Safety and Immunogenicity of a Heterologous Booster of Protein Subunit Vaccine MVC-COV1901 after Two Doses of Adenoviral Vector Vaccine AZD1222
Abstract
1. Introduction
2. Methods
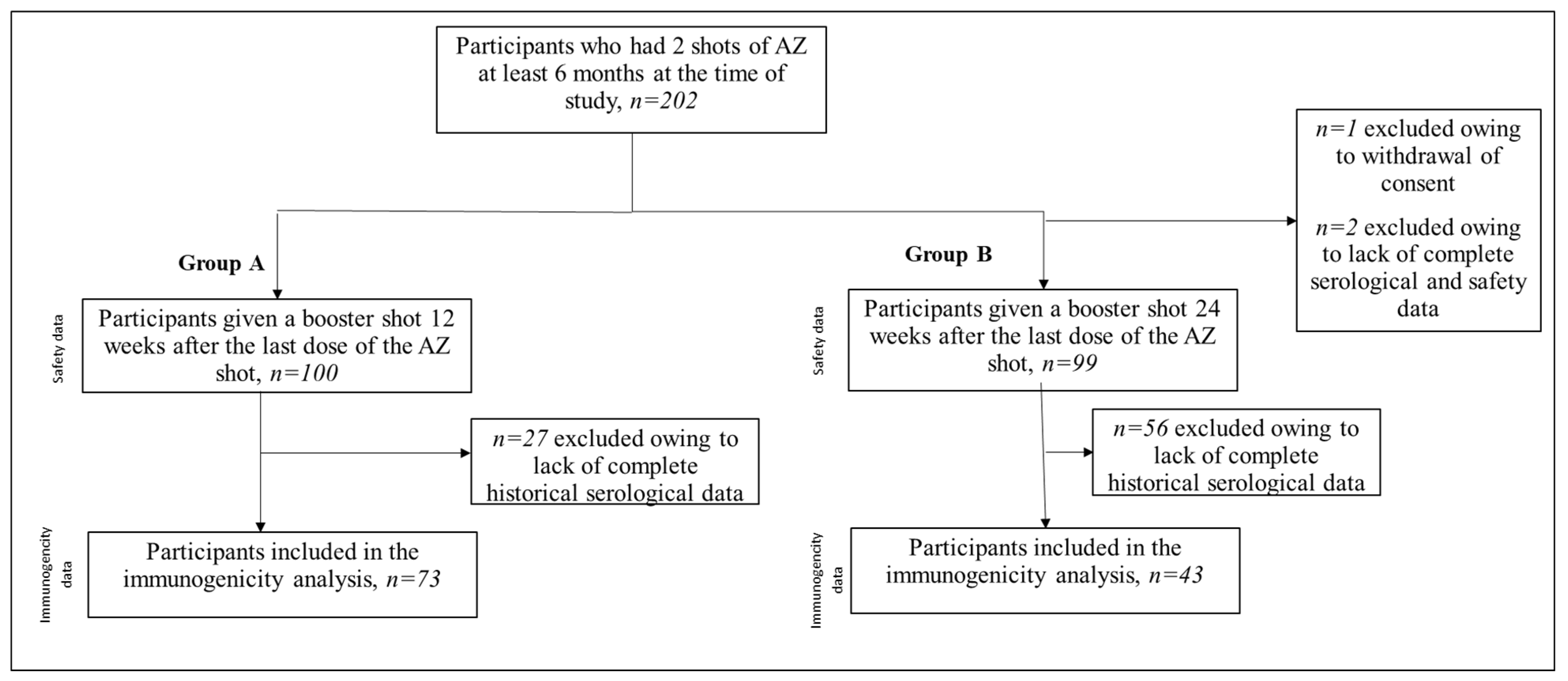
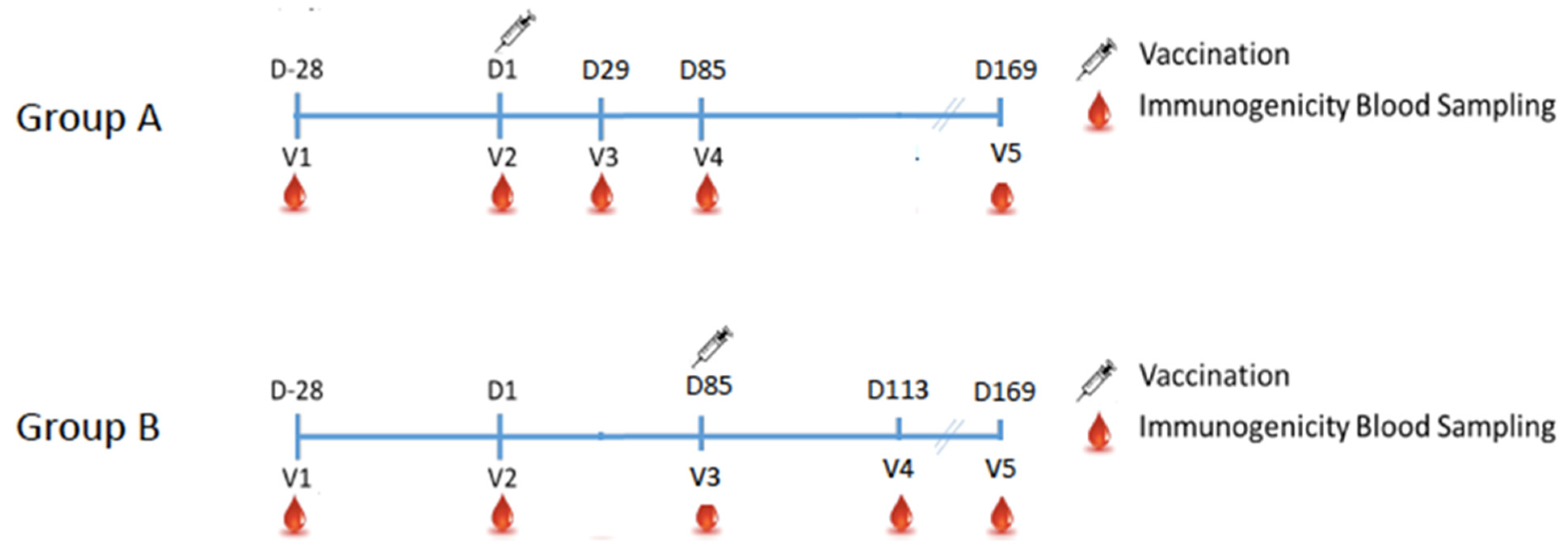
2.1. Study Design
2.2. Outcomes
2.3. Laboratory Methods
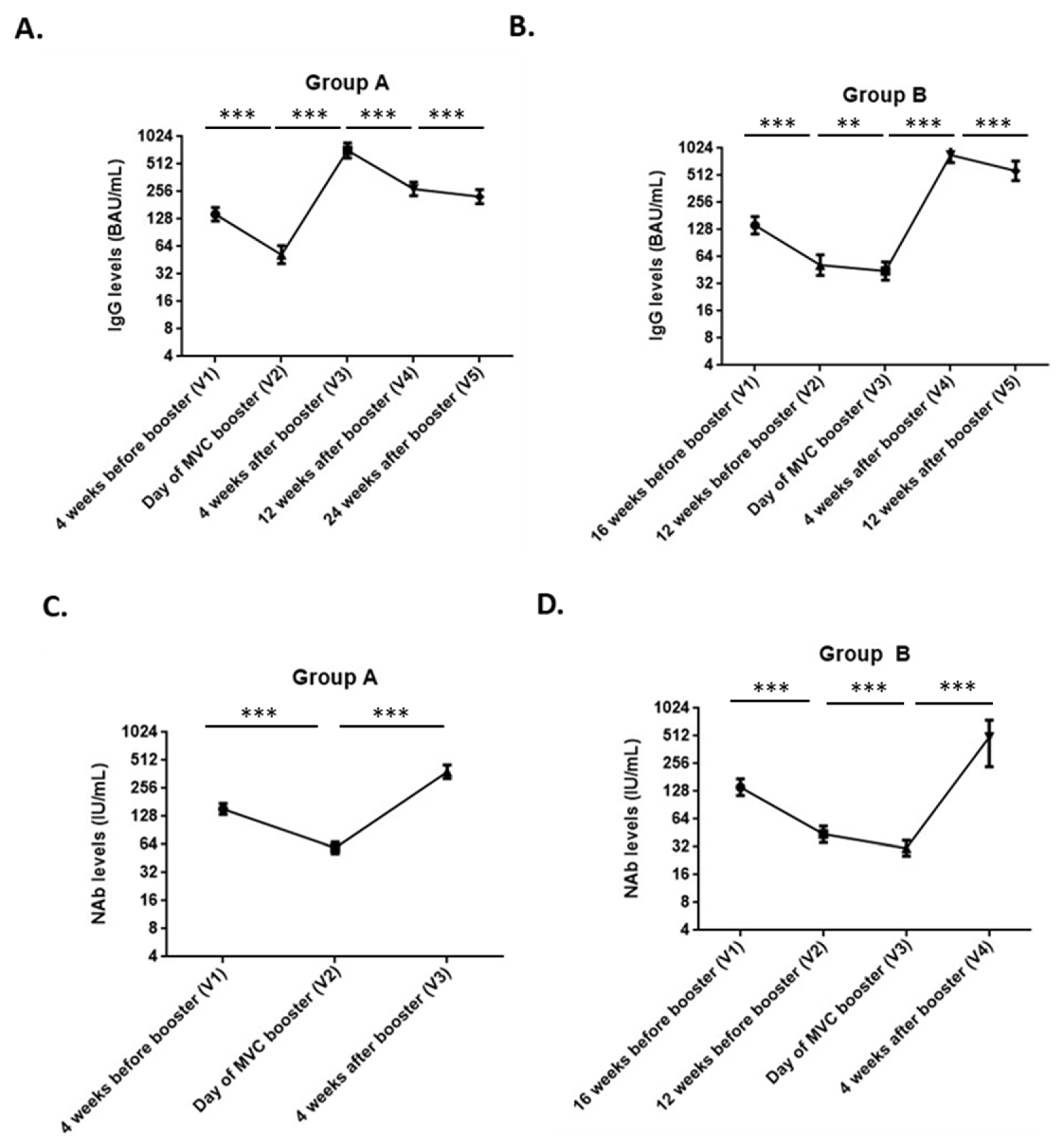
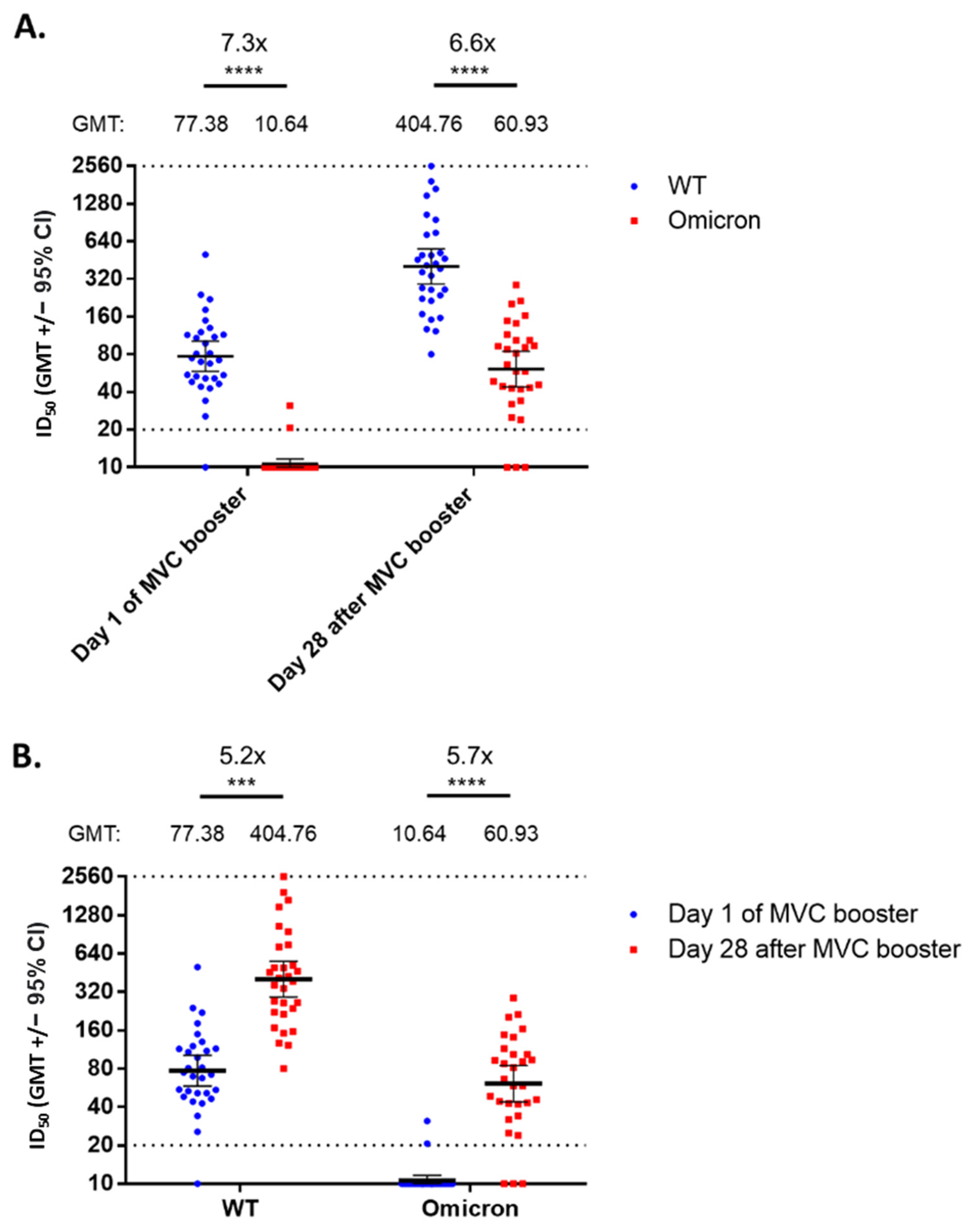
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March–August 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1337. [Google Scholar]
- US Centers for Disease Control and Prevention. Myocarditis and Pericarditis after mRNA COVID-19 Vaccination. 12 November 2021. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html (accessed on 29 November 2021).
- Simpson, C.R.; Shi, T.; Vasileiou, E.; Katikireddi, S.V.; Kerr, S.; Moore, E.; McCowan, C.; Agrawal, U.; Shah, S.A.; Ritchie, L.D.; et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021, 27, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Benhassine, F.G.; Planchais, C.; Porrot, F.; Staropoli, I.; Lemoine, F.; Pere, H.; Veyer, D.; et al. Considerable escape of SARS-CoV-2 variant Omicron to antibody neutralization. bioRxiv 2021. [Google Scholar] [CrossRef]
- Erice, A.; Varillas-Delgado, D.; Caballero, C. Decline of antibody titers 3 months after two doses of BNT162b2 in non-immunocompromised adults. Clin. Microbiol. Infect. 2022, 28, 139.e1–139.e4. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Understanding breakthrough infections following mRNA SARS-CoV-2 vaccination. JAMA 2021, 326, 2018–2020. [Google Scholar] [CrossRef]
- Sagonowsky, E. Moderna, Citing Variants and Waning Immunity, Expects COVID-19 Boosters to Become a Fact of Life. 5 August 2021. Available online: https://www.fiercepharma.com/pharma/moderna-citing-variants-and-waning-immunity-expects-covid-19-boosters-to-become-a-fact-life (accessed on 17 September 2021).
- Kollelwe, J.; The Guardian. COVID-19 Vaccines: The Contracts, Prices, and Profits. 2021. Available online: https://www.theguardian.com/world/2021/aug/11/covid-19-vaccines-the-contracts-prices-and-profits (accessed on 6 December 2021).
- BBC News. “COVID: What Do We Know about China’s Coronavirus Vaccines?” 14 January 2021. Available online: https://www.bbc.com/news/world-asia-china-55212787 (accessed on 28 November 2021).
- Munro, A.P.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Hsieh, S.M.; Liu, M.C.; Chen, Y.H.; Lee, W.S.; Hwang, S.J.; Cheng, S.H.; Ko, W.C.; Hwang, K.P.; Wang, N.C.; Lee, Y.L.; et al. Safety and immunogenicity of CpG 1018 and aluminum hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: Interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir. Med. 2021, 9, 1396–1406. [Google Scholar] [CrossRef]
- Taiwan Centers for Disease Control. V-Watch Bulletin Report, 21 November 2021. Available online: https://www.cdc.gov.tw/File/Get/Xwv8akaYldqmvlSsN3_H5Q (accessed on 29 November 2021).
- Our World in Data. Statistics and Research Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 2 October 2022).
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Hsieh, S.; Chang, S.; Cheng, H.; Shih, S.; Lien, C. The reactogenicity and immunogenicity of a booster dose after the second dose of a protein subunit vaccine MVC-COV1901. MedRxiv 2021. [Google Scholar] [CrossRef]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: An interim analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef]
- Flaxman, A.; Marchevsky, N.G.; Jenkin, D.; Aboagye, J.; Aley, P.K.; Angus, B.; Belij-Rammerstorfer, S.; Bibi, S.; Bittaye, M.; Cappuccini, F.; et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: A substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021, 398, 981–990. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.; Tian, Y.; Bruxvoort, K.; Tupert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. medRxiv 2022. [Google Scholar] [CrossRef]
- Doria-Rose, N.A.; Shen, X.; Schmidt, S.D.; O’Dell, S.; McDanal, C.; Feng, W.; Tong, J.; Eaton, A.; Maglinao, M.; Tang, H.; et al. Booster of mRNA-1273 Strengthens SARS-CoV-2 Omicron Neutralization. medRxiv 2021. [CrossRef]
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef] [PubMed]
- Lu, S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, T.; Huang, M.; Liu, S.; Su, X.; Li, G.; Song, T.; Li, W.; Zhong, N.; Xu, M.; et al. Heterologous boosting with third dose of coronavirus disease recombinant subunit vaccine increases neutralizing antibodies and T cell immunity against different severe acute respiratory syndrome coronavirus 2 variants. Emerg. Microbes Infect. 2022, 11, 829–840. [Google Scholar] [CrossRef]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef]
- Hsieh, S.-M.; Liu, W.-D.; Huang, Y.-S.; Lin, Y.-J.; Hsieh, E.-F.; Lian, W.-C.; Chen, C.; Janssen, R.; Shih, S.-R.; Huang, C.-G.; et al. Safety and immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVC COV1901) Adjuvanted with CpG 1018 and Aluminum Hydroxide in healthy adults: A Phase 1, dose-escalation study. eClinicalMedicine 2021, 38, 100989. [Google Scholar]
- Wang, Z.; Röst, G.; Moghadas, S.M. Delay in booster schedule as a control parameter in vaccination dynamics. J. Math. Biol. 2019, 79, 2157–2182. [Google Scholar] [CrossRef]
- Pettini, E.; Pastore, G.; Fiorino, F.; Medaglini, D.; Ciabattini, A. Short or Long Interval between Priming and Boosting: Does It Impact on the Vaccine Immunogenicity? Vaccines 2021, 9, 289. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, F.; Mantile, F.; De Berardinis, P.; Prisco, A. How the Interval between Prime and Boost Injection Affects the Immune Response in a Computational Model of the Immune System. Comput. Math. Methods Med. 2012, 2012, 842329. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.H.; Liu, X.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Effect of Priming Interval on Reactogenicity, Peak Immunological Response, and Waning after Homologous and Heterologous COVID-19 Vaccine Schedules: Exploratory Analyses of Com-COV, a Randomised Control Trial. Lancet Respir. Med. 2022. [Google Scholar] [CrossRef]
- Holder, J. Tracking Coronavirus Vaccinations Around the World. The New York Times. 7 August 2022. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 11 August 2022).
| Item | Vaccine Group | p Value | |
|---|---|---|---|
| Group A | Group B | ||
| Age (years) | |||
| n (Missing) | 100 (0) | 99 (0) | 0.763 |
| Mean (SD) | 41.4 (10.3) | 41.8 (10.8) | |
| Median (IQR) | 40 (13.0) | 40 (18.0) | |
| Q1–Q3 | 34.5–47.5 | 33–51 | |
| Min–Max | 24–66 | 23–64 | |
| Gender | |||
| n (Missing) | 100 (0) | 99 (0) | 0.679 |
| Male | 32 (32.0) | 29 (29.3) | |
| Female | 68 (68.0) | 70 (70.7) | |
| BMI (kg/m2) | |||
| n (Missing) | 100 (0) | 99 (0) | 0.836 |
| Mean (SD) | 24.4 (4.4) | 24.6 (4.2) | |
| Median (IQR) | 23.97 (6.03) | 23.8 (6.3) | |
| Q1–Q3 | 21.2–27.2 | 21.3–27.6 | |
| Min–Max | 17.6–35.3 | 18.0–37.8 | |
| BMI group | |||
| n (Missing) | 100 (0) | 99 (0) | 0.978 |
| <30 kg/m2 | 87 (87.0) | 86 (86.9) | |
| ≥30 kg/m2 | 13 (13.0) | 13 (13.1) | |
| Comorbidity Category | |||
| n (Missing) | 100 (0) | 99 (0) | 0.224 |
| Yes | 32 (32.0) | 24 (24.2) | |
| No | 68 (68.0) | 75 (75.8) | |
| Item | Group A | Group B | ||||
|---|---|---|---|---|---|---|
| Event | Subject | Percentage | Event | Subject | Percentage | |
| N (missing) | 100 (0) | 99 (0) | ||||
| At least one event | 257 | 82 | 82% | 208 | 76 | 78% |
| Local | 120 | 74 | 74% | 94 | 70 | 71% |
| Pain/Tenderness | 72 | 72 | 72% | 68 | 68 | 69% |
| Grade 1 | 71 | 71 | 71% | 68 | 68 | 69% |
| Grade 2 | 1 | 1 | 1% | 0 | 0 | 0% |
| Erythema/Redness | 2 | 2 | 2% | 0 | 0 | 0% |
| Grade 1 | 2 | 2 | 2% | 0 | 0 | 0% |
| Grade 2 | 0 | 0 | 0% | 0 | 0 | 0% |
| Induration/Swelling | 46 | 46 | 46% | 26 | 26 | 26% |
| Grade 1 | 46 | 46 | 46% | 26 | 26 | 26% |
| Grade 2 | 0 | 0 | 0% | 0 | 0 | 0% |
| Systemic | 137 | 63 | 63% | 114 | 54 | 55% |
| Malaise/Fatigue | 51 | 51 | 51% | 38 | 38 | 38% |
| Grade 1 | 48 | 48 | 48% | 32 | 32 | 32% |
| Grade 2 | 3 | 3 | 3% | 6 | 6 | 6% |
| Myalgia | 39 | 39 | 39% | 36 | 36 | 36% |
| Grade 1 | 35 | 35 | 35% | 31 | 31 | 31% |
| Grade 2 | 4 | 4 | 4% | 5 | 5 | 5% |
| Headache | 30 | 30 | 30% | 17 | 17 | 17% |
| Grade 1 | 29 | 29 | 29% | 15 | 15 | 15% |
| Grade 2 | 1 | 1 | 1% | 2 | 2 | 2% |
| Diarrhea | 7 | 7 | 7% | 12 | 12 | 12% |
| Grade 1 | 7 | 7 | 7% | 11 | 11 | 11% |
| Grade 2 | 0 | 0 | 0% | 0 | 0 | 0% |
| Nausea/Vomiting | 10 | 10 | 10% | 11 | 11 | 11% |
| Grade 1 | 10 | 10 | 10% | 8 | 8 | 8% |
| Grade 2 | 0 | 0 | 0% | 3 | 3 | 3% |
| Fever | 0 | 0 | 0% | 0 | 0 | 0% |
| Grade 1 | 0 | 0 | 0% | 0 | 0 | 0% |
| Grade 2 | 0 | 0 | 0% | 0 | 0 | 0% |
| Group A | Unit | V2 (n = 73) | V3 (n = 73) | Fold Change V3/V2 | * p-Value |
|---|---|---|---|---|---|
| Anti-SARS-CoV-2 spike IgG | IgG GMT | 571.4 (456.6–715.1) | 7948.7 (6558.5–9633.6) | 13.9 (10.5–18.4) | <0.0001 |
| BAU/mL GMT | 52.1 (41.6–65.2) | 724.9 (598.1–878.6) | 13.9 (10.5–18.4) | <0.0001 | |
| Neutralizing antibody | NT50 GMT | 66.6 (56.6–78.4) | 569.7 (471.7–688.0) | 8.6 (7.0–10.5) | <0.0001 |
| IU/mL GMT | 59.0 (51.2–68.0) | 385.4 (326.8–454.5) | 6.5 (5.5–7.8) | <0.0001 | |
| Group B | Unit | V3 (n = 43) | V4 (n = 43) | Fold change V4/V3 | p-value |
| Anti-SARS-CoV-2 spike IgG | IgG GMT | 487.4 (384.9–617.0) | 9504.1 (7817.9–11,554.0) | 19.5 (14.4–26.4) | <0.0001 |
| BAU/mL GMT | 44.4 (35.1–56.3) | 866.8 (713.0–1053.7) | 19.5 (14.4–26.4) | <0.0001 | |
| Neutralizing antibody | NT50 GMT | 31.9 (25.4–40.1) | 651.9 (535.1–794.2) | 20.4 (15.2–27.5) | <0.0001 |
| IU/mL GMT | 31.0 (25.4–37.8) | 433.6 (364.9–515.4) | 14.0 (10.8–18.1) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.-H.; Lin, Y.-C.; Chen, C.-P.; Cheng, C.-Y. Safety and Immunogenicity of a Heterologous Booster of Protein Subunit Vaccine MVC-COV1901 after Two Doses of Adenoviral Vector Vaccine AZD1222. Vaccines 2022, 10, 1701. https://doi.org/10.3390/vaccines10101701
Cheng S-H, Lin Y-C, Chen C-P, Cheng C-Y. Safety and Immunogenicity of a Heterologous Booster of Protein Subunit Vaccine MVC-COV1901 after Two Doses of Adenoviral Vector Vaccine AZD1222. Vaccines. 2022; 10(10):1701. https://doi.org/10.3390/vaccines10101701
Chicago/Turabian StyleCheng, Shu-Hsing, Yi-Chun Lin, Cheng-Pin Chen, and Chien-Yu Cheng. 2022. "Safety and Immunogenicity of a Heterologous Booster of Protein Subunit Vaccine MVC-COV1901 after Two Doses of Adenoviral Vector Vaccine AZD1222" Vaccines 10, no. 10: 1701. https://doi.org/10.3390/vaccines10101701
APA StyleCheng, S.-H., Lin, Y.-C., Chen, C.-P., & Cheng, C.-Y. (2022). Safety and Immunogenicity of a Heterologous Booster of Protein Subunit Vaccine MVC-COV1901 after Two Doses of Adenoviral Vector Vaccine AZD1222. Vaccines, 10(10), 1701. https://doi.org/10.3390/vaccines10101701






