Development of the Sm14/GLA-SE Schistosomiasis Vaccine Candidate: An Open, Non-Placebo-Controlled, Standardized-Dose Immunization Phase Ib Clinical Trial Targeting Healthy Young Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Antigen and Adjuvant
2.2. Preclinical Experiments on Safety and Immunogenicity in Pregnant Rabbits and Respective Offspring following Sm14 Vaccination of the Mothers
2.3. Phase Ib Clinical Trial in Healthy Women
3. Results
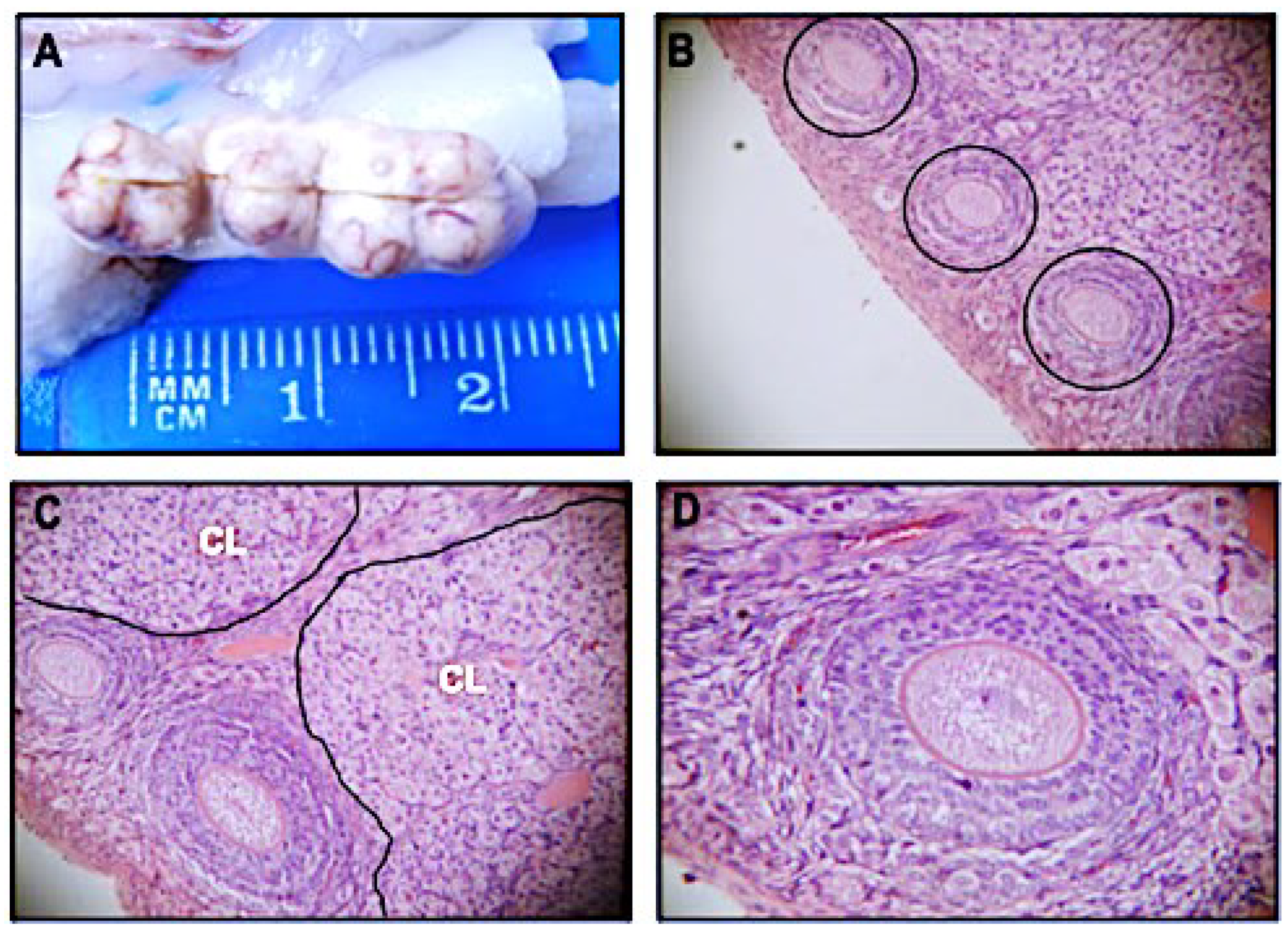
3.1. Sm14 Vaccine Candidate Does Not Induce Any Toxicological Effects in Pregnant Rabbits
3.2. Sm14 Vaccine Candidate Does Not Induce Any Major Adverse Effects (AEs) in Healthy Women
3.3. Sm14 Does Induce Adaptive Immune Responses in Healthy Women
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poole, H.; Terlouw, D.J.; Naunje, A.; Mzembe, K.; Stanton, M.; Betson, M.; Lalloo, D.G.; Stothard, J.R. Schistosomiasis in pre-school-age children and their mothers in Chikhwawa district, Malawi with notes on characterization of schistosomes and snails. Parasites Vectors 2014, 7, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osakunor, D.N.M.; Woolhouse, M.; Mutapi, F. Paediatric schistosomiasis: What we know and what we need to know. PLoS Negl. Trop. Dis. 2018, 12, e0006144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Guideline on Control and Elimination of Human Schistosomiasis; World Health Organization: Geneva, Switzerland, 2022.
- Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. Vigilância da Esquistossomose Mansoni: Diretrizes Técnicas, 4th ed.; Ministério da Saúde: Brasília, Brazil, 2014.
- World Health Organization. (Ed.). Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers; World Health Organization: Geneva, Switzerland, 2006.
- Bergquist, N.R. Schistosomiasis: From risk assessment to control. Trends Parasitol. 2002, 18, 309–314. [Google Scholar] [CrossRef]
- Tendler, M.; Brito, C.A.; Vilar, M.M.; Serra-Freire, N.; Diogo, C.M.; Almeida, M.S.; Delbem, A.C.; Da Silva, J.F.; Savino, W.; Garratt, R.C.; et al. A Schistosoma mansoni fatty acid-binding protein, Sml4, is the potential basis of a dual-purpose anti-helminth vaccine. Proc. Natl. Acad. Sci. USA 1996, 93, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Tendler, M.; Almeida, M.; Simpson, A. Development of the Brazilian Anti Schistosomiasis Vaccine Based on the Recombinant Fatty Acid Binding Protein Sm14 Plus GLA-SE Adjuvant. Front. Immunol 2015, 6, 218. [Google Scholar] [CrossRef] [Green Version]
- Tendler, M.; Almeida, M.S.; Vilar, M.M.; Pinto, P.M.; Limaverde-Sousa, G. Current Status of the Sm14/GLA-SE Schistosomiasis Vaccine: Overcoming Barriers and Paradigms towards the First Anti-Parasitic Human (itarian) Vaccine. Trop. Med. Infect. Dis. 2018, 3, 121. [Google Scholar] [CrossRef] [Green Version]
- Moser, D.; Tendler, M.; Griffiths, G.; Klinkert, M.Q. A 14-kDa Schistosoma mansoni polypeptide is homologous to a gene family of fatty acid binding proteins. J. Biol. Chem. 1991, 266, 8447–8454. [Google Scholar] [CrossRef]
- Santini-Oliveira, M.; Coler, R.N.; Parra, J.; Veloso, V.; Jayashankar, L.; Pinto, P.M.; Ciol, M.A.; Bergquist, R.; Reed, S.G.; Tendler, M. Schistosomiasis vaccine candidate Sm14/GLA-SE: Phase 1 safety and immunogenicity clinical trial in healthy, male adults. Vaccine 2016, 34, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.R.R.; Vilar, M.M.; Nascimento, A.L.; Ho, P.L.; Thaumaturgo, N.; Edelenyi, R.; Almeida, M.; Dias, W.D.O.; Diogo, C.M.; Tendler, M. r-Sm14-pRSETA efficacy in experimental animals. Mem. Inst. Oswaldo Cruz. 2001, 96 (Suppl.), 131–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergquist, N.R.; Colley, D.G. Schistosomiasis Vaccine:Research to Development. Parasitol. Today 1998, 14, 99–104. [Google Scholar] [CrossRef]
- Al-Sherbiny, M.; Osman, A.; Barakat, R.; El Morshedy, H.; Bergquist, R.; Olds, R. In vitro cellular and humoral responses to Schistosoma mansoni vaccine candidate antigens. Acta Trop. 2003, 88, 117–130. [Google Scholar] [CrossRef]
- Tendler, M.; Simpson, A.J.G. The biotechnology-value chain: Development of Sm14 as a schistosomiasis vaccine. Acta Trop. 2008, 108, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Molehin, A.J.; Rojo, J.U.; Siddiqui, S.Z.; Gray, S.A.; Carter, D.; Siddiqui, A.A. Development of a schistosomiasis vaccine. Expert Rev. Vaccines 2016, 15, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Hotez, P.J.; Bottazzi, M.E.; Bethony, J.; Diemert, D.D. Advancing the Development of a Human Schistosomiasis Vaccine. Trends Parasitol. 2019, 35, 104–108. [Google Scholar] [CrossRef]
- Tendler, M.; Pinto, R.M.; Côrtes, M.; Gebara, G. Schistosoma mansoni: Comparative evaluation of different routes of experimental infection. Rev. Inst. Med. Trop. São Paulo 1985, 27, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tendler, M.; Pinto, R.M.; Oliveira Lima, A.; Gebara, G.; Katz, N. Schistosoma mansoni: Vaccination with adult worm antigens. Int. J. Parasitol. 1986, 16, 347–352. [Google Scholar] [CrossRef]
- Tendler, M.; Pinto, R.M.; Lima A de, O.; Savino, W.; Katz, N. Vaccination in murine schistosomiasis with adult worm-derived antigens: Variables influencing protection in outbred mice. Int. J. Parasitol. 1991, 21, 299–306. [Google Scholar] [CrossRef]
- Tendler, M.; Almeida, M.S.; Pinto, R.M.; Noronha, D.; Katz, N. Schistosoma mansoni-New Zealand rabbit model: Resistance induced by infection followed by active immunization with protective antigens. J. Parasitol. 1991, 77, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Olveda, R.M.; Daniel, B.L.; Ramirez, B.D.L.; Aligui, G.; Acosta, L.P.; Fevidal, P.; Tiu, E.; De Veyra, F.; Peters, P.A.; Romulo, R.; et al. Schistosomiasis Japonica in the Philippines: The Long-Term Impact of Population-Based Chemotherapy on Infection, Transmission, and Morbidity. J. Infect. Dis. 1996, 174, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimert, C.M.; Tukahebwa, E.M.; Kabatereine, N.B.; Dunne, D.W.; Vennervald, B.J. Assessment of Schistosoma mansoni induced intestinal inflammation by means of eosinophil cationic protein, eosinophil protein X and myeloperoxidase before and after treatment with praziquantel. Acta Trop. 2008, 105, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Global Pharmaceutical Contract Research Organization (CRO)|PPD; PPD Inc. Available online: https://www.ppd.com (accessed on 20 June 2022).
- Draize, J.H.; Division of Pharmacology Food and Drug Administration Department of Health Education and Welfare. Dermal Toxicity. In Appraisal of the Safety of Chemicals in Foods, Drugs, and Cosmetics; The Association of Food & Drug Officials of the United States: Galveston, TX, USA, 1959; pp. 46–59. [Google Scholar]
- Montenegro, M.R.; Franco, M. Patologia: Processos Gerais, 4th ed.; Atheneu: São Paulo, Brazil, 2004. [Google Scholar]
- Engvall, E. The ELISA, Enzyme-Linked Immunosorbent Assay. Clin. Chem. 2010, 56, 319–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, C.F.; Fonseca, C.T.; Goes, A.M.; Azevedo, V.; Simpson, A.J.; Oliveira, S.C. Human IgG1 and IgG3 recognition of Schistosoma mansoni 14kDa fatty acid-binding recombinant protein. Parasite Immunol. 2000, 22, 41–48. [Google Scholar] [PubMed]
- Oswaldo Cruz Foundation. Phase 1 Study to Evaluate the Safety of the Vaccine Prepared sm14 against Schistosomiasis. 2016. Available online: clinicaltrials.gov (accessed on 30 July 2022).
- Food and Drug Administration (FDA). Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials; Center for Biologics Evaluation and Research: Rockville, MD, USA, 2007.
- Clopper, C.J.; Pearson, E.S. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- Jones, T.C.; Hunt, R.D.; King, N.W. Patologia Veterinária, 6th ed.; Manole: São Paulo, Brazil, 2000. [Google Scholar]
- Mouwen, J.M.V.M.; van Dijk, J.E.; Gruys, E. Atlas Colorido de Patologia Veterinária, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Bacha, W.J.; Bacha, L.M. Color Atlas of Veterinary Histology, 3rd ed.; Wiley-Blackwell: Philadelphia, PA, USA, 2012. [Google Scholar]
- Annex 1-WHO Guidelines on Non Clinical Evaluation of Vaccines. Available online: https://www.who.int/publications/m/item/annex1-nonclinical.p31-63 (accessed on 9 March 2022).


| Experimental Groups | Number of Fetuses | Size of Fetuses (cm) | Weight of Fetuses (g) | Number of Corpora Lutea | Number of Placentae (Deployments) | Number of Live Fetuses | Percentage of Live Male Fetuses/Litter |
|---|---|---|---|---|---|---|---|
| Sm14+GLA-SE | 6.60 ± 2.78 | 6.85 ± 2.40 | 23.43 ± 11.32 | 6.33 ± 3.14 | 7.58 ± 3.02 | 83 | 56.25 ± 30.24 |
| PBS Control | 7.00 ± 3.24 | 7.10 ± 0.83 | 21.58 ± 7.32 | 7.25 ± 2.89 | 8.42 ± 2.99 | 84 | 55.60 ± 34.91 |
| Proportion (95% Confidence Interval) | |||
|---|---|---|---|
| Type of Adverse Event | First Dose (Day 1) | Second Dose (Day 31) | Third Dose (Day 61) |
| Number of participants | 10 | 10 * | 10 |
| Serious adverse events | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) |
| Reactions at the site of injection Reaction to touch | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) |
| Erythema | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) |
| Induration | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) |
| Edema | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) |
| Local pain | 0.900 (0.555, 0.997) | 0.556 (0.212, 0.863) | 0.400 (0.122, 0.738) |
| Involuntary muscle contractions, mild | 0.100 (0.003, 0.445) | 0.000 (0.000, 0.308) | 0.000 (0.000, 0.308) |
| Day of Exam | New Symptoms since Previous Exam (Duration) | Relationship to the Sm14 Vaccine |
|---|---|---|
| D0 * | #10: Vomit, mild (1 day). #13: Sleepiness, mild (3 days). | Not related to the vaccine. |
| D7 | #7: Fever (2 days). #8: Fever (1 day). | Fever in patients #7 and #8 probably related to vaccine |
| D30 | #12: Gallstones, mild. | Not related to the vaccine. |
| D37 | #10: Local hyperemia, mild (3 days). #13: Light hepatic steatosis; mild headache (1 day each on separate occasions) | Patient #10 probably related to vaccine; #13 possibly related to vaccine. |
| D60 | #3: Cold (6 days). #10: Flu, mild (3 days). #14: Urinary tract infection, mild (3 days). | Not related to the vaccine. |
| D67 | #1: Cervicalgia, mild (unknown duration). #5: Pain in right heel, mild (10 days). | Not related to the vaccine |
| D90 | #7: Vaginal discharge, mild (3 days). #13: Flu, mild (5 days). | Not related to the vaccine |
| D120 | #2: Slight elevation of GGT (1 day). #3: Moderate eosinophilia (1 day). #10: Mild headache (1 day). | Not related to the vaccine |
| Type of Test | Tests Outside the Reference Values (RVs) by Participant Number (#) and Day of Occurrence | Toxicity Evaluation |
|---|---|---|
| Hematologic tests | ||
| MCV (fl)-RV= (80–100) | #3 at D120 (MCV = 79.7) | No toxicity observed. |
| Total Leukocytes (TL) (/µL) RV = (3600–11,000) ICTDR G1: 13,000–15,000 /mm3 | #2 at baseline (TL = 11,170) #10 at baseline (TL = 10,270) #13 at D90 (TL = 11,310) and D120 (TL = 10,940) | No toxicity observed. |
| Eosinophil (E) (%) RV= (0–5) | #2: at D37 (E = 8), D67 (E = 6), and D120 (E = 6) #3 at baseline (E = 12), D7 (E = 10), D67 (E = 14), D90 (E = 21), and D120 (E = 19) #7 at baseline (E = 8), D7 (E = 12), and D90 (E = 7) #12 at baseline (E = 7), D37 (E = 7), D67 (E = 6), and D90 (E = 6) | ICTDR has no recommendations for toxicity. Elevated values at all follow-ups were probably not related to vaccine. |
| Neutrophil (%)–RV = (0–5) | No values outside the RV. | No toxicity observed. |
| Segmented neutrophil (SN) (%) RV = (40–78) | #2 at D90 (SN = 80, NCS) #3 at baseline (SN = 31) | ICTDR has no recommendations for toxicity. Elevated value at D90 was probably not related to vaccine. |
| Lymphocytes (L) (%) RV = (20–50) | #2 at baseline (L = 16, NCS) and D90 (L = 13, NCS) #7 at D37 (L = 19, NCS) #10 at baseline (L = 17, NCS) and D90 (L = 16, NCS) | ICTDR has no recommendations for toxicity. No toxicity observed. |
| Atypical lymphocyte | None observed | No toxicity observed. |
| Monocytes (M) (%) RV = (2–10) | #1 at D120 (M = 13, NCS) #12 at D37 (M = 12, NCS) | ICTDR has no recommendations for toxicity. |
| Hemoglobin (H) (g/dL) RV = (12.0–16.0) | #2 at baseline (H = 11.6, NCS) #3 at D7 (H = 11.6, NCS), D67 (H = 11.8, NCS), and D90 (H = 11.8, NCS) #5 at baseline (H = 11.6, NCS), D7 (H = 11.4, NCS), D37 (H = 11.7, NCS), D67 (H = 11.6, NCS), D90 (H = 11.4, NCS), and D120 (H = 11.9, NCS) #8 at D7 (H = 11.8, NCS), D37 (H = 11.8, NCS), and D120 (H = 11.7, NCS) #10 at D7 (H = 11.9, NCS) #13 at baseline (H = 11.6, NCS), D7 (H = 11.4, NCS), D37 (H = 11.0, NCS), D67 (H = 11.3, NCS), D90 (H = 11.6, NCS), and D120 (H = 11.3, NCS) | No toxicity observed. |
| Platelets (/µL) RV = (140,000–400,000) | #14 at D37 (Platelets = 438,000, NCS) | No toxicity observed. |
| Red blood cells (RBC) (M/µL) RV = (3.9–5.3) | #5 at baseline (RBC = 3.84), D7 (RBC = 3.79), and D90 (RBC = 3.85) #10 at D7 (RBC = 3.87) | No toxicity observed. |
| Hematocrit (HM) (%) RV= (36–48) | #3 at baseline (HM = 35.4), D7 (HM = 35.9), and D67 (HM = 35.5) #5 at baseline (HM = 34.6), D7 (HM = 34.1), D37 (HM = 34.6), D67 (HM = 35.4), and D90 (HM = 34.3) #8 at D7 (HM = 35.9), D67 (HM = 35.9), and D120 (HM = 34.0) #10 at D7 (35.3) #13 at baseline (HM = 35.2), D7 (HM = 33.5), D37 (HM = 32.4), D67 (HM = 34.0), D90 (HM = 35.8), and D120 (HM = 32.9) | No toxicity observed. |
| Basophil (%)–RV = (0–2), Myelocyte (%)–RV = 0, Metamyelocyte (%)–RV = 0 | None found in any participant during the entire study. | |
| Activated partial thromboplastin time PTT (sec)-RV = (25.1–36.5) | #10 at D67 (PTT = 23.9) #14 at baseline (PTT = 24.3), D37 (PTT = 24.3), D90 (PTT = 23.8), and D120 (PTT = 24.0) | No toxicity observed. |
| Prothrombin time (sec) RV = (9.4–12.5) | No values outside the RV. | No toxicity observed. |
| Prothrombin ratio (PR) (%) RV = (70–100) | #2 at baseline (PR = 101, NCS), D7 (PR = 117, NCS), D37 (PR = 112, NCS), and D67 (PR = 113, NCS) #7 at D7 (PR = 103, NCS), and D90 (103, NCS) #10 at baseline (PR = 104, NCS), and D67 (PR = 101, NCS) #12 at baseline (PR = 104, NCS) #13 at baseline (PR = 112, NCS) #14 at baseline (PR = 115, NCS), and D7 (PR = 114, NCS), D37 (PR = 106, NCS), D90 (PR = 108, NCS), and D120 (PR = 115, NCS). | No toxicity observed. |
| International Normalized Ratio (INR)–RV = (1.0–1.2) | No values were above the LR RV. | ICTDR has no recommendations for toxicity. No toxicity observed. |
| Blood Chemistry | ||
| Sodium (mmol/L) RV = (136–145) | No values were above the RV. | No toxicity observed. |
| Potassium (K) (mmol/L) RV = (3.5–5.1) ICTDR G1: 5.6 to 6.0 mEq/L ICTDR G2: 6.0 to 6.5 mmol/L. | #1 at D37 (K = 5.4, NCS) and D67 (K = 5.7, G1). #10 at D37 (K = 5.2, NCS). | #1 did not show other symptoms or other abnormal laboratory findings and was considered to have no toxicity, according to medical chart. |
| Liver function | ||
| Total bilirubin (mg/dL) RV ≤ 1.2 | No values were above the RV. | No toxicity observed. |
| Direct bilirubin (DB) (mg/dL) RV ≤ 0.3 | #1 at baseline (DB = 0.34) | No toxicity observed. |
| Indirect bilirubin (mg/dL)–RV ≤ 0.7 | No values were above the LR RV. | No toxicity observed |
| Alkaline Phosphatase (AP) (U/L) RV = (35–104) ICTDR G1 Lower Limit = 130 | #12 at D90 (AP = 105) #13 at baseline (AP = 119), D7 (AP = 118), D37 (AP = 106), D67 (AP = 106), D90 (AP = 123), and D120 (AP = 104) | No toxicity observed according to medical charts. High values for #13 are probably not due to vaccine (was high at baseline). |
| AST (U/L)–RV ≤ 32 | #12 at D90 (AST = 37, NCS) | No toxicity observed. |
| ALT (U/L) RV ≤ 33 ICTDR G1: (40–80) | #12 at D90 (ALT = 66, G1, NCS) #13 at D37 (ALT = 35), D67 (ALT = 35), and D90 (ALT = 33) | #12 above ICTDR threshold for grade 1 toxicity, but not clinically significant according to medical chart. No other toxicity observed. |
| GGT (U/L) RV ≤ 40 ICTDR G1: (50–100) ICTDR G2: (101–200) ICTDR G3: (201–400) ICTDR G4: > 400 | #2 at baseline (GGT = 52, G1), D7 (GGT = 50), D37 (GGT = 58, G1), D67 (GGT = 57, G1), D90 (GGT = 68, G1), and D120 (GGT = 69, G1) #12 at baseline (GGT = 99, G1), D7(GGT = 106, G2), D37(GGT = 113, G2), D67(GGT = 95, G1), D90 (GGT = 240, G3), and D120 (GGT = 107, G2) #13 at baseline (GGT = 122, G2), D7(GGT = 123, G2), D37 (GGT = 112, G2), D67(GGT = 106, G2), D90 (GGT = 130, G2), and D120 (GGT = 102, G2) | Three participants had elevated GGT at baseline and all follow-ups, which were most likely not due to the vaccine. |
| Kidney function | ||
| Creatinine (C) (mg/dL) RV= (0.5–0.9) ICTDR G1: 0.99–1.35 | #13 at D67 (C = 0.95) and D120 (C = 0.94) | No toxicity observed. |
| Urea (mg/dL) RV < 50 (for age ≤ 65) | No values were above the RV. | No toxicity observed. |
| Other blood tests | ||
| Calcitonin (pg/mL) RV ≤ 11.5 | No values were above the RV | No toxicity observed. |
| Abdomen physical exam | ||
| Abnormalities | None observed. | No toxicity observed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santini-Oliveira, M.; Machado Pinto, P.; Santos, T.d.; Vilar, M.M.; Grinsztejn, B.; Veloso, V.; Paes-de-Almeida, E.C.; Amaral, M.A.Z.; Ramos, C.R.; Marroquin-Quelopana, M.; et al. Development of the Sm14/GLA-SE Schistosomiasis Vaccine Candidate: An Open, Non-Placebo-Controlled, Standardized-Dose Immunization Phase Ib Clinical Trial Targeting Healthy Young Women. Vaccines 2022, 10, 1724. https://doi.org/10.3390/vaccines10101724
Santini-Oliveira M, Machado Pinto P, Santos Td, Vilar MM, Grinsztejn B, Veloso V, Paes-de-Almeida EC, Amaral MAZ, Ramos CR, Marroquin-Quelopana M, et al. Development of the Sm14/GLA-SE Schistosomiasis Vaccine Candidate: An Open, Non-Placebo-Controlled, Standardized-Dose Immunization Phase Ib Clinical Trial Targeting Healthy Young Women. Vaccines. 2022; 10(10):1724. https://doi.org/10.3390/vaccines10101724
Chicago/Turabian StyleSantini-Oliveira, Marília, Patrícia Machado Pinto, Tatiane dos Santos, Mônica Magno Vilar, Beatriz Grinsztejn, Valdilea Veloso, Elan C. Paes-de-Almeida, Maria A. Z. Amaral, Celso R. Ramos, Miryam Marroquin-Quelopana, and et al. 2022. "Development of the Sm14/GLA-SE Schistosomiasis Vaccine Candidate: An Open, Non-Placebo-Controlled, Standardized-Dose Immunization Phase Ib Clinical Trial Targeting Healthy Young Women" Vaccines 10, no. 10: 1724. https://doi.org/10.3390/vaccines10101724
APA StyleSantini-Oliveira, M., Machado Pinto, P., Santos, T. d., Vilar, M. M., Grinsztejn, B., Veloso, V., Paes-de-Almeida, E. C., Amaral, M. A. Z., Ramos, C. R., Marroquin-Quelopana, M., Coler, R., Reed, S., Ciol, M. A., Savino, W., Parra, J. d. C., Almeida, M. S. d. S., & Tendler, M. (2022). Development of the Sm14/GLA-SE Schistosomiasis Vaccine Candidate: An Open, Non-Placebo-Controlled, Standardized-Dose Immunization Phase Ib Clinical Trial Targeting Healthy Young Women. Vaccines, 10(10), 1724. https://doi.org/10.3390/vaccines10101724






