNA expression during stress-induced apoptosis could be a useful therapeutic strategy for treating CVD. F-silica-miR-24 vectors are easily uptaken, biocompatible, and exhibit 78% gene transfer efficiency. It did this by evading degradation in endolysosomes and releasing miRNA molecules, which then disrupted the target gene (Bim) in cultured cardiomyocytes. Reduced ventricular remodelling with long-term improvement in cardiac function were observed in mice with the F-silica-miR-24 delivery method in early cases of acute myocardial infarction due to Bim inhibition [159]. Nanoparticles having a stable shelf life and a size appropriate for the physiological delivery of miR therapies in vivo are required. The miR nanoparticles containing the cardioprotective miR-199a-3p were synthesized with a DSPE-PEG shell and a CPP conjugation. The fluorescent core of the miNP was made of poly(9,9-dioctylfluorene-co-benzothiadiazole), which allows particle identification and tracking. MiNPs typically measured approximately 110 nm in size, with little to no fluctuation, making them comparable in size to exosomes that are produced naturally (30–120 nm). The miNPs were stable in terms of size, surface charge, and dispersion even after being subjected to many freeze–thaw cycles. Since miNPs exhibited little cellular toxicity, the delivery to cardiomyocytes at higher miRs concentrations can be achieved without causing damage [160]. Transfection efficiency is low when using nonviral vectors in the case of nondividing cells such as cardiomyocytes. The TAT CPP was integrated into the miNP shell to resolve these uptake problems. The transactivation motif of HIV-1 (TAT) is a cationic moiety that facilitates HIV-1 absorption into cells. Uptake of miNP by human embryonic stem cell (hESC)-derived CMs and hESC-derived endothelial cells (ECs) was enhanced by the addition of TAT CPP (ECs). These results demonstrate the feasibility of nonviral miR delivery to cardiac tissues, which had been an earlier challenge but is now seen as promising for in vivo translation of the treatment [161]. Mimicking cardiac macrophages after MI, miRNA-21 given via nanoparticles reduces inflammation. Upregulation of miRNA-21 by peritoneal macrophages after apoptosis reduces inflammation. Thus, miRNA-21 was formulated as nanoparticles (NPs) due to the formation of a complex of hyaluronan-sulphate (HAS) and nucleic acid via calcium ion bridges. Laser capture microdissection (LCM) was used to monitor the LV posterior wall. LCM allowed researchers to study cardiac macrophages in their native milieu without processing of cells or in vitro culture affecting their activation. After coronary ligation in mice, cardiac macrophages expressed miRNA21. MiRNA-21 has inconsistent effects on macrophage polarisation according to studies. MiRNA-21 is upregulated within macrophages after engulfing cells apoptotic in nature, enhancing inflammation resolution, while pri-miR-21 controls early inflammation. This modulation increases angiogenesis, reduces apoptotic cells, and slows left ventricular remodelling following MI. Current modulation did not enhance systolic function and will likely need more improvement to do so [162]. Whether or not Au nanoparticles might be functionalized with a ligand capable of attaching firmly and precisely to a biomarker of endothelial cells (disturbed flow, or d-flow) was investigated. The presence of VCAM1 on inflamed endothelial cells prompted the functionalization of Au nanoparticles with a VCAM1-binding peptide (VHSPNKKGGSKGC) to minimise non-specific accumulation and negative consequences due to the presence of VCAM1. The highly flow-sensitive pro-atherogenic miR-712 is increased in endothelial cells in response to d-flow, as established by in vitro and in vivo studies. In this study, Au nanospheres of 5 nm size were used for targeted delivery of anti-miR-712 to inhibit miR-712 expression and activity. Using a complementary DNA carrier, anti-miR-712 was hybridised to an Au nanosphere. Derivatization of Au nanospheres with mPEG-SH and NH2-PEG-SH at varying ratios reduces the protein corona, subsequently functionalizing the nanospheres with carrier DNA that has a 3’-end thiol group and 10 adenine (A) nucleotides. Following this, anti-miR-712 is hybridised to the Au nanosphere through the carrier DNA. To prevent its release into the body’s extracellular fluid inadvertently, anti-miR712 only weakly attaches to carrier DNA. Because of improved interaction with the carrier DNA, anti-miR-712 is quickly released inside cells after being introduced. For targeted delivery of anti-miR-712 to active endothelial cells, a VCAM1-binding peptide might be incorporated into Au nanospheres [163].
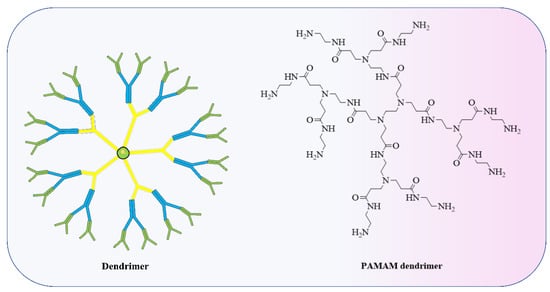
Figure 4.
Dendrimers are specific polymeric structures made up of a set number of molecules arranged around a central PAMAM, poly(amidoamine) used to form LNP.
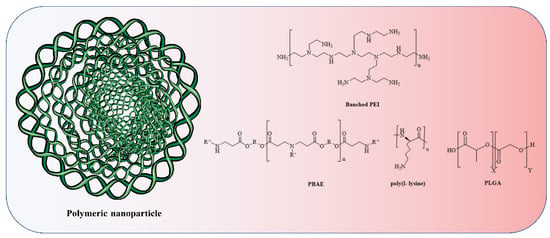
Figure 5.
The anionic phosphodiester part of RNA is complexed by cationic amine groups (due to attachment of a proton) in polymeric nanoparticles and polymers based on poly(ethylenimine) (PEI), poly(l-lysine) (PLL), and poly(beta-amino ester) (PBAE). Polymers derived from poly(lactic-co-glycolic acid) (PLGA) are commonly modified to incorporate discrete cationic groups.
Optimising multifunctional nanoparticles is difficult because they need a high loading efficiency, a hidden cationic domain to enable lysosomal escape, and dense, stable, targeting moieties. Vascular cell adhesion molecule 1 (VCAM1) was targeted by loading anti-miR-712 (1400 molecules, >95% loading efficiency) onto cationic lipoparticles (CCLs) coated with 5 mol% of peptide (VHPK). In vitro and in vivo studies confirmed disease-specific accumulation of anti-miR-712 in endothelial cells from inflamed mice aortas. With the help of VHPK-CCL-anti-miR-712, the endothelium’s metalloproteinase activity was suppressed by reducing the miR-712 expression that was increased by d-flow and restoring the expression of its target genes, TIMP3 and RECK. VHPK-CCLs transported anti-miR-712 to inflamed endothelium in pro-atherogenic d-flow areas. This may also be used in case of mimics, miRNA inhibitors, plasmids, siRNAs, and pharmaceuticals. VCAM1-targeted cationic nanoparticles containing anti-miRs can be used for tailored antiatherogenic treatment with little off-target effects [164]. Functional miRNA mimics were delivered to macrophages in vivo by chitosan nanoparticles (chNPs). chNPs, a cross-linked chitosan polysaccharide polymer, have been shown in a number of in vivo and in vitro investigations to potentially act as a shield and transmitter of exogenous miR-33 to naive macrophages, hence influencing ABCA1 expression. Macrophages treated with chNPs expressing miR-33 exhibited reduced cholesterol efflux to apoA1 and reduced RCT. Delivery of the efflux-promoting miRNAs miR-206 and miR-223 into cells via nanoparticles led to elevated expression of ABCA1 and the RCT pathway. With the results of this study in hand, it is possible that miRNAs might be employed in vivo to selectively target atherosclerotic lesions by being transported to macrophages by chNPs and regulating ABCA1 expression and cholesterol efflux [165].
4.3. Device-Based Methods for RNA Vaccines
As a preliminary attempt to boost concentration in the heart or vasculature, local RNA treatment delivery devices have been tested. A pig ischemia/reperfusion model was utilised to test the efficacy of LNA-92a at doses of 0.03 mg/kg b.w. intravenously, intracoronally (antegrade), and retrogradely. While miR-92a expression was reduced by all three treatment routes, antegrade and retrograde infusions were more effective at enhancing heart function and decreasing apoptosis as well as infarct size than intravenous administration [17]. The LNA concentration in this study was low (0.03 mg/kg b.w.), especially when compared with previous studies utilising LNAs (0.5–25 mg/kg b.w.), and further increasing the dose to 0.15 mg/kg (intracoronary injection) did not further boost therapeutic effectiveness [53,54,166]. Injecting antimiRs by catheter into the heart successfully reduced the systemic inhibitory effects of miR-92a, but this was not the case for other miRs. Intramuscular injection is an alternative delivery strategy for RNA therapy since it has been shown to be successful in cell therapy. Another option is to employ drug-eluting stents or balloons to deliver RNA therapies to the arterial wall. It has been shown that anti-21-coated stents can locally decrease miR-21 expression just as effectively as systemic miR-21 inhibition, with fewer adverse effects [167].
4.4. RNA Viral Vector-Mediated Vaccine Delivery
Moreover, tissue-specific accumulation of miRNAs or lncRNAs can be accomplished by the use of viral vectors for cell-type-specific delivery [168]. For example, AAV serotype 9 has been utilised to overexpress miR-199a and miR-590 in cardiomyocytes for an extended period of time [169]. One further use of this idea is the restoration of miR-1 levels to prevent ventricular hypertrophy [24]. Cardiomyocytes are the only cell type that AAV serotype 9 viruses target, hence these viruses have been widely utilised in small animals to overexpress miRNAs, shRNAs, and mRNAs [24,43,169,170]. While there is hope for AAV-mediated gene therapy, its lacklustre performance in human clinical research (CUPID trial (Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease)) implies there is substantial room for improvement in vector efficiency. If vasculature-specific tools are considered, the methods are less sophisticated [171]. Though vectors targeting endothelial cells do exist, they have so far only been shown to have an effect on the liver and not the endothelium lining the heart [172]. A novel strategy for introducing viruses to tissues has just been reported in the scientific literature. A technique called split-intein-mediated protein trans-splicing was used to attach large polypeptides to the AAV particle surface by covalent bonding. Gene transfer to specific cell types was achieved by the use of scFvs (single-chain variable fragments) or DARPins (designed ankyrin repeat proteins) that have a high affinity to cell surface receptors expressed exclusively on the cell membrane of target cells. Furthermore, compared with AAVs expressing the same targeting ligand but not genetically connected, protein trans-splicing-based AAVs transmit far less of their genes into target receptor-negative cells [173].
4.5. Vaccine Based on Tissue Enrichment via RNA Therapeutics Modification
Chemically modifying the ASO is another option for improving tissue-specific absorption of RNA therapies or generating locally restricted activity. Improved wound healing was observed in diabetic mice when miR 92a was inhibited by light-induced RNA interference (RNAi) [174]. Light-inducible antimiR-92a’s activation and delivery has been demonstrated to be equally effective as conventional, non-activatable antimiRs at decreasing miR-92a expression in mouse skin. The levels of miR-92a in other organs were not significantly altered by local activatable antimiRs, in contrast to conventional antimiRs. Light induction of RNA treatments needs more study before it can be employed on well-perfused visceral organs such as the heart. It is conceivable to enrich a specific antimiR or miRNA mimic in a cell-type-specific manner by linking miRNAs to aptamers. Tumour models and endothelial cells have both benefited from aptamer-mediated miRNA delivery. One study established a connection between miR-126 and an aptamer that binds the widely expressed transferrin receptor [175]. Currently, there is insufficient information to conclude that this is even possible in an in vivo cardiovascular system. Transferrin receptor miR-126 chimeras, or the delivery mechanism enhanced with additional endothelial-specific aptamers, are promising therapeutic options for the treatment of vascular disorders. Using aptamers to target a specific cell population and microparticles to enhance cellular uptake of miRNA mimics proved effective in treating atherosclerosis. Specific miR-181b and miR-146a delivery to activated endothelial cells was achieved by loading nanoporous microparticles constructed of porous silicon multistage vectors [19].
4.6. miRNA Encapsulation
Improving cellular uptake of RNA treatments by encapsulating the pharmaceuticals might be an option. Many different approaches of molecular targeting have been studied experimentally so far. Upon intracoronary administration in pigs, antagomir-92a made of 9 μm poly-d,l-lactide-co-glycolide microspheres selectively target the vasculature and improve functions of the heart [18]. Overexpression of miR-126-5p was shown to be possible using miRNA mimics delivered in a nanoparticle-based system. MiR-126 upregulation inhibited atherosclerosis and rescued endothelial cell proliferation [176]. Atherosclerosis development was inhibited through a cell-specific route when miR-181b mimics conjugated with lipofectamine were injected into the vascular endothelium [177]. In the aforementioned investigation, overexpression of miR-146a and miR-181b as a treatment for atherosclerosis was tested by delivering the RNAs to the inflammatory endothelium that surrounds atherosclerotic plaque using a multistage vector that targets E-selectin. Polyethylene glycol-polyethyleneimine nanoparticles contained multistage vector microparticles targeting E-selectin loaded with Cy5-conjugated miR-146a and miR-181b [19]. An intracardiac vaccine based on a synthetic lipid delivery system and loaded with hsa-miR-199a-3p and hsamiR-590-3p mimics was shown to enhance ventricular function just after an episode of AMI. Such a synthetic miRNA lipid drug delivery vaccine system is sufficient enough for the restoration of cardiac functions [178]. However, there are currently few established protocols for improving noncoding RNA therapeutics in cardiac tissue, especially ASOs, despite the aforementioned promising instances. In contrast, hepatic tissue may be the focus of LNP (lipid nanoparticle) administration methods using ionizable amino lipids, which have recently emerged as the principal siRNA delivery approach with many products now in clinical trials. siRNA-loaded LNPs were able to cause silencing of genes in the hepatocytes when its vaccine was injected intravenously at low doses of 0.005 mg/kg b.w. in animals [120]. The liposomal nanoparticle vaccine formulation known as SMARTICLES, with a size of roughly 120 nm, has been used in phase I clinical studies to deliver the tumour-suppressing miR-34. As for efficacy, biodistribution, and safety, all were maximised compared with other delivery strategies [179]. The efficacy of the vaccine formulated by conjugating ASOs with GN3 (N-acetyl galactosamine), a high-affinity ligand for the hepatocyte-specific ASGPR (asialoglycoprotein receptor), was tested in the liver of mice and was found to increase by 6–10 fold [180].
5. Challenges Associated with RNA Vaccine Therapy
Although miRNA inhibition has shown therapeutic advantages in mice and large animals, targeting cardiac tissue is more difficult than kidney, liver, or other organs of interest [181]. The anti-imiRs vaccination can be given intravenously, intramuscularly, or subcutaneously for long-term inhibition. Studies in mice or rats using LNA-based antimiR typically used doses between ≈ 0.5 and 25 mg/kg. To suppress miRNAs in cardiomyocytes or the vasculature, however, significantly greater doses of AntagomiR (8–80 mg/kg body weight) were required. Both chemical composition and the sequence of targets might affect the appropriate doses and dosing strategy (the number of injections). Studies in mice showed that very low doses of LNA-92a (0.5 mg/kg) or antagomiR-92a (8 mg/kg) were sufficient to effectively suppress miR-92a in the cardiomyocytes [17]. Targeting miRNAs such as miR-34a or miR-29 required a significantly higher dose (5 mg/kg LNA-based antimiRs, 20 mg/kg antagomiRs), despite the fact that the tissues they acted on were the same [13,53]. In contrast, vaccines formulated with siRNA delivery have shown efficacy at concentrations as low as 0.01 mg/kg bodyweight, which is essential to inhibit the hepatic genetic expression [120], whilst in the first clinical study, 3–7 mg/kg of body weight was applied to an antimiR that targets the miR-122 that is highly expressed in the liver (5 weekly injections) [120,182]. The appropriate dose of a given miRNA inhibitor appears to be affected by a number of factors. Both the miRNA and the inhibitor sequence play crucial roles. Furthermore, biodistribution and bioavailability, cellular uptake process, targeted miRNA, and associated genes are also important. By developing novel chemical changes, bioavailability was increased by decreasing clearance from the circulation. This means that antimiR activity is no longer restricted to highly accumulated tissues such as the liver and kidney, but may be found in a wider variety of organs. It is unclear, however, whether or not assessing ASO uptake alone is connected to their biological action [183]. The endocytotic mechanisms that are responsible for the ASOs uptake can be classified as either productive or nonproductive. Although uptake via productive routes results in ASO binding to its target, uptake via nonproductive pathways may cause accumulation of the ASO in late endosomes or lysosomes [184]. Even if the mechanisms behind the route towards production as opposed to nonproduction uptake are not entirely understood, TSG101, a component of the ESCRT-I (endosomal sorting complexes required for transport I), is a critical modulator of nonproductive uptake of anti-miR-21 [185]. Productive uptake was enhanced in both in vitro and in vivo trials when TSG101 was inhibited. Evidence suggests that surface protein expression and binding capacities regulate the uptake process [184]. Furthermore, the effective dose may change depending on how much the corresponding target genes and miRNAs are expressed. In the past, evaluating knockdown effectiveness frequently required removing the RNA from the entire tissue, which could produce inconsistent results. Since the presence of the antimiR in the tissue may directly modify the PCR, this method of evaluating miRNAs may overestimate the inhibitory effects.
Therefore, Northern blotting should be employed to validate the level of inhibition, with a focus on evaluating target derepression. Additionally, assessing the actual biological significance of miR inhibition may be complicated by cellular heterogeneity. The heart is composed of many different cell types, including cardiomyocytes, endothelial cells, immune cells and fibroblasts, each of which can express miRNAs and their targets uniquely. This can make it challenging to discern effects in whole heart tissue. It may be required to examine the effects of antimiR therapy on a subset of cells. The expression levels of miRNAs and their targets can undergo drastic alterations in pathological circumstances.
The potential for harmful effects from ASOs and antimiRs increases with the dose. Hybridization-dependent toxicity and hybridization-independent toxicity are the two main types of toxicity seen after oligonucleotide therapy. The sequence of the oligonucleotide is responsible for the toxicity caused by hybridization, which can be caused either by direct modulation of on-target effects or nonspecific binding with other nucleotide sequences with comparable characteristics (off-target effects). Preclinical and clinical experiments take into account the target’s mechanism of action and expression profile/level, and toxicity caused due to it can be evaluated and possibly mitigated. Off-target effects in miRNAs could be caused by the seed sequence being too similar to the target RNA or by random interactions with other molecules.
Although studies have been conducted on antimiRs that target the 3′ end of the miRNA, the results have been widely contested, making it unclear which approach is most successful [37]. Furthermore, targeting of an entire miRNA family, such as in the case of miR-21 where conflicting outcomes were found, may be advantageous but may not be appropriate for other miRNAs [186]. Hybridization to double-stranded DNA sections might further produce off-target effects, possibly generating site-specific mutagenesis through the triplex formation. Standard assays, including AMES and micronucleus testing, found no evidence for genotoxicity in a panel of oligonucleotides, albeit this outcome is theoretically plausible [187]. Newly modified oligonucleotides should still be subjected to genotoxicity testing. Because of the oligonucleotide’s base pair structure and any alterations made to it, it can be hazardous even when not hybridised. Conventionally, RNA oligonucleotides stimulate the immune mechanism by way of TLR (toll-like receptor) signalling [188]. Phosphorothioate-modified oligonucleotides activate the complementary system in non-human primates, particularly at high plasma concentrations [189,190,191]. The peak plasma levels that cause these adverse effects can be minimised with gradual infusion as opposed to bolus administration. Human serum did not show any evidence of complement activation in response to oligonucleotide therapy, which is interesting. Platelet activation, aggregation, and thrombus formation have all been demonstrated to occur in vitro and in vivo when phosphorothioate-modified oligonucleotides bind to platelets [192]. Recently, it was shown that blocking ASO binding to platelet proteins by LNA insertion significantly attenuated platelet activation [193]. However, it is crucial to incorporate adequate immunologic testing into both the preclinical and clinical phases in the development of the pharmaceutical. Raised levels of the transaminases alanine aminotransferase (ALT) and aspartate aminotransferase (AST) present in liver have been associated with the use of several LNA-modified oligonucleotides which is an indicator of possible hepatotoxicity [194]. Hepatotoxicity can be attributed to a specific sequence rather than a conventional one, as several LNA-based oligonucleotides exhibited non-toxic effects in the liver (in vivo). Trinucleotide motifs, which increase p53 and NRF2 (nuclear factor [erythroid]-derived 2]-like 2) pathway activation in vivo, are intriguing because they may be predictive of potential hepatotoxicity [195].
6. Six Qualities of Vaccine Delivery Systems to Be Clinically Relevant
There has been a constant effort to develop nanoparticle-based RNA delivery using reported vehicles. A detailed pharmacokinetic and clearance clinical data have been reported regarding patisiran [196,197]. The approved animal models, toxicity readouts, and time points for patisiran were detailed in a report from the FDA Center for Drug Evaluation and Research [198]. These data provide a thorough roadmap for characterising nanoparticle-based RNA delivery of appropriate clinical outcome. In a broader sense, the 3 RNA drug delivery systems that have received FDA approval [108,199] or an EUA grant [200] thus far seem to have six qualities (Figure 6) that, when taken together, might be considered hallmarks of authorised delivery vehicles. To begin with, biodegradable, scalable chemistry is typically used in the fabrication of therapeutic delivery systems. The LNP safety of Moderna, Acuitas, and Alnylam lipids, all of which are used in humans, was enhanced by introducing ester linkages, which may breakdown in water, to form ionizable lipids [201]. The RNA-based delivery technique must be chemically simple to enable human-scale production; for instance, let us consider a clinical trial setting where injecting a vaccine into a patient weighing 100 kg was administered with 6 mg/kg lipid and 0.3 mg/kg RNA (that is, a lipid:RNA mass ratio of 20:1), roughly 1 g of lipid would be needed for a single vaccine unit, assuming that there is no loss of lipid during formulation development. GalNAc conjugates, attached to siRNAs or ASOs independently, can be manufactured on a human scale (with the capacity to manufacture a large batch that complies with cGMP or Current Good Manufacturing Practice). Only LNPs with four lipid components and no targeting ligands were approved by regulators. It is essential that those developing therapeutic nanoparticles should know the benefits and drawbacks of incorporating targeted ligands [202]. Third, there needs to be a good on-target to off-target delivery ratio using the RNA delivery system. Biodistribution and function are two metrics that may be used to assess on- and off-target delivery. Biodistribution is required for effective cytoplasmic RNA delivery, but it is not adequate because endosomes retain more than 95% of RNA [203]. Additionally, studies have demonstrated that very low siRNA copy numbers are effective for gene knockdown in vitro [204], suggesting that siRNA functions catalytically. Fourthly, the dose of RNA that is effective must be far smaller than the dose that causes harm. Due to undetermined factors, mice have not shown to be reliable models of RNA toxicity; therefore, this can be seen in non-human primates. Fifth, even after shipping, the drug’s efficacy must be uniform throughout the batches. Work is being conducted to stabilise mRNA-based drugs for this reason. Storage of lyophilized CureVac mRNA at 40 °C for up to 6 months at room temperature maintained for 3 years showed no loss of functionality [205]. Furthermore, unless cryoprotectants such as sucrose are utilised, lyophilization may reduce stability for LNP- encapsulated mRNA by encouraging lipid crystallisation [206]. With the addition of 10% sucrose, for instance, RNA-complexed lipid nanoparticles may be lyophilized and kept for up to ≥ 8 months without refrigeration [207].
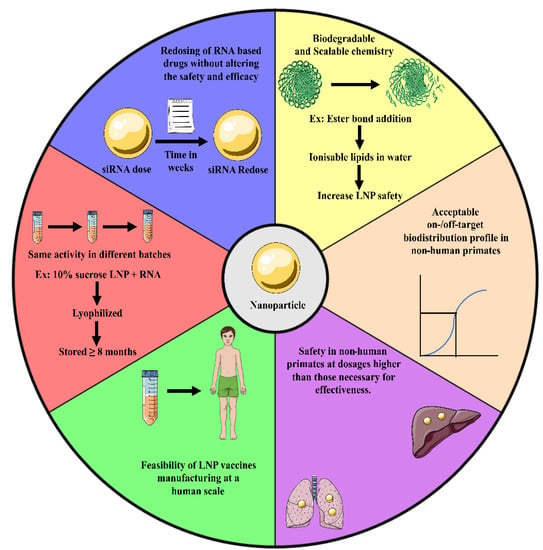
Figure 6.
Any RNA-based vaccine delivery during its development must possess the 6 mentioned ideal characteristics (approved by FDA/EUA) so as to exert clinical relevancy and reach the pharmaceutical market.
Sixth, in most clinical contexts, re-dosing the RNA drug so as to not compromise with the efficacy or safety is of outmost importance in maintaining the biological impact or “dose to effect.” Patients can safely be re-dosed with siRNA drugs when doses are spaced out by 3 weeks [108].
There has been successful double-dosing of mRNA vaccines, with either a 3-week or 4-weeks gap [200,208]. This impact is produced by a subpopulation of B lymphocytes, and it has been demonstrated that weekly re-dosing of mRNA reduces its efficiency in mice [209].
7. Clinical Angle
The potential exists to create new therapeutic approaches for CVD by targeting noncoding RNAs. While there have been obstacles in the past to the development of ASO and siRNAs delivery, recently conducted clinical trials reveal that chemical modifications along with targeting approaches have boosted efficiency, while at the same time they have decreased risk associated with undesired adverse effects. ORION-1 is a phase 2, multi-centric, double-blind, placebo-controlled, dose-escalation trial that recently reported its results on lowering LDL (low-density lipoprotein) level through the uptake of long-acting siRNA inclisiran, a subcutaneous vaccine, or PCSK9 into hepatocytes, facilitated due to its conjugation with triantennary N-acetylgalactosamine carbohydrates, which has high binding affinity for the ASGPRs in the liver. Phosphorothioate, 2′-O-methyl, and 2-fluoro nucleotide modifications are added to the siRNA to augment its stability as a molecule. With 2300 mg doses of inclisiran, PCSK9 and LDL cholesterol were significantly reduced and remained down for at least 240 days. Both the placebo and therapy groups experienced a comparable percentage of mild and severe adverse events (76% in both cases) [210]. Immune activation symptoms, a common worry associated with RNA therapies, were uncommon, and platelet counts were unaffected. Three patients treated with inclisiran exhibited transient increases in hepatic enzyme levels. A total of 4% and 7% of patients receiving a single dose and 2 doses of inclisiran experienced injection-site responses. These results are in line with those reported with monoclonal antibody therapy targeting PCSK9, but significantly different from those seen with earlier ASO therapies (wherein 80% of patients reported injection-site reaction) (e.g., trial results for ODYSEE showed a 5.9% success rate (To examine if it could minimise the risk associated with cardiovascular events in individuals experiencing an acute coronary syndrome; a randomized, double-blind, placebo-controlled, parallel-group study was conducted)) [211]. When compared with the 80% found in past ASO therapies’ injection-site studies, the lower figure is not surprising (e.g., 76% with mipomersen) [212]. However, the study did find that newer generations of siRNAs were substantially more effective and safe, and this was true even if this siRNA did not target a microRNA or long non-coding RNA in the cardiovascular system. To date, only one clinical study has been published demonstrating the safety and efficacy of LNA-based antimiRs (mirvarsen) for liver miR-122 targeting. Renal impairment due to Alport syndrome (kidney fibrosis) is being evaluated in another clinical trial using an antimiR against miR-21 (RG-012). In a multiple-dose phase 1 study, the concerned test substance was assessed in patients as well as healthy volunteers for a phase 2 placebo-controlled study (HERA study (Herceptin Adjuvant); NCT02855268) [182]. A list of vaccines that have reached various phases of clinical trials are listed in Table 3. Though few noncoding vaccines have reached clinical trial and eventually the market, some guidelines exist that suggest patients can extract benefit from such translational therapy. To begin with, patients with high priority disease, where molecular target cannot be addressed using conventional therapies, are to be considered. A large population of patients with severe clinical phenotypes may qualify for this therapy. Factors such as patients being unresponsive to conventional therapy, or the therapy itself being not efficient enough, or the therapy involves invasiveness, may be considered. CVD patients with high-priority diseases should have a unique pathomechanism at the centre of the condition, e.g., PCSK9 implicated in atherosclerosis and Ca2+ in heart failure. This kind of therapy can only be initiated in CVD patients with no irreversible damage such as myocardial fibrosis, myocardial infarction, and destruction of matrix.

Table 3.
Enlisted vaccines in clinical trials.
8. Challenges Associated with Long Non-Coding RNA Therapeutics
As gene therapy targeting epigenetic modulators is still a relatively new idea and method compared with typical pharmacological targets and proteins, there are still some concerns regarding its potential side effects. The most significant concern is the lack of fundamental research on the function of miRNAs, lncRNAs, and their possible downstream consequences; as a result, the clinical usage of lncRNA-targeted medicines may result in unforeseen hazards and inappropriate pathological effects. In particular, off-target impacts may result in negative side effects. Therefore, highly specialised targeting strategies and delivery methods must be enhanced to ensure that the targeted epigenetic regulators are impacted. Highly cardio-specific targeted drug delivery methods pose a significant obstacle to the therapeutic application of lncRNAs. Another research suggested that in vitro differentiation of MSCs (mesenchymal stem cells) is inhibited by HOTAIR, especially differentiation toward adipogenic lineage. Despite poor sequence conservation, lncRNA possesses great tissue specificity. RNA-seq and unsupervised cluster analysis reveal that most lncRNAs exhibit tissue-specific expression. Furthermore, the effectiveness and safety of lncRNA medicines in humans remain uncertain because of a lack of significant evidence from clinical studies [225,226,227].
9. Conclusions
Important recent developments and therapeutic prospects of ncRNA research were addressed, with a focus on the translation of findings from fundamental science and in vivo/in vitro research into clinically useful new diagnostics and therapies. Meticulous patient selection turned out to be an important component in the effectiveness of clinical trials using ncRNAs as targets (miRs) and those using ncRNAs as tools (siRNAs) for therapeutic outcome. Consequently, given the rarity of validation of claimed outcomes, clinical trials conducted at large-scale are necessary for appraising the potential of ncRNAs as therapeutic agents in clinical contexts. Characterized selection criteria and adequate clinical outcome parameters, as well as carefully defined patient cohorts in which one molecular mechanism is responsible for pathogenesis and the main reason behind the onset and progression of any CVD, needs to be given careful thought for potential future clinical applications. Patients who are more likely to benefit from the work of ncRNA therapeutic approaches will be the primary focus for the success of future clinical trials [228].
The lncRNA is still at in its early stages with regard to how it acts at the molecular level in the body. Understanding the interaction between the delivery vehicles and RNA payload is the need of the hour. The interaction between these may affect tolerability and cellular targeting which needs attention and exploration. There is reported evidence that with change in the RNA payload, the nanoparticle movement changes. This is basically caused by the modification of both the nanoparticle and biomolecules as well as their interactions. Changes made in the RNA payload can affect the tropism associated with nanoparticles [141]. Moreover, it is the intrinsic properties of a cell that generate exogenous mRNA, which might not be suitable enough to produce SiRNA-mediated silencing of mRNA [229]. The chemical modifications made to ASOs and SiRNA have been found to be very effective in comparison with the unmodified ones [230]. The cell-specific activity can be tailored by modifying the untranslated portion of the mRNA [231,232].
This report suggests that RNA payloads can further be explored and improved for better results and to be effective enough to reach target patients. Another issue which we found during the review was that the extrapolation of tolerability and therapeutic effectiveness in rats and mice (small animal models) to humans and non-human primates requires appropriate study. There are stringent ethical issues associated with exploring drug delivery systems in non-human primates. Therefore, the development of appropriate small-animal-based models is suggested and the results can be scientifically translated to non-human primates.
Author Contributions
Conceptualization, M.A.S. and R.B.; methodology, M.A.S.; software, R.B.; validation, M.A.S., F.A.A.-A. and S.A.A.; formal analysis, A.M.A.; investigation, A.S.; resources, I.K.; data curation, S.K.; writing—original draft preparation, M.A.S., R.B. and A.S.; writing—review and editing, M.A.S. and R.B.; visualization, F.A.A.-A. and S.A.A.; supervision, I.K. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge CCRUM, Ministry of AYUSH (Govt. of India), Bio-Equivalence Study Centre, UGC-UPE-II, Department of Science and Technology (Govt. of India), RUSA 2.0, UGC (RGNF).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
RNA: ribonucleic acid; lncRNA, long non-coding RNA; shRNA, short hairpin RNA; miRNA, micro RNA; mRNA, messenger RNA; DGCR, DiGeorge syndrome chromosomal region; ASO, antisense oligonucleotides; siRNA, small interfering RNA; LNP, lipid nanoparticle; AMI, acute myocardial infarction; DNA, deoxyribonucleic acid; ERK-MAP kinase, extracellular signal-regulated kinase-mitogen-activated protein kinase; TAC, transverse aortic constriction; SERCA2a, sarco/endoplasmic reticulum Ca2+-ATPase 2a; Hand2, heart- and neural crest derivatives-expressed protein 2; MHC, myosin heavy chain; PTEN, phosphatase and TENsin homolog deleted on chromosome 10; SREBF, sterol regulatory element binding proteins; HDL, high-density lipoprotein; Dlk1, delta-Like 1 homolog; NF-κB, nuclear factor-kappa B; TRAF6, tumour necrosis factor receptor-associated factor 6; LNA, locked nucleic acid; ESCRT-I, endosomal sorting complexes required for transport I; TSG101, tumour susceptibility gene 101 protein; MIAT, myocardial infarction-associated transcript; TGF-1, transforming growth factor-1; MIAT, myocardial infarction-associated transcript; CARL, cardiac apoptosis-related lncRNA; MDRL, mitochondrial dynamic-related lncRNA; APF, autophagy-promoting factor; CHRF, cardiac hypertrophy-related factor; Mhrt, myosin heavy chain-associated RNA transcript; ANRIL, antisense noncoding RNA in the INK4 (inhibitor of CDK4) locus; GATA2, GATA-binding factor 2; PCSK9, proprotein convertase subtilisin/kexin type 9; Malat-1, metastasis-associated lung adenocarcinoma transcript 1; CRISPR/Cas9, clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat-associated 9; PHB2, prohibitin 2; CUPID, calcium upregulation by percutaneous administration of gene therapy in cardiac disease; scFvs, single-chain variable fragments; DARPins, designed ankyrin repeat proteins.
References
- Small, E.M.; Olson, E.N. Pervasive Roles of MicroRNAs in Cardiovascular Biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. Correction: MicroRNAs: Small RNAs with a Big Role in Gene Regulation. Nat. Rev. Genet. 2004, 5, 631. [Google Scholar] [CrossRef]
- Thum, T. MicroRNA Therapeutics in Cardiovascular Medicine. EMBO Mol. Med. 2012, 4, 3–14. [Google Scholar] [CrossRef]
- Boon, R.A.; Dimmeler, S. MicroRNAs in Myocardial Infarction. Nat. Rev. Cardiol. 2015, 12, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The Landscape of Long Noncoding RNAs in the Human Transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef]
- Boon, R.A.; Jaé, N.; Holdt, L.; Dimmeler, S. Long Noncoding RNAs: From Clinical Genetics to Therapeutic Targets? J. Am. Coll. Cardiol. 2016, 67, 1214–1226. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, D.P. LincRNAs: Genomics, Evolution, and Mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef]
- Perry, R.B.-T.; Ulitsky, I. The Functions of Long Noncoding RNAs in Development and Stem Cells. Development 2016, 143, 3882–3894. [Google Scholar] [CrossRef]
- Van Rooij, E.; Olson, E.N. MicroRNA Therapeutics for Cardiovascular Disease: Opportunities and Obstacles. Nat. Rev. Drug Discov. 2012, 11, 860–872. [Google Scholar] [CrossRef]
- Hullinger, T.G.; Montgomery, R.L.; Seto, A.G.; Dickinson, B.A.; Semus, H.M.; Lynch, J.M.; Dalby, C.M.; Robinson, K.; Stack, C.; Latimer, P.A.; et al. Inhibition of MiR-15 Protects against Cardiac Ischemic Injury. Circ. Res. 2012, 110, 71–81. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of Neonatal and Adult Mammalian Heart Regeneration by the MiR-15 Family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef]
- Fiedler, J.; Jazbutyte, V.; Kirchmaier, B.C.; Gupta, S.K.; Lorenzen, J.; Hartmann, D.; Galuppo, P.; Kneitz, S.; Pena, J.T.G.; Sohn-Lee, C.; et al. MicroRNA-24 Regulates Vascularity after Myocardial Infarction. Circulation 2011, 124, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Boon, R.A.; Iekushi, K.; Lechner, S.; Seeger, T.; Fischer, A.; Heydt, S.; Kaluza, D.; Tréguer, K.; Carmona, G.; Bonauer, A.; et al. MicroRNA-34a Regulates Cardiac Ageing and Function. Nature 2013, 495, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Gao, X.-M.; Winbanks, C.E.; Boey, E.J.H.; Tham, Y.K.; Kiriazis, H.; Gregorevic, P.; Obad, S.; Kauppinen, S.; Du, X.-J.; et al. Therapeutic Inhibition of the MiR-34 Family Attenuates Pathological Cardiac Remodeling and Improves Heart Function. Proc. Natl. Acad. Sci. USA 2012, 109, 17615–17620. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, H.-W.; Qiu, Y.; Dupee, D.; Noonan, M.; Lin, Y.-D.; Fisch, S.; Unno, K.; Sereti, K.-I.; Liao, R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ. Res. 2015, 117, 450–459. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef]
- Hinkel, R.; Penzkofer, D.; Zühlke, S.; Fischer, A.; Husada, W.; Xu, Q.F.; Baloch, E.; Van Rooij, E.; Zeiher, A.M.; Kupatt, C.; et al. Inhibition of MicroRNA-92a Protects against Ischemia/Reperfusion Injury in a Large-Animal Model. Circulation 2013, 128, 1066–1075. [Google Scholar] [CrossRef]
- Bellera, N.; Barba, I.; Rodriguez-Sinovas, A.; Ferret, E.; Asín, M.A.; Gonzalez-Alujas, M.T.; Pérez-Rodon, J.; Esteves, M.; Fonseca, C.; Toran, N.; et al. Single Intracoronary Injection of Encapsulated Antagomir-92a Promotes Angiogenesis and Prevents Adverse Infarct Remodeling. J. Am. Heart Assoc. 2014, 3, e000946. [Google Scholar] [CrossRef]
- Ma, S.; Tian, X.Y.; Zhang, Y.; Mu, C.; Shen, H.; Bismuth, J.; Pownall, H.J.; Huang, Y.; Wong, W.T. E-Selectin-Targeting Delivery of MicroRNAs by Microparticles Ameliorates Endothelial Inflammation and Atherosclerosis. Sci. Rep. 2016, 6, 22910. [Google Scholar] [CrossRef]
- Cao, L.; Chai, S. Is Involved in Morphine Pre Conditioning to Protect Rat Cardiomyocytes from Ischemia/Reperfusion Injury through Targeting Akt3. Mol. Med. Rep. 2020, 320, 1480–1488. [Google Scholar] [CrossRef]
- Tan, J.; Pan, W.; Chen, H.; Du, Y.; Jiang, P.; Zeng, D.; Wu, J.; Peng, K. Circ_0124644 Serves as a CeRNA for MiR-590-3p to Promote Hypoxia-Induced Cardiomyocytes Injury via Regulating SOX4. Front. Genet. 2021, 12, 667724. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; Zhang, X.; Lughmani, H.Y.; Kennedy, D.J.; Haller, S.T.; Pierre, S.V.; Shapiro, J.I.; Tian, J. A Strategic Expression Method of MiR-29b and Its Anti-Fibrotic Effect Based on RNA-Sequencing Analysis. PLoS ONE 2020, 15, e0244065. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 Contributes to Myocardial Disease by Stimulating MAP Kinase Signalling in Fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef]
- Karakikes, I.; Chaanine, A.H.; Kang, S.; Mukete, B.N.; Jeong, D.; Zhang, S.; Hajjar, R.J.; Lebeche, D. Therapeutic Cardiac-Targeted Delivery of MiR-1 Reverses Pressure Overload-Induced Cardiac Hypertrophy and Attenuates Pathological Remodeling. J. Am. Heart Assoc. 2013, 2, e000078. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.; Tuazon, J.P.; Borlongan, C.V.; Yu, G. MicroRNA-133a and Myocardial Infarction. Cell Transplant. 2019, 28, 831–838. [Google Scholar] [CrossRef]
- Zhang, X.-T.; Xu, M.-G. Potential Link between MicroRNA-208 and Cardiovascular Diseases. J. Xiangya Med. 2021, 6, 12. [Google Scholar] [CrossRef]
- Wahlquist, C.; Jeong, D.; Rojas-Muñoz, A.; Kho, C.; Lee, A.; Mitsuyama, S.; van Mil, A.; Park, W.J.; Sluijter, J.P.G.; Doevendans, P.A.F.; et al. Inhibition of MiR-25 Improves Cardiac Contractility in the Failing Heart. Nature 2014, 508, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wahlquist, C.; El Azzouzi, H.; Deddens, J.C.; Kuster, D.; van Mil, A.; Rojas-Munoz, A.; Huibers, M.M.; Mercola, M.; de Weger, R.; et al. MiR-132/212 Impairs Cardiomyocytes Contractility in the Failing Heart by Suppressing SERCA2a. Front. Cardiovasc. Med. 2021, 8, 592362. [Google Scholar] [CrossRef] [PubMed]
- Loyer, X.; Potteaux, S.; Vion, A.-C.; Guérin, C.L.; Boulkroun, S.; Rautou, P.-E.; Ramkhelawon, B.; Esposito, B.; Dalloz, M.; Paul, J.-L.; et al. Inhibition of MicroRNA-92a Prevents Endothelial Dysfunction and Atherosclerosis in Mice. Circ. Res. 2014, 114, 434–443. [Google Scholar] [CrossRef]
- Mondadori dos Santos, A.; Metzinger, L.; Haddad, O.; M’baya-Moutoula, E.; Taïbi, F.; Charnaux, N.; Massy, Z.A.; Hlawaty, H.; Metzinger-Le Meuth, V. MiR-126 Is Involved in Vascular Remodeling under Laminar Shear Stress. Biomed. Res. Int. 2015, 2015, 497280. [Google Scholar] [CrossRef]
- Petrkova, J.; Borucka, J.; Kalab, M.; Klevcova, P.; Michalek, J.; Taborsky, M.; Petrek, M. Increased Expression of MiR-146a in Valvular Tissue from Patients with Aortic Valve Stenosis. Front. Cardiovasc. Med. 2019, 6, 86. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, J.; Zhang, F.; Lei, Q.; Zhang, T.; Li, K.; Guo, J.; Hong, Y.; Bu, G.; Lv, X.; et al. MicroRNA-181a-5p and MicroRNA-181a-3p Cooperatively Restrict Vascular Inflammation and Atherosclerosis. Cell Death Dis. 2019, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Thum, T. Noncoding RNAs and Myocardial Fibrosis. Nat. Rev. Cardiol. 2014, 11, 655–663. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of Stress-Dependent Cardiac Growth and Gene Expression by a MicroRNA. Science 2007, 316, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Schütz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjärn, M.; Hansen, H.F.; Berger, U.; et al. LNA-Mediated MicroRNA Silencing in Nonhuman Primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Silahtaroglu, A.; Bak, M.; Christensen, M.; Lind-Thomsen, A.; Hedtjärn, M.; Hansen, J.B.; Hansen, H.F.; Straarup, E.M.; et al. Antagonism of MicroRNA-122 in Mice by Systemically Administered LNA-AntimiR Leads to up-Regulation of a Large Set of Predicted Target MRNAs in the Liver. Nucleic Acids Res. 2008, 36, 1153–1162. [Google Scholar] [CrossRef]
- Obad, S.; dos Santos, C.O.; Petri, A.; Heidenblad, M.; Broom, O.; Ruse, C.; Fu, C.; Lindow, M.; Stenvang, J.; Straarup, E.M.; et al. Silencing of MicroRNA Families by Seed-Targeting Tiny LNAs. Nat. Genet. 2011, 43, 371–378. [Google Scholar] [CrossRef]
- Nahar, S.; Singh, A.; Morihiro, K.; Moai, Y.; Kodama, T.; Obika, S.; Maiti, S. Systematic Evaluation of Biophysical and Functional Characteristics of Selenomethylene-Locked Nucleic Acid-Mediated Inhibition of MiR-21. Biochemistry 2016, 55, 7023–7032. [Google Scholar] [CrossRef]
- Meng, L.; Liu, C.; Lü, J.; Zhao, Q.; Deng, S.; Wang, G.; Qiao, J.; Zhang, C.; Zhen, L.; Lu, Y.; et al. Small RNA Zippers Lock MiRNA Molecules and Block MiRNA Function in Mammalian Cells. Nat. Commun. 2017, 8, 13964. [Google Scholar] [CrossRef]
- Fabani, M.M.; Abreu-Goodger, C.; Williams, D.; Lyons, P.A.; Torres, A.G.; Smith, K.G.C.; Enright, A.J.; Gait, M.J.; Vigorito, E. Efficient Inhibition of MiR-155 Function in Vivo by Peptide Nucleic Acids. Nucleic Acids Res. 2010, 38, 4466–4475. [Google Scholar] [CrossRef]
- Paulasova, P.; Pellestor, F. The Peptide Nucleic Acids (PNAs): A New Generation of Probes for Genetic and Cytogenetic Analyses. Ann. Genet. 2004, 47, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Behlke, M.A. Chemical Modification of SiRNAs for in Vivo Use. Oligonucleotides 2008, 18, 305–319. [Google Scholar] [CrossRef]
- Li, Y.-G.; Zhang, P.-P.; Jiao, K.-L.; Zou, Y.-Z. Knockdown of MicroRNA-181 by Lentivirus Mediated SiRNA Expression Vector Decreases the Arrhythmogenic Effect of Skeletal Myoblast Transplantation in Rat with Myocardial Infarction. Microvasc. Res. 2009, 78, 393–404. [Google Scholar] [CrossRef]
- Choi, W.-Y.; Giraldez, A.J.; Schier, A.F. Target Protectors Reveal Dampening and Balancing of Nodal Agonist and Antagonist by MiR-430. Science 2007, 318, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Langlet, F.; Chachlaki, K.; Roa, J.; Rasika, S.; Jouy, N.; Gallet, S.; Gaytan, F.; Parkash, J.; Tena-Sempere, M.; et al. Corrigendum: A MicroRNA Switch Regulates the Rise in Hypothalamic GnRH Production before Puberty. Nat. Neurosci. 2016, 19, 1115. [Google Scholar] [CrossRef]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of MicroRNAs in Vivo with “Antagomirs”. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Iaconetti, C.; Polimeni, A.; Sorrentino, S.; Sabatino, J.; Pironti, G.; Esposito, G.; Curcio, A.; Indolfi, C. Inhibition of MiR-92a Increases Endothelial Proliferation and Migration in Vitro as Well as Reduces Neointimal Proliferation in Vivo after Vascular Injury. Basic Res. Cardiol. 2012, 107, 296. [Google Scholar] [CrossRef]
- Jeong, D.; Yoo, J.; Lee, P.; Kepreotis, S.V.; Lee, A.; Wahlquist, C.; Brown, B.D.; Kho, C.; Mercola, M.; Hajjar, R.J. MiR-25 Tough Decoy Enhances Cardiac Function in Heart Failure. Mol. Ther. 2018, 26, 718–729. [Google Scholar] [CrossRef]
- Dirkx, E.; Gladka, M.M.; Philippen, L.E.; Armand, A.-S.; Kinet, V.; Leptidis, S.; El Azzouzi, H.; Salic, K.; Bourajjaj, M.; da Silva, G.J.J.; et al. Nfat and MiR-25 Cooperate to Reactivate the Transcription Factor Hand2 in Heart Failure. Nat. Cell Biol. 2013, 15, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.P.; Wu, J.; Wang, X.; Sartor, M.A.; Jones, K.; Qian, J.; Nicolaou, P.; Pritchard, T.J.; Fan, G.C. MicroRNA-320 Is Involved in the Regulation of Cardiac Ischemia/Reperfusion Injury by Targeting Heat-Shock Protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The MiRNA-212/132 Family Regulates Both Cardiac Hypertrophy and Cardiomyocyte Autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Azuma, J.; Toh, R.; Merk, D.R.; Deng, A.; Chin, J.T.; Raaz, U.; Schoelmerich, A.M.; Raiesdana, A.; Leeper, N.J.; et al. Inhibition of MicroRNA-29b Reduces Murine Abdominal Aortic Aneurysm Development. J. Clin. Investig. 2012, 122, 497–506. [Google Scholar] [CrossRef]
- Boon, R.A.; Seeger, T.; Heydt, S.; Fischer, A.; Hergenreider, E.; Horrevoets, A.; Vinciguerra, M.; Rosenthal, N.; Sciacca, S.; Pilato, M.; et al. MicroRNA-29 in Aortic Dilation:Implications for Aneurysm Formation. Circ. Res. 2011, 109, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.L.; Hullinger, T.G.; Semus, H.M.; Dickinson, B.A.; Seto, A.G.; Lynch, J.M.; Stack, C.; Latimer, P.A.; Olson, E.N.; van Rooij, E. Therapeutic Inhibition of MiR-208a Improves Cardiac Function and Survival during Heart Failure. Circulation 2011, 124, 1537–1547. [Google Scholar] [CrossRef]
- Wahlestedt, C.; Salmi, P.; Good, L.; Kela, J.; Johnsson, T.; Hökfelt, T.; Broberger, C.; Porreca, F.; Lai, J.; Ren, K.; et al. Potent and Nontoxic Antisense Oligonucleotides Containing Locked Nucleic Acids. Proc. Natl. Acad. Sci. USA 2000, 97, 5633–5638. [Google Scholar] [CrossRef]
- Mangos, M.M.; Min, K.-L.; Viazovkina, E.; Galarneau, A.; Elzagheid, M.I.; Parniak, M.A.; Damha, M.J. Efficient RNase H-Directed Cleavage of RNA Promoted by Antisense DNA or 2’F-ANA Constructs Containing Acyclic Nucleotide Inserts. J. Am. Chem. Soc. 2003, 125, 654–661. [Google Scholar] [CrossRef]
- Krieg, A.M. Targeting LDL Cholesterol with LNA. Mol. Ther. Nucleic Acids 2012, 1, e6. [Google Scholar] [CrossRef] [PubMed]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zörnig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long Noncoding RNA MALAT1 Regulates Endothelial Cell Function and Vessel Growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.-T.; Gupta, S.K.; Viereck, J.; Foinquinos, A.; Samolovac, S.; Kramer, F.L.; Garg, A.; Remke, J.; Zimmer, K.; Batkai, S.; et al. Inhibition of the Cardiac Fibroblast-Enriched LncRNA Meg3 Prevents Cardiac Fibrosis and Diastolic Dysfunction. Circ. Res. 2017, 121, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cai, J.; Han, Y.; Chen, J.; Huang, Z.-P.; Chen, C.; Cai, Y.; Huang, H.; Yang, Y.; Liu, Y.; et al. LincRNA-P21 Regulates Neointima Formation, Vascular Smooth Muscle Cell Proliferation, Apoptosis, and Atherosclerosis by Enhancing P53 Activity. Circulation 2014, 130, 1452–1465. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, C.-Y.; Zhou, L.-Y.; Wang, J.-X.; Wang, M.; Zhao, B.; Zhao, W.-K.; Xu, S.-J.; Fan, L.-H.; Zhang, X.-J.; et al. APF LncRNA Regulates Autophagy and Myocardial Infarction by Targeting MiR-188-3p. Nat. Commun. 2015, 6, 6779. [Google Scholar] [CrossRef] [PubMed]
- Burel, S.A.; Hart, C.E.; Cauntay, P.; Hsiao, J.; Machemer, T.; Katz, M.; Watt, A.; Bui, H.-H.; Younis, H.; Sabripour, M.; et al. Hepatotoxicity of High Affinity Gapmer Antisense Oligonucleotides Is Mediated by RNase H1 Dependent Promiscuous Reduction of Very Long Pre-MRNA Transcripts. Nucleic Acids Res. 2016, 44, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, L.; Song, J.; Wang, Z.; Huang, X.; Guo, Z.; Chen, F.; Zhao, X. Long Noncoding RNA MALAT1 Mediates Cardiac Fibrosis in Experimental Postinfarct Myocardium Mice Model. J. Cell. Physiol. 2019, 234, 2997–3006. [Google Scholar] [CrossRef]
- Qu, X.; Du, Y.; Shu, Y.; Gao, M.; Sun, F.; Luo, S.; Yang, T.; Zhan, L.; Yuan, Y.; Chu, W.; et al. MIAT Is a Pro-Fibrotic Long Non-Coding RNA Governing Cardiac Fibrosis in Post-Infarct Myocardium. Sci. Rep. 2017, 7, 42657. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.-J.; Zhang, H.-S. LncRNA-CARl in a Rat Model of Myocardial Infarction. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4332–4340. [Google Scholar] [CrossRef]
- Wang, K.; Liu, F.; Zhou, L.-Y.; Long, B.; Yuan, S.-M.; Wang, Y.; Liu, C.-Y.; Sun, T.; Zhang, X.-J.; Li, P.-F. The Long Noncoding RNA CHRF Regulates Cardiac Hypertrophy by Targeting MiR-489. Circ. Res. 2014, 114, 1377–1388. [Google Scholar] [CrossRef]
- Viereck, J.; Kumarswamy, R.; Foinquinos, A.; Xiao, K.; Avramopoulos, P.; Kunz, M.; Dittrich, M.; Maetzig, T.; Zimmer, K.; Remke, J.; et al. Long Noncoding RNA Chast Promotes Cardiac Remodeling. Sci. Transl. Med. 2016, 8, 326ra22. [Google Scholar] [CrossRef]
- Han, P.; Li, W.; Lin, C.-H.; Yang, J.; Shang, C.; Nuernberg, S.T.; Jin, K.K.; Xu, W.; Lin, C.-Y.; Lin, C.-J.; et al. A Long Noncoding RNA Protects the Heart from Pathological Hypertrophy. Nature 2014, 514, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Hobuß, L.; Bär, C.; Thum, T. Long Non-Coding RNAs: At the Heart of Cardiac Dysfunction? Front. Physiol. 2019, 10, 30. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Huang, X.; Guo, X.; Liu, Y.; Zhong, J.; Yuan, J. STAT3-Induced Upregulation of LncRNA MEG3 Regulates the Growth of Cardiac Hypertrophy through MiR-361-5p/HDAC9 Axis. Sci. Rep. 2019, 9, 460. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, M.; Wang, H.; Zhao, S.; Zhao, D.; Yang, Y.; Wang, Z.M.; Wang, F.; Yang, Z.J.; Lu, X.; et al. Association of Polymorphisms in Long Noncoding RNA H19 with Coronary Artery Disease Risk in a Chinese Population. Mutat. Res. 2015, 772, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Breckwoldt, K.; Remmele, C.W.; Hartmann, D.; Dittrich, M.; Pfanne, A.; Just, A.; Xiao, K.; Kunz, M.; Müller, T.; et al. Development of Long Noncoding RNA Based Strategies to Modulate Tissue Vascularization. J. Am. Coll. Cardiol. 2015, 66, 2005–2015. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Erratum: Knocking down Barriers: Advances in SiRNA Delivery. Nat. Rev. Drug Discov. 2010, 9, 412. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Bumcrot, D.; Manoharan, M.; Koteliansky, V.; Sah, D.W.Y. RNAi Therapeutics: A Potential New Class of Pharmaceutical Drugs. Nat. Chem. Biol. 2006, 2, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Santel, A.; Aleku, M.; Keil, O.; Endruschat, J.; Esche, V.; Fisch, G.; Dames, S.; Löffler, K.; Fechtner, M.; Arnold, W.; et al. A Novel SiRNA-Lipoplex Technology for RNA Interference in the Mouse Vascular Endothelium. Gene Ther. 2006, 13, 1222–1234. [Google Scholar] [CrossRef]
- Van de Water, F.M.; Boerman, O.C.; Wouterse, A.C.; Peters, J.G.P.; Russel, F.G.M.; Masereeuw, R. Intravenously Administered Short Interfering RNA Accumulates in the Kidney and Selectively Suppresses Gene Function in Renal Proximal Tubules. Drug Metab. Dispos. 2006, 34, 1393–1397. [Google Scholar] [CrossRef]
- Takakura, Y.; Mahato, R.I.; Hashida, M. Extravasation of Macromolecules. Adv. Drug Deliv. Rev. 1998, 34, 93–108. [Google Scholar] [CrossRef]
- Li, W.; Szoka, F.C., Jr. Lipid-Based Nanoparticles for Nucleic Acid Delivery. Pharm. Res. 2007, 24, 438–449. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Poh, M.-Z.; Insin, N.; Bawendi, M.G.; Fukumura, D.; Munn, L.L.; Jain, R.K. Diffusion of Particles in the Extracellular Matrix: The Effect of Repulsive Electrostatic Interactions. Biophys. J. 2010, 99, 1342–1349. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Regulated Portals of Entry into the Cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Swanson, J.A.; Watts, C. Macropinocytosis. Trends Cell Biol. 1995, 5, 424–428. [Google Scholar] [CrossRef]
- Bareford, L.M.; Swaan, P.W. Endocytic Mechanisms for Targeted Drug Delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Partlow, K.C.; Lanza, G.M.; Wickline, S.A. Exploiting Lipid Raft Transport with Membrane Targeted Nanoparticles: A Strategy for Cytosolic Drug Delivery. Biomaterials 2008, 29, 3367–3375. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Masuda, T.; Katayama, K.; Yoshikawa, T.; Tsutsumi, Y.; Akashi, M.; Mayumi, T.; Nakagawa, S. Fusogenic Liposome Delivers Encapsulated Nanoparticles for Cytosolic Controlled Gene Release. J. Control. Release 2005, 105, 344–353. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, W.; Jin, H.; Lovell, J.F.; Yang, M.; Ding, L.; Chen, J.; Corbin, I.; Luo, Q.; Zheng, G. Biomimetic Nanocarrier for Direct Cytosolic Drug Delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 9171–9175. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A Combinatorial Library of Lipid-like Materials for Delivery of RNAi Therapeutics. Nat. Biotechnol. 2008, 26, 561–569. [Google Scholar] [CrossRef]
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M.; et al. Mechanisms and Optimization of in Vivo Delivery of Lipophilic SiRNAs. Nat. Biotechnol. 2007, 25, 1149–1157. [Google Scholar] [CrossRef]
- Morrissey, D.V.; Lockridge, J.A.; Shaw, L.; Blanchard, K.; Jensen, K.; Breen, W.; Hartsough, K.; Machemer, L.; Radka, S.; Jadhav, V.; et al. Potent and Persistent in Vivo Anti-HBV Activity of Chemically Modified SiRNAs. Nat. Biotechnol. 2005, 23, 1002–1007. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational Design of Cationic Lipids for SiRNA Delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef]
- Truong, N.P.; Gu, W.; Prasadam, I.; Jia, Z.; Crawford, R.; Xiao, Y.; Monteiro, M.J. An Influenza Virus-Inspired Polymer System for the Timed Release of SiRNA. Nat. Commun. 2013, 4, 1902. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Z.; Dou, S.; Wang, Y.-C.; Long, H.-Y.; Xiong, M.-H.; Mao, C.-Q.; Yao, Y.-D.; Wang, J. Single-Step Assembly of Cationic Lipid-Polymer Hybrid Nanoparticles for Systemic Delivery of SiRNA. ACS Nano 2012, 6, 4955–4965. [Google Scholar] [CrossRef]
- Dunn, S.S.; Tian, S.; Blake, S.; Wang, J.; Galloway, A.L.; Murphy, A.; Pohlhaus, P.D.; Rolland, J.P.; Napier, M.E.; DeSimone, J.M. Reductively Responsive SiRNA-Conjugated Hydrogel Nanoparticles for Gene Silencing. J. Am. Chem. Soc. 2012, 134, 7423–7430. [Google Scholar] [CrossRef] [PubMed]
- Endo-Takahashi, Y.; Negishi, Y.; Nakamura, A.; Suzuki, D.; Ukai, S.; Sugimoto, K.; Moriyasu, F.; Takagi, N.; Suzuki, R.; Maruyama, K.; et al. PDNA-Loaded Bubble Liposomes as Potential Ultrasound Imaging and Gene Delivery Agents. Biomaterials 2013, 34, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Suma, T.; Miyata, K.; Anraku, Y.; Watanabe, S.; Christie, R.J.; Takemoto, H.; Shioyama, M.; Gouda, N.; Ishii, T.; Nishiyama, N.; et al. Smart Multilayered Assembly for Biocompatible SiRNA Delivery Featuring Dissolvable Silica, Endosome-Disrupting Polycation, and Detachable PEG. ACS Nano 2012, 6, 6693–6705. [Google Scholar] [CrossRef]
- McMahon, K.M.; Mutharasan, R.K.; Tripathy, S.; Veliceasa, D.; Bobeica, M.; Shumaker, D.K.; Luthi, A.J.; Helfand, B.T.; Ardehali, H.; Mirkin, C.A.; et al. Biomimetic High Density Lipoprotein Nanoparticles for Nucleic Acid Delivery. Nano Lett. 2011, 11, 1208–1214. [Google Scholar] [CrossRef]
- Qi, L.; Gao, X. Quantum Dot-Amphipol Nanocomplex for Intracellular Delivery and Real-Time Imaging of SiRNA. ACS Nano 2008, 2, 1403–1410. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, K.; Moon, S.H.; Lee, Y.; Park, T.G.; Cheon, J. All-in-One Target-Cell-Specific Magnetic Nanoparticles for Simultaneous Molecular Imaging and SiRNA Delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 4174–4179. [Google Scholar] [CrossRef]
- Jiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-Coated Iron Oxide Nanoparticles for Efficient DNA and SiRNA Delivery. Nano Lett. 2013, 13, 1059–1064. [Google Scholar] [CrossRef]
- Posadas, I.; Guerra, F.J.; Ceña, V. Nonviral Vectors for the Delivery of Small Interfering RNAs to the CNS. Nanomedicine 2010, 5, 1219–1236. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Gherardini, L.; Bardi, G.; Nunes, A.; Guo, C.; Bussy, C.; Herrero, M.A.; Bianco, A.; Prato, M.; Kostarelos, K.; et al. Functional Motor Recovery from Brain Ischemic Insult by Carbon Nanotube-Mediated SiRNA Silencing. Proc. Natl. Acad. Sci. USA 2011, 108, 10952–10957. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lytton-Jean, A.K.R.; Chen, Y.; Love, K.T.; Park, A.I.; Karagiannis, E.D.; Sehgal, A.; Querbes, W.; Zurenko, C.S.; Jayaraman, M.; et al. Molecularly Self-Assembled Nucleic Acid Nanoparticles for Targeted in Vivo SiRNA Delivery. Nat. Nanotechnol. 2012, 7, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. MiR-15 and MiR-16 Induce Apoptosis by Targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef]
- Hermeking, H. The MiR-34 Family in Cancer and Apoptosis. Cell Death Differ. 2010, 17, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Knight, R.A. MiR-34: From Bench to Bedside. Oncotarget 2014, 5, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and MRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, C.; Paunovska, K.; Hatit, M.Z.C.; Lokugamage, M.P.; Dahlman, J.E. Therapeutic RNA Delivery for COVID and Other Diseases. Adv. Healthc. Mater. 2021, 10, e2002022. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Lipid Bilayers and Vesicles. Biochim. Biophys. Acta 1977, 470, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Darjuan, M.M.; Mercer, J.E.; Chen, S.; van der Meel, R.; Thewalt, J.L.; Tam, Y.Y.C.; Cullis, P.R. On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and SiRNA. ACS Nano 2018, 12, 4787–4795. [Google Scholar] [CrossRef]
- Herrera, M.; Kim, J.; Eygeris, Y.; Jozic, A.; Sahay, G. Illuminating Endosomal Escape of Polymorphic Lipid Nanoparticles That Boost MRNA Delivery. Biomater. Sci. 2021, 9, 4289–4300. [Google Scholar] [CrossRef]
- Malamas, A.S.; Gujrati, M.; Kummitha, C.M.; Xu, R.; Lu, Z.-R. Design and Evaluation of New PH-Sensitive Amphiphilic Cationic Lipids for SiRNA Delivery. J. Control. Release 2013, 171, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Altınoglu, S.; Wang, M.; Xu, Q. Combinatorial Library Strategies for Synthesis of Cationic Lipid-like Nanoparticles and Their Potential Medical Applications. Nanomedicine 2015, 10, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef]
- Love, K.T.; Mahon, K.P.; Levins, C.G.; Whitehead, K.A.; Querbes, W.; Dorkin, J.R.; Qin, J.; Cantley, W.; Qin, L.L.; Racie, T.; et al. Lipid-like Materials for Low-Dose, in Vivo Gene Silencing. Proc. Natl. Acad. Sci. USA 2010, 107, 1864–1869. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Lee, A.C.H.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-Mediated Gene Silencing in Non-Human Primates. Nature 2006, 441, 111–114. [Google Scholar] [CrossRef]
- Dong, Y.; Love, K.T.; Dorkin, J.R.; Sirirungruang, S.; Zhang, Y.; Chen, D.; Bogorad, R.L.; Yin, H.; Chen, Y.; Vegas, A.J.; et al. Lipopeptide Nanoparticles for Potent and Selective SiRNA Delivery in Rodents and Nonhuman Primates. Proc. Natl. Acad. Sci. USA 2014, 111, 3955–3960. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the Potency of SiRNA Lipid Nanoparticles for Hepatic Gene Silencing in Vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8529–8533. [Google Scholar] [CrossRef]
- Paunovska, K.; Da Silva Sanchez, A.J.; Sago, C.D.; Gan, Z.; Lokugamage, M.P.; Islam, F.Z.; Kalathoor, S.; Krupczak, B.R.; Dahlman, J.E. Nanoparticles Containing Oxidized Cholesterol Deliver MRNA to the Liver Microenvironment at Clinically Relevant Doses. Adv. Mater. 2019, 31, e1807748. [Google Scholar] [CrossRef]
- Kauffman, K.J. Rapid, Single-Cell Analysis and Discovery of Vectored MRNA Transfection in Vivo with a LoxP-Flanked Tdtomato Reporter Mouse. Mol. Ther. Nucleic Acids 2018, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Dorkin, J.R.; Yang, J.H.; Heartlein, M.W.; DeRosa, F.; Mir, F.F.; Fenton, O.S.; Anderson, D.G. Optimization of Lipid Nanoparticle Formulations for MRNA Delivery in Vivo with Fractional Factorial and Definitive Screening Designs. Nano Lett. 2015, 15, 7300–7306. [Google Scholar] [CrossRef] [PubMed]
- Sedic, M. Safety Evaluation of Lipid Nanoparticleformulated Modified MRNA in the Sprague-Dawley Rat and Cynomolgus Monkey. Vet. Pathol. 2018, 55, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent in Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]
- Benenato, K.E.; Kumarasinghe, E.S.; Cornebise, M. Compounds and Compositions for Intracellular Delivery of Therapeutic Agents. WO2017049245A3, 18 May 2017. [Google Scholar]
- Zhang, X.; Zhao, W.; Nguyen, G.N.; Zhang, C.; Zeng, C.; Yan, J.; Du, S.; Hou, X.; Li, W.; Jiang, J.; et al. Functionalized Lipid-like Nanoparticles for in Vivo MRNA Delivery and Base Editing. Sci. Adv. 2020, 6, 34. [Google Scholar] [CrossRef]
- Musunuru, K.; Chadwick, A.C.; Mizoguchi, T.; Garcia, S.P.; DeNizio, J.E.; Reiss, C.W.; Wang, K.; Iyer, S.; Dutta, C.; Clendaniel, V.; et al. In Vivo CRISPR Base Editing of PCSK9 Durably Lowers Cholesterol in Primates. Nature 2021, 593, 429–434. [Google Scholar] [CrossRef]
- Rothgangl, T.; Dennis, M.K.; Lin, P.J.C.; Oka, R.; Witzigmann, D.; Villiger, L.; Qi, W.; Hruzova, M.; Kissling, L.; Lenggenhager, D.; et al. In Vivo Adenine Base Editing of PCSK9 in Macaques Reduces LDL Cholesterol Levels. Nat. Biotechnol. 2021, 39, 949–957. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Investor Overview. Beam Therapeutics. Available online: https://investors.beamtx.com/ (accessed on 26 December 2022).
- Luigetti, M.; Romano, A.; Di Paolantonio, A.; Bisogni, G.; Sabatelli, M. Diagnosis and Treatment of Hereditary Transthyretin Amyloidosis (hATTR) Polyneuropathy: Current Perspectives on Improving Patient Care. Ther. Clin. Risk Manag. 2020, 16, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid Ther. 2018, 28, 146–157. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The Role of Helper Lipids in Lipid Nanoparticles (LNPs) Designed for Oligonucleotide Delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 129–137. [Google Scholar] [CrossRef]
- Dahlman, J.E.; Barnes, C.; Khan, O.; Thiriot, A.; Jhunjunwala, S.; Shaw, T.E.; Xing, Y.; Sager, H.B.; Sahay, G.; Speciner, L.; et al. In Vivo Endothelial SiRNA Delivery Using Polymeric Nanoparticles with Low Molecular Weight. Nat. Nanotechnol. 2014, 9, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Khan, O.F.; Kowalski, P.S.; Doloff, J.C.; Tsosie, J.K.; Bakthavatchalu, V.; Winn, C.B.; Haupt, J.; Jamiel, M.; Langer, R.; Anderson, D.G. Endothelial SiRNA Delivery in Nonhuman Primates Using Ionizable Low–Molecular Weight Polymeric Nanoparticles. Sci. Adv. 2018, 4, eaar8409. [Google Scholar] [CrossRef]
- Sago, C.D.; Lokugamage, M.P.; Islam, F.Z.; Krupczak, B.R.; Sato, M.; Dahlman, J.E. Nanoparticles That Deliver RNA to Bone Marrow Identified by in Vivo Directed Evolution. J. Am. Chem. Soc. 2018, 140, 17095–17105. [Google Scholar] [CrossRef]
- Paunovska, K.; Gil, C.J.; Lokugamage, M.P.; Sago, C.D.; Sato, M.; Lando, G.N.; Gamboa Castro, M.; Bryksin, A.V.; Dahlman, J.E. Analyzing 2000 in Vivo Drug Delivery Data Points Reveals Cholesterol Structure Impacts Nanoparticle Delivery. ACS Nano 2018, 12, 8341–8349. [Google Scholar] [CrossRef]
- Sago, C.D.; Lokugamage, M.P.; Paunovska, K.; Vanover, D.A.; Monaco, C.M.; Shah, N.N.; Gamboa Castro, M.; Anderson, S.E.; Rudoltz, T.G.; Lando, G.N.; et al. High-Throughput in Vivo Screen of Functional MRNA Delivery Identifies Nanoparticles for Endothelial Cell Gene Editing. Proc. Natl. Acad. Sci. USA 2018, 115, E9944–E9952. [Google Scholar] [CrossRef]
- Lokugamage, M.P.; Vanover, D.; Beyersdorf, J.; Hatit, M.Z.C.; Rotolo, L.; Echeverri, E.S.; Peck, H.E.; Ni, H.; Yoon, J.-K.; Kim, Y.; et al. Optimization of Lipid Nanoparticles for the Delivery of Nebulized Therapeutic MRNA to the Lungs. Nat. Biomed. Eng. 2021, 5, 1059–1068. [Google Scholar] [CrossRef]
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.C.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of SiRNA Lipid Nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139. [Google Scholar] [CrossRef] [PubMed]
- Ryals, R.C.; Patel, S.; Acosta, C.; McKinney, M.; Pennesi, M.E.; Sahay, G. The Effects of PEGylation on LNP Based MRNA Delivery to the Eye. PLoS ONE 2020, 15, e0241006. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Eygeris, Y.; Patel, S.; Jozic, A.; Sahay, G. Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett. 2020, 20, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA Delivery to Dendritic Cells Exploits Antiviral Defence for Cancer Immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Cheng, Q. Selective Organ Targeting (SORT) Nanoparticles for Tissue-Specific MRNA Delivery and CRISPR-Cas Gene Editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Intellia Therapeutics Presents Preclinical Proof of Concept for CRISPR-Based In Vivo Editing of Bone Marrow at Keystone eSymposium—Intellia Therapeutics. Available online: https://ir.intelliatx.com/news-releases/news-release-details/intellia-therapeutics-presents-preclinical-proof-concept-crispr (accessed on 15 January 2023).
- Zhou, G.; Hu, T.; Du, Q.; Huang, W.; Yao, C. Nanoparticle-Delivered MicroRNA-153-3p Alleviates Myocardial Infarction-Induced Myocardial Injury in a Rat Model. ACS Biomater. Sci. Eng. 2022, 8, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric Nanoparticles: A Study on the Preparation Variables and Characterization Methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, Z.; Viennois, E.; Kang, Y.; Zhang, M.; Han, M.K.; Chen, J.; Merlin, D. Combination Therapy for Ulcerative Colitis: Orally Targeted Nanoparticles Prevent Mucosal Damage and Relieve Inflammation. Theranostics 2016, 6, 2250–2266. [Google Scholar] [CrossRef] [PubMed]
- Koenig, O.; Walker, T.; Perle, N.; Zech, A.; Neumann, B.; Schlensak, C.; Wendel, H.-P.; Nolte, A. New Aspects of Gene-Silencing for the Treatment of Cardiovascular Diseases. Pharmaceuticals 2013, 6, 881–914. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Lintermans, L.L.; Morselt, H.W.M.; Leus, N.G.J.; Ruiters, M.H.J.; Molema, G.; Kamps, J.A.A.M. Anti-VCAM-1 and Anti-E-Selectin SAINT-O-Somes for Selective Delivery of SiRNA into Inflammation-Activated Primary Endothelial Cells. Mol. Pharm. 2013, 10, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Ku, S.H.; Lee, M.S.; Jeong, J.H.; Mok, H.; Choi, D.; Kim, S.H. Cardiac RNAi Therapy Using RAGE SiRNA/Deoxycholic Acid-Modified Polyethylenimine Complexes for Myocardial Infarction. Biomaterials 2014, 35, 7562–7573. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, Y.; Zhang, R.; Shen, M.; Zhu, Y.; Zhang, Q.; Liu, H.; Han, D.; Shi, X.; Zhang, J. Inhibition of Cardiomyocyte Apoptosis Post-Acute Myocardial Infarction through the Efficient Delivery of MicroRNA-24 by Silica Nanoparticles. Nanoscale Adv. 2021, 3, 6379–6385. [Google Scholar] [CrossRef]
- Yang, H.; Qin, X.; Wang, H.; Zhao, X.; Liu, Y.; Wo, H.-T.; Liu, C.; Nishiga, M.; Chen, H.; Ge, J.; et al. An in Vivo MiRNA Delivery System for Restoring Infarcted Myocardium. ACS Nano 2019, 13, 9880–9894. [Google Scholar] [CrossRef]
- Bheri, S.; Davis, M.E. Nanoparticle-Hydrogel System for Post-Myocardial Infarction Delivery of MicroRNA. ACS Nano 2019, 13, 9702–9706. [Google Scholar] [CrossRef]
- Bejerano, T.; Etzion, S.; Elyagon, S.; Etzion, Y.; Cohen, S. Nanoparticle Delivery of MiRNA-21 Mimic to Cardiac Macrophages Improves Myocardial Remodeling after Myocardial Infarction. Nano Lett. 2018, 18, 5885–5891. [Google Scholar] [CrossRef]
- Sun, T.; Simmons, R.; Huo, D.; Pang, B.; Zhao, X.; Kim, C.W.; Jo, H.; Xia, Y. Targeted Delivery of Anti-MiR-712 by VCAM1-Binding Au Nanospheres for Atherosclerosis Therapy. ChemNanoMat 2016, 2, 400–406. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Kim, C.W.; Seo, J.W.; Kumar, S.; Son, D.J.; Gagnon, M.K.J.; Ingham, E.S.; Ferrara, K.W.; Jo, H. Multifunctional Nanoparticles Facilitate Molecular Targeting and MiRNA Delivery to Inhibit Atherosclerosis in ApoE(−/−) Mice. ACS Nano 2015, 9, 8885–8897. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.-C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in Vitro and in Vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.-M.; Penzkofer, D.; Teske, R.; Dutzmann, J.; Koch, A.; Bielenberg, W.; Bonauer, A.; Boon, R.A.; Fischer, A.; Bauersachs, J.; et al. Inhibition of MiR-92a Improves Re-Endothelialization and Prevents Neointima Formation Following Vascular Injury. Cardiovasc. Res. 2014, 103, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Deuse, T.; Stubbendorff, M.; Chernogubova, E.; Erben, R.G.; Eken, S.M.; Jin, H.; Li, Y.; Busch, A.; Heeger, C.-H.; et al. Local MicroRNA Modulation Using a Novel Anti-MiR-21-Eluting Stent Effectively Prevents Experimental in-Stent Restenosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1945–1953. [Google Scholar] [CrossRef]
- Lucas, T.; Dimmeler, S. RNA Therapeutics for Treatment of Cardiovascular Diseases: Promises and Challenges: Promises and Challenges. Circ. Res. 2016, 119, 794–797. [Google Scholar] [CrossRef]
- Eulalio, A.; Mano, M.; Dal Ferro, M.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional Screening Identifies MiRNAs Inducing Cardiac Regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 Controls Cardiac Hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Desai, A.S.; Barnard, D.; Bouchard, A.; Jaski, B.; Lyon, A.R.; et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Patients with Cardiac Disease (CUPID 2): A Randomised, Multinational, Double-Blind, Placebo-Controlled, Phase 2b Trial. Lancet 2016, 387, 1178–1186. [Google Scholar] [CrossRef]
- Buchholz, C.J.; Friedel, T.; Büning, H. Surface-Engineered Viral Vectors for Selective and Cell Type-Specific Gene Delivery. Trends Biotechnol. 2015, 33, 777–790. [Google Scholar] [CrossRef]
- Muik, A.; Reul, J.; Friedel, T.; Muth, A.; Hartmann, K.P.; Schneider, I.C.; Münch, R.C.; Buchholz, C.J. Covalent Coupling of High-Affinity Ligands to the Surface of Viral Vector Particles by Protein Transsplicing Mediates Cell Type-Specific Gene Transfer. Biomaterials 2017, 144, 84–94. [Google Scholar] [CrossRef]
- Lucas, T.; Schäfer, F.; Müller, P.; Eming, S.A.; Heckel, A.; Dimmeler, S. Lightinducible AntimiR-92a as a Therapeutic Strategy to Promote Skin Repair in Healing-Impaired Diabetic Mice. Nat. Commun. 2017, 8, 15162. [Google Scholar] [CrossRef]
- Rohde, J.-H.; Weigand, J.E.; Suess, B.; Dimmeler, S. A Universal Aptamer Chimera for the Delivery of Functional MicroRNA-126. Nucleic Acid Ther. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Schober, A.; Nazari-Jahantigh, M.; Wei, Y.; Bidzhekov, K.; Gremse, F.; Grommes, J.; Megens, R.T.A.; Heyll, K.; Noels, H.; Hristov, M.; et al. MicroRNA-126-5p Promotes Endothelial Proliferation and Limits Atherosclerosis by Suppressing Dlk1. Nat. Med. 2014, 20, 368–376. [Google Scholar] [CrossRef]
- Sun, X.; He, S.; Wara, A.K.M.; Icli, B.; Shvartz, E.; Tesmenitsky, Y.; Belkin, N.; Li, D.; Blackwell, T.S.; Sukhova, G.K.; et al. Systemic Delivery of MicroRNA-181b Inhibits Nuclear Factor-ΚB Activation, Vascular Inflammation, and Atherosclerosis in Apolipoprotein E-Deficient Mice. Circ. Res. 2014, 114, 32–40. [Google Scholar] [CrossRef]
- Lesizza, P.; Prosdocimo, G.; Martinelli, V.; Sinagra, G.; Zacchigna, S.; Giacca, M. Single-Dose Intracardiac Injection of pro-Regenerative MicroRNAs Improves Cardiac Function after Myocardial Infarction. Circ. Res. 2017, 120, 1298–1304. [Google Scholar] [CrossRef]
- Bader, A.G. MiR-34—A MicroRNA Replacement Therapy Is Headed to the Clinic. Front. Genet. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P.; Graham, M.J.; Yu, J.; Carty, R.; Low, A.; Chappell, A.; Schmidt, K.; Zhao, C.; Aghajan, M.; Murray, H.F.; et al. Targeted Delivery of Antisense Oligonucleotides to Hepatocytes Using Triantennary N-Acetyl Galactosamine Improves Potency 10-Fold in Mice. Nucleic Acids Res. 2014, 42, 8796–8807. [Google Scholar] [CrossRef]
- Fernandez-Prado, R.; Perez-Gomez, M.V.; Ortiz, A. Pelacarsen for Lowering Lipoprotein(a): Implications for Patients with Chronic Kidney Disease. Clin. Kidney J. 2020, 13, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.A.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV Infection by Targeting MicroRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S.; Wancewicz, E.; Matson, J.; Pearce, M.; Siwkowski, A.; Swayze, E.; Bennett, F. Effect of Dose and Plasma Concentration on Liver Uptake and Pharmacologic Activity of a 2’-Methoxyethyl Modified Chimeric Antisense Oligonucleotide Targeting PTEN. Biochem. Pharmacol. 2009, 78, 284–291. [Google Scholar] [CrossRef]
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X.-H. Cellular Uptake and Trafficking of Antisense Oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237. [Google Scholar] [CrossRef]
- Wagenaar, T.R.; Tolstykh, T.; Shi, C.; Jiang, L.; Zhang, J.; Li, Z.; Yu, Q.; Qu, H.; Sun, F.; Cao, H.; et al. Identification of the Endosomal Sorting Complex Required for Transport-I (ESCRT-I) as an Important Modulator of Anti-MiR Uptake by Cancer Cells. Nucleic Acids Res. 2015, 43, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Chau, N.; Bhat, B.; Gupta, S.K.; Linsley, P.S.; Bauersachs, J.; Engelhardt, S. Comparison of Different MiR-21 Inhibitor Chemistries in a Cardiac Disease Model. J. Clin. Investig. 2011, 121, 461–462. [Google Scholar] [CrossRef] [PubMed]
- Berman, C.L.; Barros, S.A.; Galloway, S.M.; Kasper, P.; Oleson, F.B.; Priestley, C.C.; Sweder, K.S.; Schlosser, M.J.; Sobol, Z. OSWG Recommendations for Genotoxicity Testing of Novel Oligonucleotide-Based Therapeutics. Nucleic Acid Ther. 2016, 26, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Peacock, H.; Fucini, R.V.; Jayalath, P.; Ibarra-Soza, J.M.; Haringsma, H.J.; Flanagan, W.M.; Willingham, A.; Beal, P.A. Nucleobase and Ribose Modifications Control Immunostimulation by a MicroRNA-122-Mimetic RNA. J. Am. Chem. Soc. 2011, 133, 9200–9203. [Google Scholar] [CrossRef]
- Monteith, D.K.; Geary, R.S.; Leeds, J.M.; Johnston, J.; Monia, B.P.; Levin, A.A. Preclinical Evaluation of the Effects of a Novel Antisense Compound Targeting C-Raf Kinase in Mice and Monkeys. Toxicol. Sci. 1998, 46, 365–375. [Google Scholar] [CrossRef]
- Levin, A.A. A Review of Issues in the Pharmacokinetics and Toxicology of Phosphorothioate Antisense Oligonucleotides. Biochim. Biophys. Acta Gene Struct. Expr. 1999, 1489, 69–84. [Google Scholar] [CrossRef]
- Henry, S.P.; Beattie, G.; Yeh, G.; Chappel, A.; Giclas, P.; Mortari, A.; Jagels, M.A.; Kornbrust, D.J.; Levin, A.A. Complement Activation Is Responsible for Acute Toxicities in Rhesus Monkeys Treated with a Phosphorothioate Oligodeoxynucleotide. Int. Immunopharmacol. 2002, 2, 1657–1666. [Google Scholar] [CrossRef]
- Flierl, U.; Nero, T.L.; Lim, B.; Arthur, J.F.; Yao, Y.; Jung, S.M.; Gitz, E.; Pollitt, A.Y.; Zaldivia, M.T.K.; Jandrot-Perrus, M.; et al. Phosphorothioate Backbone Modifications of Nucleotide-Based Drugs Are Potent Platelet Activators. J. Exp. Med. 2015, 212, 129–137. [Google Scholar] [CrossRef]
- Sewing, S.; Roth, A.B.; Winter, M.; Dieckmann, A.; Bertinetti-Lapatki, C.; Tessier, Y.; McGinnis, C.; Huber, S.; Koller, E.; Ploix, C.; et al. Assessing Single-Stranded Oligonucleotide Drug-Induced Effects in Vitro Reveals Key Risk Factors for Thrombocytopenia. PLoS ONE 2017, 12, e0187574. [Google Scholar] [CrossRef]
- Swayze, E.E.; Siwkowski, A.M.; Wancewicz, E.V.; Migawa, M.T.; Wyrzykiewicz, T.K.; Hung, G.; Monia, B.P.; Bennett, C.F. Antisense Oligonucleotides Containing Locked Nucleic Acid Improve Potency but Cause Significant Hepatotoxicity in Animals. Nucleic Acids Res. 2007, 35, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Burdick, A.D.; Sciabola, S.; Mantena, S.R.; Hollingshead, B.D.; Stanton, R.; Warneke, J.A.; Zeng, M.; Martsen, E.; Medvedev, A.; Makarov, S.S.; et al. Sequence Motifs Associated with Hepatotoxicity of Locked Nucleic Acid-Modified Antisense Oligonucleotides. Nucleic Acids Res. 2014, 42, 4882–4891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goel, V.; Robbie, G.J. Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients with Hereditary Transthyretin-Mediated Amyloidosis. J. Clin. Pharmacol. 2020, 60, 573–585. [Google Scholar] [CrossRef]
- Zhang, X.; Goel, V.; Attarwala, H.; Sweetser, M.T.; Clausen, V.A.; Robbie, G.J. Patisiran Pharmacokinetics, Pharmacodynamics, and Exposure-Response Analyses in the Phase 3 APOLLO Trial in Patients with Hereditary Transthyretin-Mediated (HATTR) Amyloidosis. J. Clin. Pharmacol. 2020, 60, 37–49. [Google Scholar] [CrossRef]
- MULTI-DISCIPLINE REVIEW. Summary Review Office Director cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210922Orig1s000MultiR.pdf (accessed on 26 December 2022).
- Parums, D.V. Editorial: First Full Regulatory Approval of a COVID-19 Vaccine, the BNT162b2 Pfizer-BioNTech Vaccine, and the Real-World Implications for Public Health Policy. Med. Sci. Monit. 2021, 27, e934625. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable Lipids Enabling Rapidly Eliminated Lipid Nanoparticles for Systemic Delivery of RNAi Therapeutics. Mol. Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional Nanoparticles: Cost versus Benefit of Adding Targeting and Imaging Capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef]
- Gilleron, J.; Querbes, W.; Zeigerer, A.; Borodovsky, A.; Marsico, G.; Schubert, U.; Manygoats, K.; Seifert, S.; Andree, C.; Stöter, M.; et al. Image-Based Analysis of Lipid Nanoparticle-Mediated SiRNA Delivery, Intracellular Trafficking and Endosomal Escape. Nat. Biotechnol. 2013, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Ai, A.; Liu, X.; Hamar, P.; Trifonova, R.; Charisse, K.; Manoharan, M.; Kirchhausen, T.; Lieberman, J. Visualizing Lipid-Formulated SiRNA Release from Endosomes and Target Gene Knockdown. Nat. Biotechnol. 2015, 33, 870–876. [Google Scholar] [CrossRef]
- Alberer, M. Safety and Immunogenicity of a MRNA Rabies Vaccine in Healthy Adults: An Open-Label, Non-Randomised, Prospective, First-in-Human Phase 1 Clinical Trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-Term Storage of Lipid-like Nanoparticles for MRNA Delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Gerhardt, A.; Voigt, E.; Archer, M.; Reed, S.; Larson, E.; Van Hoeven, N.; Kramer, R.; Fox, C.; Casper, C. A Thermostable, Flexible RNA Vaccine Delivery Platform for Pandemic Response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Besin, G.; Milton, J.; Sabnis, S.; Howell, R.; Mihai, C.; Burke, K.; Benenato, K.E.; Stanton, M.; Smith, P.; Senn, J.; et al. Accelerated Blood Clearance of Lipid Nanoparticles Entails a Biphasic Humoral Response of B-1 Followed by B-2 Lymphocytes to Distinct Antigenic Moieties. ImmunoHorizons 2019, 3, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.T.; Turner, T.; Visseren, F.L.J.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Krempf, M. ODYSSEY LONG TERM Investigators. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Raal, F.J.; Santos, R.D.; Blom, D.J.; Marais, A.D.; Charng, M.-J.; Cromwell, W.C.; Lachmann, R.H.; Gaudet, D.; Tan, J.L.; Chasan-Taber, S.; et al. Mipomersen, an Apolipoprotein B Synthesis Inhibitor, for Lowering of LDL Cholesterol Concentrations in Patients with Homozygous Familial Hypercholesterolaemia: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2010, 375, 998–1006. [Google Scholar] [CrossRef]
- Boada, C.; Sukhovershin, R.; Pettigrew, R.; Cooke, J.P. RNA Therapeutics for Cardiovascular Disease. Curr. Opin. Cardiol. 2021, 36, 256–263. [Google Scholar] [CrossRef]
- Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Bär, C.; Borchert, T.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. CDR132L Improves Systolic and Diastolic Function in a Large Animal Model of Chronic Heart Failure. Eur. Heart J. 2021, 42, 192–201. [Google Scholar] [CrossRef]
- Bejar, N.; Tat, T.T.; Kiss, D.L. RNA Therapeutics: The next Generation of Drugs for Cardiovascular Diseases. Curr. Atheroscler. Rep. 2022, 24, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, M.W.; Elmén, J.; Fisker, N.; Hansen, H.F.; Persson, R.; Møller, M.R.; Rosenbohm, C.; Ørum, H.; Straarup, E.M.; Koch, T. PCSK9 LNA Antisense Oligonucleotides Induce Sustained Reduction of LDL Cholesterol in Nonhuman Primates. Mol. Ther. 2012, 20, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, C.; Freemantle, N.; Hughes, S.G.; Furniss, S.; Sulke, N. The Effect of C-Reactive Protein Reduction with a Highly Specific Antisense Oligonucleotide on Atrial Fibrillation Assessed Using Beat-to-Beat Pacemaker Holter Follow-Up. J. Interv. Card. Electrophysiol. 2015, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wolska, A.; Yang, Z.-H.; Remaley, A.T. Hypertriglyceridemia: New Approaches in Management and Treatment: New Approaches in Management and Treatment. Curr. Opin. Lipidol. 2020, 31, 331–339. [Google Scholar] [CrossRef]
- Krychtiuk, K.A.; Rader, D.J.; Granger, C.B. RNA-Targeted Therapeutics in Cardiovascular Disease: The Time Is Now. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 9, 94–99. [Google Scholar] [CrossRef]
- Koren, M.J.; Moriarty, P.M.; Baum, S.J.; Neutel, J.; Hernandez-Illas, M.; Weintraub, H.S.; Florio, M.; Kassahun, H.; Melquist, S.; Varrieur, T.; et al. Preclinical Development and Phase 1 Trial of a Novel SiRNA Targeting Lipoprotein(a). Nat. Med. 2022, 28, 96–103. [Google Scholar] [CrossRef]
- A Study of LY3561774 in Participants with Dyslipidemia. Available online: https://clinicaltrials.gov/ct2/show/NCT04644809?term=LY+3561774&draw=2&rank=1 (accessed on 26 December 2022).
- Bhatia, H.S.; Wilkinson, M.J. Lipoprotein(a): Evidence for Role as a Causal Risk Factor in Cardiovascular Disease and Emerging Therapies. J. Clin. Med. 2022, 11, 6040. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Michael, O.S.; Rathee, S.; Singh, K.R.B.; Ajayi, O.O.; Adetunji, J.B.; Ojha, A.; Singh, J.; Singh, R.P. Potentialities of Nanomaterials for the Management and Treatment of Metabolic Syndrome: A New Insight. Mater. Today Adv. 2022, 13, 100198. [Google Scholar] [CrossRef]
- Laggerbauer, B.; Engelhardt, S. MicroRNAs as Therapeutic Targets in Cardiovascular Disease. J. Clin. Investig. 2022, 132, e159179. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long Non-Coding RNAs: From Disease Code to Drug Role. Acta Pharm. Sin. B 2021, 11, 340–354. [Google Scholar] [CrossRef]
- Kalwa, M.; Hänzelmann, S.; Otto, S.; Kuo, C.-C.; Franzen, J.; Joussen, S.; Fernandez-Rebollo, E.; Rath, B.; Koch, C.; Hofmann, A.; et al. The lncRNA HOTAIR Impacts on Mesenchymal Stem Cellsviatriple Helix Formation. Nucleic Acids Res. 2016, 44, 10631–10643. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Q.; Mao, J.; Zhang, J.; Li, L. The Roles of lncRNA in Myocardial Infarction: Molecular Mechanisms, Diagnosis Biomarkers, and Therapeutic Perspectives. Front. Cell Dev. Biol. 2021, 9, 680713. [Google Scholar] [CrossRef]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.-M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-Coding RNAs in Cardiovascular Diseases: Diagnostic and Therapeutic Perspectives. Eur. Heart J. 2017, 39, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.M.; Echeverria, D.; Hassler, M.; Ly, S.; Khvorova, A. Cell Type Impacts Accessibility of mRNA to Silencing by RNA Interference. Mol. Ther. Nucleic Acids 2020, 21, 384–393. [Google Scholar] [CrossRef]
- Translate Bio Announces Results from Second Interim Data Analysis from Ongoing Phase 1/2 Clinical Trial of MRT5005 in Patients with Cystic Fibrosis (CF). Available online: https://www.globenewswire.com/en/news-release/2021/03/17/2194916/0/en/Translate-Bio-Announces-Results-from-Second-Interim-Data-Analysis-from-Ongoing-Phase-1-2-Clinical-Trial-of-MRT5005-in-Patients-with-Cystic-Fibrosis-CF.html (accessed on 15 January 2023).
- Orlandini von Niessen, A.G.; Poleganov, M.A.; Rechner, C.; Plaschke, A.; Kranz, L.M.; Fesser, S.; Diken, M.; Löwer, M.; Vallazza, B.; Beissert, T.; et al. Improving mRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3′ UTRs Identified by Cellular Library Screening. Mol. Ther. 2019, 27, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Frederick, J.P.; Huang, E.Y.; Burke, K.E.; Mauger, D.M.; Andrianova, E.A.; Farlow, S.J.; Siddiqui, S.; Pimentel, J.; Cheung-Ong, K.; et al. MicroRNAs Enable mRNA Therapeutics to Selectively Program Cancer Cells to Self-Destruct. Nucleic Acid Ther. 2018, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).