Cost-Effectiveness of COVID-19 Sequential Vaccination Strategies in Inactivated Vaccinated Individuals in China
Abstract
:1. Introduction
2. Methods
2.1. Study Design
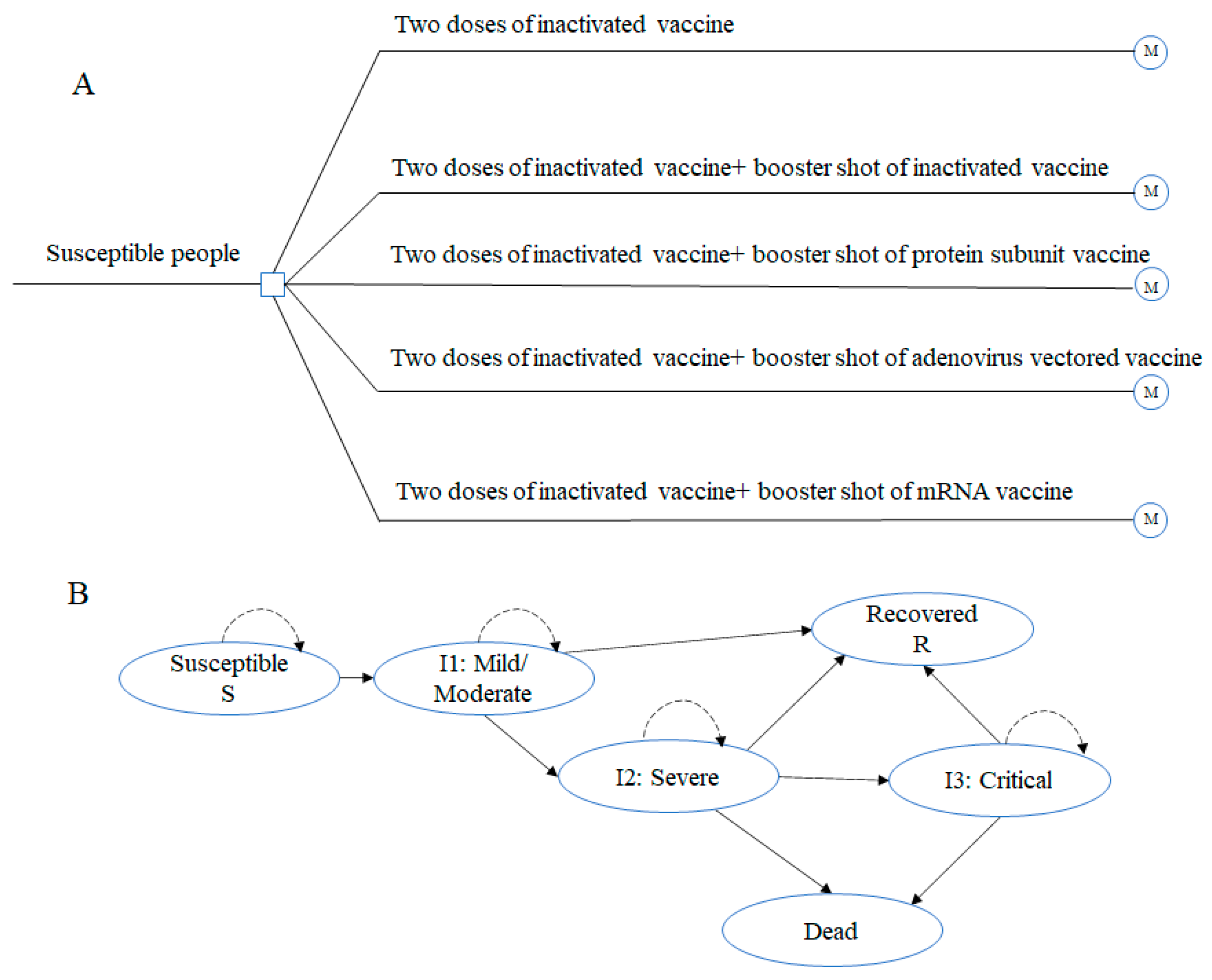
2.2. Target Population and Vaccination Strategies
2.3. Model Structure and Assumptions
2.4. Model Parameters
2.4.1. Vaccine Effectiveness
2.4.2. Transition Probabilities
2.4.3. Costs
2.4.4. Health Utilities
| Parameter | Base Case Value | Lower Bound | Upper Bound | Distribution | Data Source |
|---|---|---|---|---|---|
| Vaccination effectiveness (%) | |||||
| Two doses of inactivated vaccine | |||||
| Against infection | 65.90 | 65.20 | 66.60 | Beta | Jara et al. 2021 [33] |
| Against hospitalization | 87.50 | 86.70 | 88.20 | Beta | |
| Against ICU | 90.30 | 89.10 | 91.40 | Beta | |
| Against death | 86.30 | 84.50 | 87.90 | Beta | |
| Two doses of inactivated vaccine + booster shot of inactivated vaccine | |||||
| Against infection | 78.80 | 76.80 | 80.60 | Beta | Jara et al. 2022 [34] |
| Against hospitalization | 86.30 | 83.70 | 88.50 | Beta | |
| Against ICU | 92.20 | 88.70 | 94.60 | Beta | |
| Against death | 86.70 | 80.50 | 91.00 | Beta | |
| Two doses of inactivated vaccine+ booster shot of protein subunit vaccine | |||||
| Against infection | 83.22 | 74.90 | 91.54 | Beta | Calculated |
| Against hospitalization | 91.44 | 82.30 | 100 | Beta | |
| Against ICU | 91.63 | 82.47 | 100 | Beta | |
| Against death | 91.64 | 82.48 | 100 | Beta | |
| Two doses of inactivated vaccine + booster shot of adenovirus vectored vaccine | |||||
| Against infection | 93.20 | 92.90 | 93.60 | Beta | Jara et al. 2022 [34] |
| Against hospitalization | 97.70 | 97.30 | 98.00 | Beta | |
| Against ICU | 98.90 | 98.50 | 99.20 | Beta | |
| Against death | 98.10 | 97.30 | 98.60 | Beta | |
| Two doses of inactivated vaccine+ booster shot of mRNA vaccine | |||||
| Against infection | 96.50 | 96.20 | 96.70 | Beta | Jara et al. 2022 [34] |
| Against hospitalization | 96.10 | 95.30 | 96.90 | Beta | |
| Against ICU | 96.20 | 94.60 | 97.30 | Beta | |
| Against death | 96.80 | 93.90 | 98.30 | Beta | |
| Transition probabilities without vaccination | |||||
| Natural infection rate | 0.1043 | 0.0939 | 0.1147 | Beta | Jara et al. 2021 [33] |
| I1 to I2 | 0.1450 | 0.1305 | 0.1595 | Beta | Zhao et al. 2021 [37] |
| I2 to I3 | 0.2540 | 0.2286 | 0.2794 | Beta | |
| I2 to death | 0.0005 | 0.00045 | 0.00055 | Beta | |
| I3 to death | 0.0005 | 0.00045 | 0.00055 | Beta | |
| I1 to recover | 0.7475 | 0.6728 | 0.8223 | Beta | Padula et al. 2021 [3] |
| I2 to recover | 0.6500 | 0.5850 | 0.7150 | Beta | |
| I3 to recover | 0.5300 | 0.4770 | 0.5830 | Beta | |
| Vaccination cost per dose (2021 USD) | |||||
| Inactivated vaccine | 4.00 | 4.00 | 5.50 | Gamma | WHO [39]; Calculated |
| Adenovirus vectored vaccine | 15.00 | 9.63 | 15.00 | Gamma | WHO [39]; Calculated |
| Protein subunit vaccine | 19.54 | 2.12 | 19.54 | Gamma | News [40]; Calculated |
| mRNA vaccine | 6.75 | 3.80 | 6.75 | Gamma | WHO [39]; Calculated |
| Cold-chain freight fee as a percentage of vaccine cost | 6% | / | / | / | Chen et al. 2019 [41] |
| Refrigerator storage of 2–8 ℃ incubator | 0.18 | / | / | / | Jiang et al. 2022 [8] |
| Refrigerator storage of −70 ℃ incubator | 0.39 | / | / | / | Calculated |
| Administration | 1.55 | / | / | / | National medical insurance bureau [42] |
| Medical costs of health status (2021USD) | |||||
| I1 | 876.32 | 619.11 | 1804.12 | Gamma | Jin et al. 2020 [43];Zhao et al. 2021 [37] |
| I2 | 8284.63 | 5852.95 | 17057.25 | Gamma | |
| I3/Death | 23469.03 | 16861.36 | 49139.19 | Gamma | |
| Length of hospital stay (day) | |||||
| I1 | 14 | / | / | / | Jin et al. 2020 [43];Zhao et al. 2021 [37] |
| I2 | 21 | / | / | / | |
| I3 | 42 | / | / | / | |
| Death | 42 | / | / | / | |
| Average salary per day (2021USD) | 42.55 | 32.07 | 76.04 | Gamma | National Bureau of Statistics [44] |
| Health utilities | |||||
| Susceptible | 0.946 | 0.9461 | 1 | Xie et al. 2022 | |
| I1 | 0.847 | 0.762 | 0.932 | Beta | Alinia et al. 2021 [45] |
| I2 | 0.766 | 0.689 | 0.843 | Beta | |
| I3 | 0.629 | 0.566 | 0.692 | Beta | |
| Recover | 0.896 | 0.806 | 0.986 | Beta | |
| Death | 0 | / | / | / | |
2.5. Model Analysis
2.5.1. Base Case Analysis
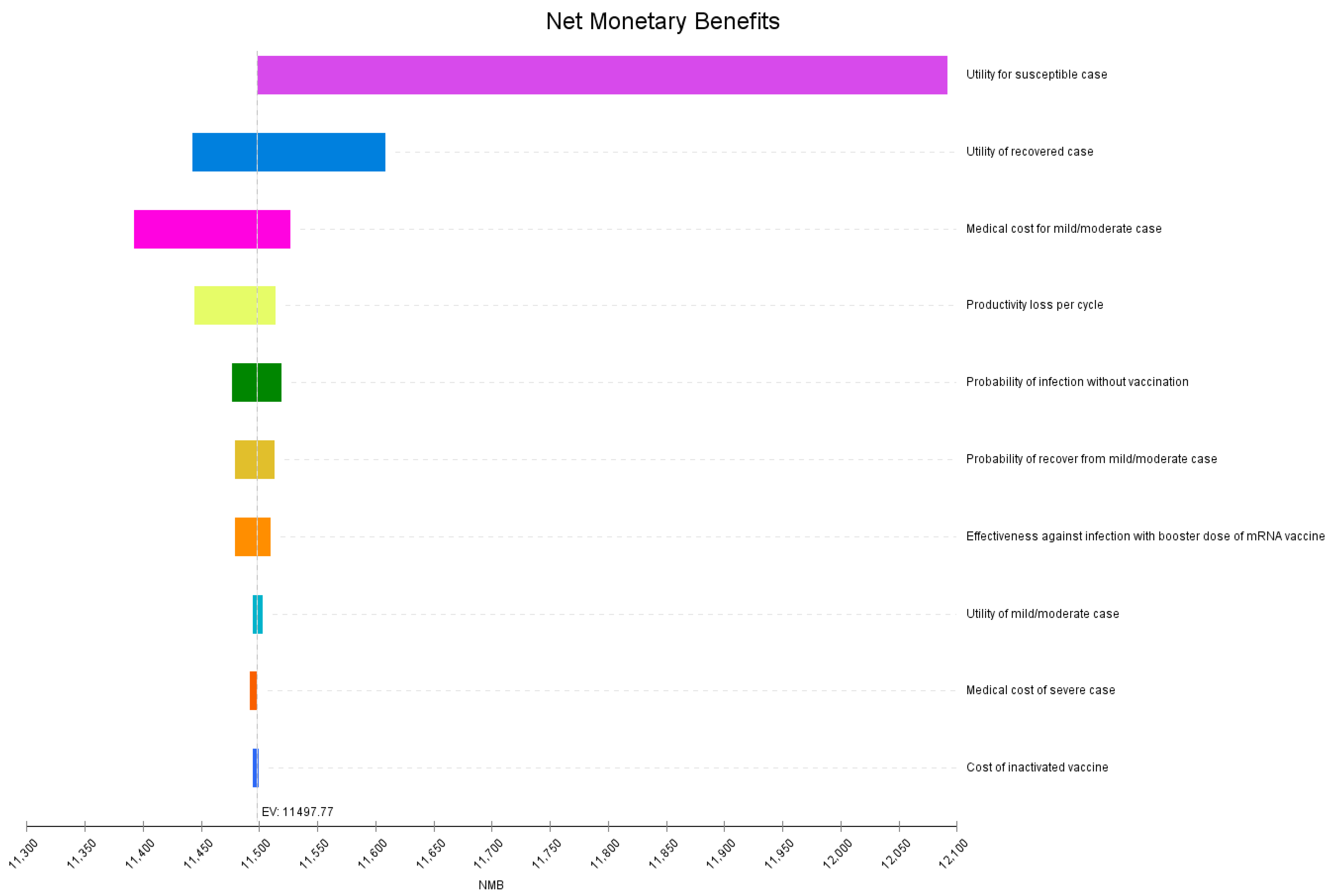
2.5.2. Sensitivity Analysis
2.5.3. Scenario Analysis
3. Results
3.1. Base Case Analysis
3.2. Sensitivity Analysis
3.3. Scenario Analysis
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Coronavirus COVID-19 Dashboard. Available online: https://covid19.who.int (accessed on 8 December 2021).
- Shaker, M.; Abrams, E.M.; Greenhawt, M. A Cost-Effectiveness Evaluation of Hospitalizations, Fatalities, and Economic Out-comes Associated with Universal Versus Anaphylaxis Risk-Stratified COVID-19 Vaccination Strategies. J. Allergy Clin. Immunol. Pract. 2021, 9, 2658–2668.e3. [Google Scholar] [CrossRef] [PubMed]
- Padula, W.V.; Malaviya, S.; Reid, N.M.; Cohen, B.G.; Chingcuanco, F.; Ballreich, J.; Tierce, J.; Alexander, G.C. Economic value of vaccines to address the COVID-19 pandemic: A U.S. cost-effectiveness and budget impact analysis. J. Med. Econ. 2021, 24, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Maschio, M.; Becker, D.; Weinstein, M.C. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: Use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine 2021, 39, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Marco-Franco, J.E.; Emilio, J.; Pita-Barros, P.; González-de-Julián, S.; Sabat, I.; Vivas-Consuelo, D. Simplified Mathematical Modeling of Uncertainty: Cost-Effectiveness of COVID-19 Vaccines in Spain. Mathematics 2021, 9, 566. [Google Scholar] [CrossRef]
- Debrabant, K.; Grønbæk, L.; Kronborg, C. The Cost-Effectiveness of a COVID-19 Vaccine in a Danish Context. Clin. Drug Investig. 2021, 41, 975–988. [Google Scholar] [CrossRef]
- Hagens, A.; Inkaya, A.; Yildirak, K.; Sancar, M.; van der Schans, J.; Sancar, A.A.; Ünal, S.; Postma, M.; Yeğenoğlu, S. COVID-19 Vaccination Scenarios: A Cost-Effectiveness Analysis for Turkey. Vaccines 2021, 9, 399. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, D.; Shi, S. Economic evaluations of inactivated COVID-19 vaccines in six Western Pacific and South East Asian countries and regions: A modeling study. Infect. Dis. Model. 2021, 7, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Belayachi, J.; Obtel, M.; Razine, R.; Abouqal, R. Long term effectiveness of inactivated vaccine BBIBP-CorV (Vero Cells) against COVID-19 associated severe and critical hospitalization in Morocco. medRxiv 2022. [Google Scholar] [CrossRef]
- Glöckner, S.; Hornung, F.; Baier, M.; Weis, S.; Pletz, M.W.; Deinhardt-Emmer, S.; Löffler, B. CoNAN Study Group Robust Neutralizing Antibody Levels Detected after Either SARS-CoV-2 Vaccination or One Year after Infec-tion. Viruses 2021, 13, 2003. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2021, 22, 483–495. [Google Scholar] [CrossRef]
- Abbasi, J. Studies Suggest COVID-19 Vaccine Boosters Save Lives. JAMA 2022, 327, 115. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Duan, K.; Zhang, Y.; Yuan, Z.; Zhang, Y.; Wang, Z.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: A ran-domized, double-blind, placebo-controlled, phase 1/2 trial. eClinicalMedicine 2021, 38, 101010. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wu, Q.; Zeng, G.; Yang, J.; Jiang, D.; Deng, X.; Chu, K.; Zheng, W.; Zhu, F.; Yu, H.; et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18-59 years: Interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Falsey, A.R.; Frenck, R.W., Jr.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Bailey, R.; Swanson, K.A.; Xu, X.; et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef]
- Saban, M.; Myers, V.; Wilf-Miron, R. Changes in infectivity, severity and vaccine effectiveness against delta COVID-19 vari-ant ten months into the vaccination program: The Israeli case. Prev. Med. 2022, 154, 106890. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Sapkota, B.; Saud, B.; Shrestha, R.; Al-Fahad, D.; Sah, R.; Shrestha, S.; Rodriguez-Morales, A.J. Heterologous prime–boost strategies for COVID-19 vaccines. J. Travel Med. 2021, 29, taab191. [Google Scholar] [CrossRef]
- Petrelli, F.; Luciani, A.; Borgonovo, K.; Ghilardi, M.; Parati, M.C.; Petrò, D.; Lonati, V.; Pesenti, A.; Cabiddu, M. Third dose of SARS-CoV-2 vaccine: A systematic review of 30 published studies. J. Med. Virol. 2022, 94, 2837–2844. [Google Scholar] [CrossRef]
- Thompson, M.G. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance-VI-SION Network, 10 States, August 2021-January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 139–145. [Google Scholar] [PubMed]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChA-dOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Reimann, P.; Ulmer, H.; Mutschlechner, B.; Benda, M.; Severgnini, L.; Volgger, A.; Lang, T.; Atzl, M.; Huynh, M.; Gasser, K.; et al. Efficacy and safety of heterologous booster vaccination with Ad26.COV2.S after BNT162b2 mRNA COVID-19 vaccine in haemato-oncological patients with no antibody response. Br. J. Haematol. 2022, 196, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Abolhassani, H.; Du, L.; Piralla, A.; Bertoglio, F.; de Campos-Mata, L.; Wan, H.; Schubert, M.; Wang, Y.; Sun, R.; et al. Heterologous immunization with inactivated vaccine followed by mRNA booster elicits strong humoral and cellular immune responses against the SARS-CoV-2 Omicron variant. medRxiv 2022. [Google Scholar] [CrossRef]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Srimuan, D.; Thatsanatorn, T.; et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and mRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines 2022, 10, 86. [Google Scholar] [CrossRef]
- Chiu, N.-C.; Chi, H.; Tu, Y.-K.; Huang, Y.-N.; Tai, Y.-L.; Weng, S.-L.; Chang, L.; Huang, D.T.-N.; Huang, F.-Y.; Lin, C.-Y. To mix or not to mix? A rapid systematic review of heterologous prime–boost covid-19 vaccination. Expert Rev. Vaccines 2021, 20, 1211–1220. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, T.; Huang, M.; Liu, S.; Su, X.; Li, G.; Song, T.; Li, W.; Zhong, N.; Xu, M.; et al. Heterologous boosting with third dose of coronavirus disease recombinant subunit vaccine increases neutralizing antibodies and T cell immunity against different severe acute respiratory syndrome coronavirus 2 variants. Emerg. Microbes Infect. 2022, 11, 829–840. [Google Scholar] [CrossRef]
- Kaku, C.I.; Champney, E.R.; Normark, J.; Garcia, M.; Johnson, C.E.; Ahlm, C.; Christ, W.; Sakharkar, M.; Ackerman, M.E.; Klingström, J.; et al. Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 vaccination. Science 2022, 375, 1041–1047. [Google Scholar] [CrossRef]
- Commission, N.H. COVID-19 Diagnosis and Treatment Guideline (Version 9). Available online: http://www.nhc.gov.cn/yzygj/s7653p/202203/b74ade1ba4494583805a3d2e40093d88.shtml (accessed on 30 March 2022).
- China, M.o.T.o.t.P.s.R.o. COVID-19 Prevention and Control Guidelines for Ports and Their Frontline Personnel (Ninth Edition). Available online: https://xxgk.mot.gov.cn/2020/jigou/syj/202203/t20220303_3644120.html (accessed on 24 March 2022).
- Zhou, D.; Shao, T.; Shao, H.; Tu, Y.; Tang, Y.; Zhou, J.; Malone, D.; Tang, W. EPH172 When Is It Valuable for COVID-19 Booster Dose?: A Transmission Dynamics Model-Based Effectiveness and Cost-Effectiveness Analysis of Two Booster Dose Vaccination Priority Strategies in Mainland China. Value Health 2022, 25, S466. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Araos, R. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; González, C.; Pizarro, A.; Acevedo, J.; Araos, R. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: A large-scale pro-spective cohort study. Lancet Glob. Health 2022, 10, e798–e806. [Google Scholar] [CrossRef]
- Zhifei Biological Products Co., L. The Coronavirus Recombinant Protein Vaccine (CHO Cells) has been Approved for Conditional Marketing. Available online: http://www.zhifeishengwu.com/news/gsyw/qyyw/2022-03-02/624.html (accessed on 21 March 2022).
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2021, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, H.; Li, X.; Jia, J.; Zhang, C.; Zhao, H.; Ma, W.; Wang, Z.; He, Y.; Lee, J.; et al. Disease Burden Attributable to the First Wave of COVID-19 in China and the Effect of Timing on the Cost-Effectiveness of Movement Restriction Policies. Value Health 2021, 24, 615–624. [Google Scholar] [CrossRef]
- Data, O. Exchange Rates. 2022. Available online: https://data.oecd.org/conversion/exchange-rates.htm (accessed on 10 April 2022).
- World Health Organization. Vaccine Purchase Data. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/vaccine-access/mi4a/mi4a-vaccine-purchase-data (accessed on 20 February 2022).
- Finance, S. In-depth Analysis of the Pharmaceutical Industry. Available online: http://stock.finance.sina.com.cn/stock/go.php/vReport_Show/kind/lastest/rptid/660793064184/index.phtml (accessed on 5 April 2022).
- Chen, C.; Liceras, F.C.; Flasche, S.; Sidharta, S.; Yoong, J.; Sundaram, N.; Jit, M. Effect and cost-effectiveness of pneumococcal conjugate vaccination: A global modelling analysis. Lancet Glob. Health 2019, 7, e58–e67. [Google Scholar] [CrossRef] [Green Version]
- Bureau, N.M.I. Notice on the Effective Implementation of Phased Liquidation of Coronavirus Vaccines and Vaccination Costs. Available online: http://www.nhsa.gov.cn/art/2021/8/23/art_53_5856.html (accessed on 20 February 2022).
- Jin, H.; Wang, H.; Li, X.; Zheng, W.; Ye, S.; Zhang, S.; Zhou, J.; Pennington, M. Economic burden of COVID-19, China, January-March, 2020: A cost-of-illness study. Bull. World Health Organ. 2021, 99, 112–124. [Google Scholar] [CrossRef]
- Statistics, N.B.o. China Statistical Data. Available online: https://data.stats.gov.cn/easyquery.htm?cn=C01 (accessed on 10 April 2022).
- Alinia, C.; Yaghmaei, S.; Abdullah, F.Z.; Ahmadi, A.; Samadi, N.; Pourteimour, S.; Safari, H.; Mahmoodi, H.; Moradi, G.; Piroozi, B. The health-related quality of life in Iranian patients with COVID-19. BMC Infect. Dis. 2021, 21, 459. [Google Scholar]
- Xie, S.; Wu, J.; Xie, F. Population Norms for SF-6Dv2 and EQ-5D-5L in China. Appl. Health Econ. Health Policy 2022, 20, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Harapan, H.; Anwar, S.; Bustamam, A.; Radiansyah, A.; Angraini, P.; Fasli, R.; Salwiyadi, S.; Bastian, R.A.; Oktiviyari, A.; Akmal, I.; et al. Willingness to pay for a dengue vaccine and its associated determinants in Indonesia: A community-based, cross-sectional survey in Aceh. Acta Trop. 2017, 166, 249–256. [Google Scholar] [CrossRef]
- Kabir, K.M.A.; Hagishima, A.; Tanimoto, J. Hypothetical assessment of efficiency, willingness-to-accept and willing-ness-to-pay for dengue vaccine and treatment: A contingent valuation survey in Bangladesh. Hum. Vaccin. Immunother. 2021, 17, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.U.o.H. COVID-19 Vaccine Effectiveness in Hong Kong. Available online: http://www.med.hku.hk/en/news/press/-/media/D9C071B122C54C3089C5319E43E5187C.ashx (accessed on 5 April 2022).
- Hong Kong Special Administrative Region Government. Archive of Statistics on 5th Wave of COVID-19. Available online: https://www.coronavirus.gov.hk/eng/5th-wave-statistics.html (accessed on 11 April 2022).
- Department of Health, The Government of the Hong Kong Special Administrative Region. Health Fact of Hong Kong in 2021. Available online: https://www.dh.gov.hk/chs/statistics/statistics_hs/statistics_hfhk.html (accessed on 12 April 2022).
- Wang, W.-C.; Fann, J.C.-Y.; Chang, R.-E.; Jeng, Y.-C.; Hsu, C.-Y.; Chen, H.-H.; Liu, J.-T.; Yen, A.M.-F. Economic evaluation for mass vaccination against COVID-19. J. Formos. Med. Assoc. 2021, 120, S95–S105. [Google Scholar] [CrossRef]
- Council, S. Vaccination Status. Available online: http://www.gov.cn/xinwen/gwylflkjz190/index.htm (accessed on 20 September 2022).
- Yu, X.; Wei, D.; Xu, W.; Li, Y.; Li, X.; Zhang, X.; Qu, J.; Yang, Z.; Chen, E. Reduced sensitivity of SARS-CoV-2 Omicron variant to booster-enhanced neutralization. medRxiv 2021. [Google Scholar] [CrossRef]
- Angkasekwinai, N.; Niyomnaitham, S.; Sewatanon, J.; Phumiamorn, S.; Sukapirom, K.; Senawong, S.; Toh, Z.Q.; Umrod, P.; Somporn, T.; Chumpol, S.; et al. The immunogenicity and reactogenicity of four COVID-19 booster vaccinations against SARS-CoV-2 variants of concerns (Delta, Beta, and Omicron) following CoronaVac or ChAdOx1 nCoV-19 primary series. medRxiv 2022. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Fu, Z.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, S.; Liu, J.; Wu, L.; Qiu, J.; Wang, N.; Ren, J.; Li, Z.; Guo, X.; Tao, F.; et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant in-fection, severe illness, and death. medRxiv 2022. [Google Scholar] [CrossRef]
- Li, R.; Liu, H.; Fairley, C.K.; Zou, Z.; Xie, L.; Li, X.; Shen, M.; Li, Y.; Zhang, L. Cost-effectiveness analysis of BNT162b2 COVID-19 booster vaccination in the United States. Int. J. Infect. Dis. 2022, 119, 87–94. [Google Scholar] [CrossRef]

| Strategy | Cost (US$) | Effect (QALYs) | NMB (US$) | Incremental Cost (US$) | Incremental Effect (QALYs) | ICER (US$/QALY) |
|---|---|---|---|---|---|---|
| Two-dose inactivated vaccine | 918.26 | 0.9062 | 9188.22 | - | - | - |
| Two doses of inactivated vaccine+ booster shot of inactivated vaccine | 755.30 | 0.9138 | 9435.36 | −162.96 | 0.0075 | −21,587.61 |
| Two doses of inactivated vaccine+ booster shot of protein subunit vaccine | 656.52 | 0.9172 | 9572.21 | −261.73 | 0.0110 | −23,875.83 |
| Two doses of inactivated vaccine+ booster shot of adenovirus vectored vaccine | 335.04 | 0.9271 | 10,003.44 | −583.21 | 0.0208 | −28,034.30 |
| Two doses of inactivated vaccine+ booster shot of mRNA vaccine | 193.77 | 0.9311 | 10,190.13 | −724.49 | 0.0249 | −29,123.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Zhao, J.; Wei, X.; Han, P.; Yang, L.; Ren, T.; Zhan, S.; Li, L. Cost-Effectiveness of COVID-19 Sequential Vaccination Strategies in Inactivated Vaccinated Individuals in China. Vaccines 2022, 10, 1712. https://doi.org/10.3390/vaccines10101712
Fu Y, Zhao J, Wei X, Han P, Yang L, Ren T, Zhan S, Li L. Cost-Effectiveness of COVID-19 Sequential Vaccination Strategies in Inactivated Vaccinated Individuals in China. Vaccines. 2022; 10(10):1712. https://doi.org/10.3390/vaccines10101712
Chicago/Turabian StyleFu, Yaqun, Jingyu Zhao, Xia Wei, Peien Han, Li Yang, Tao Ren, Siyan Zhan, and Liming Li. 2022. "Cost-Effectiveness of COVID-19 Sequential Vaccination Strategies in Inactivated Vaccinated Individuals in China" Vaccines 10, no. 10: 1712. https://doi.org/10.3390/vaccines10101712
APA StyleFu, Y., Zhao, J., Wei, X., Han, P., Yang, L., Ren, T., Zhan, S., & Li, L. (2022). Cost-Effectiveness of COVID-19 Sequential Vaccination Strategies in Inactivated Vaccinated Individuals in China. Vaccines, 10(10), 1712. https://doi.org/10.3390/vaccines10101712






