A Dual Adjuvant System for Intranasal Boosting of Local and Systemic Immunity for Influenza Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Reagents
2.3. Immunization with IIAV plus Fos47 through IM and IN Routes
2.4. Bronchoalveolar Lavage and Nasal Lavage for Collecting Mucosal Fluids and Cells
2.5. Assessment for IgG and IgA Antibody Titers against Homologous Virus
2.6. Evaluation of Cross-Reactive IgG and IgA by ELISA
2.7. Flow Cytometry Analysis of Lung Cells
2.8. Assessment of Recall T Cell Responses
2.9. Safety Studies
2.10. Statistical Analyses
3. Results
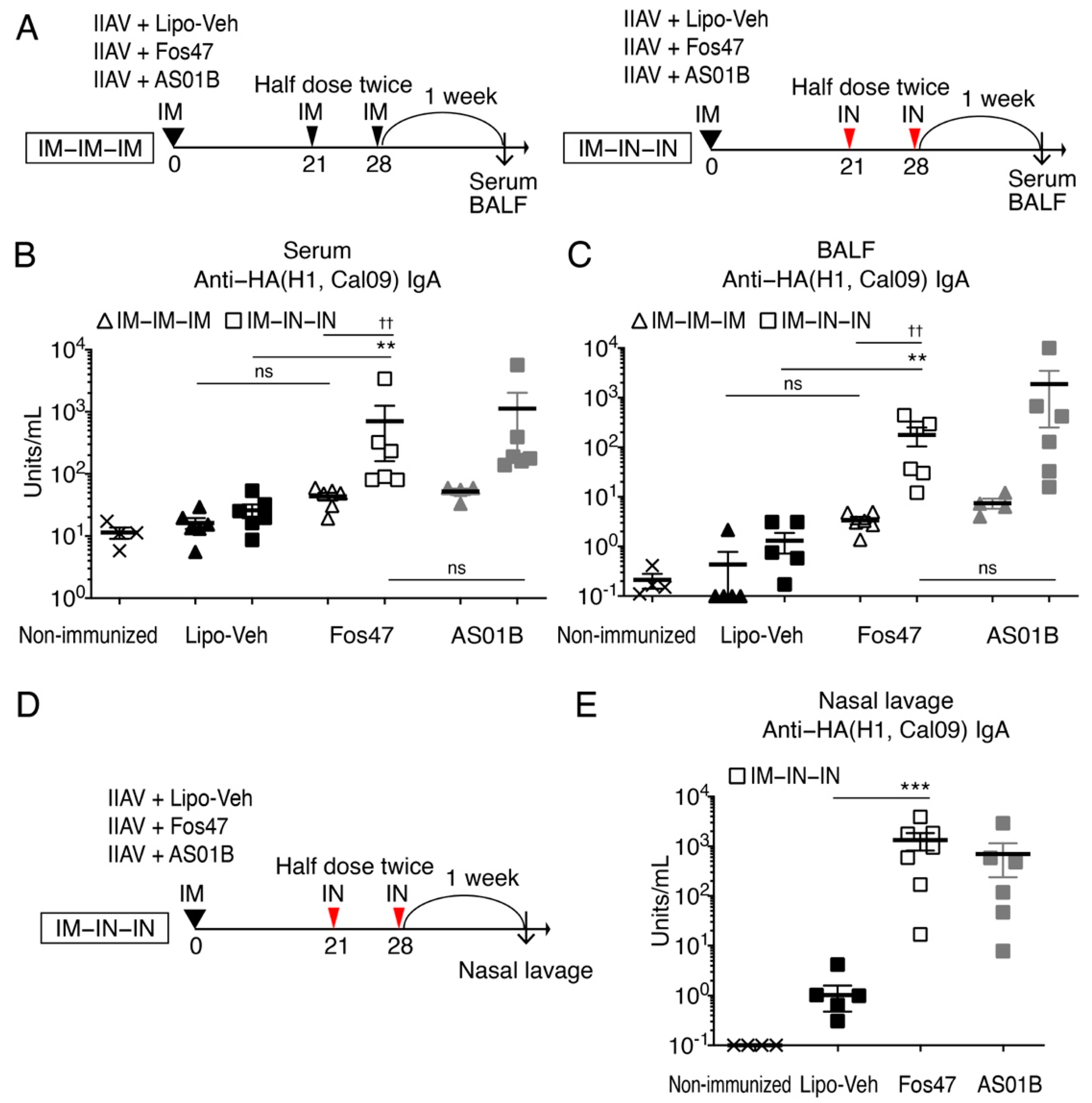
3.1. Enhancement of Local and Systemic Antigen-Specific IgA Titers by IN Boosting with Fos47
3.2. Mucosal IgA Induced by IN Boosting with Fos47 Is Cross-Reactive
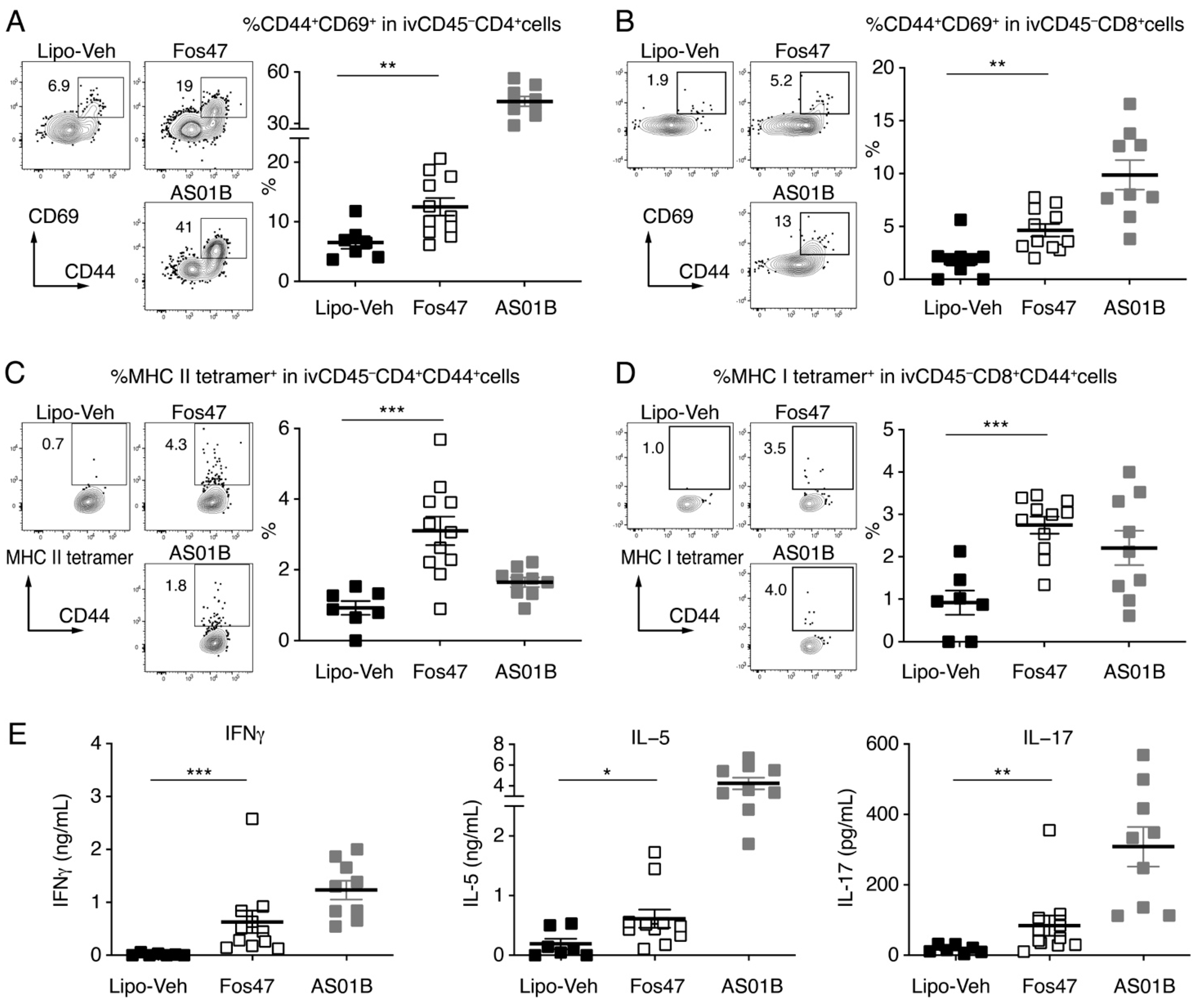
3.3. IN Boosting with Fos47 Enhanced Local and Systemic Antigen-Specific T Cell Responses
3.4. Induction of Systemic IgG by IN Boost with Fos47 Is Comparable to That Induced by Original IM-Boosting Regimen
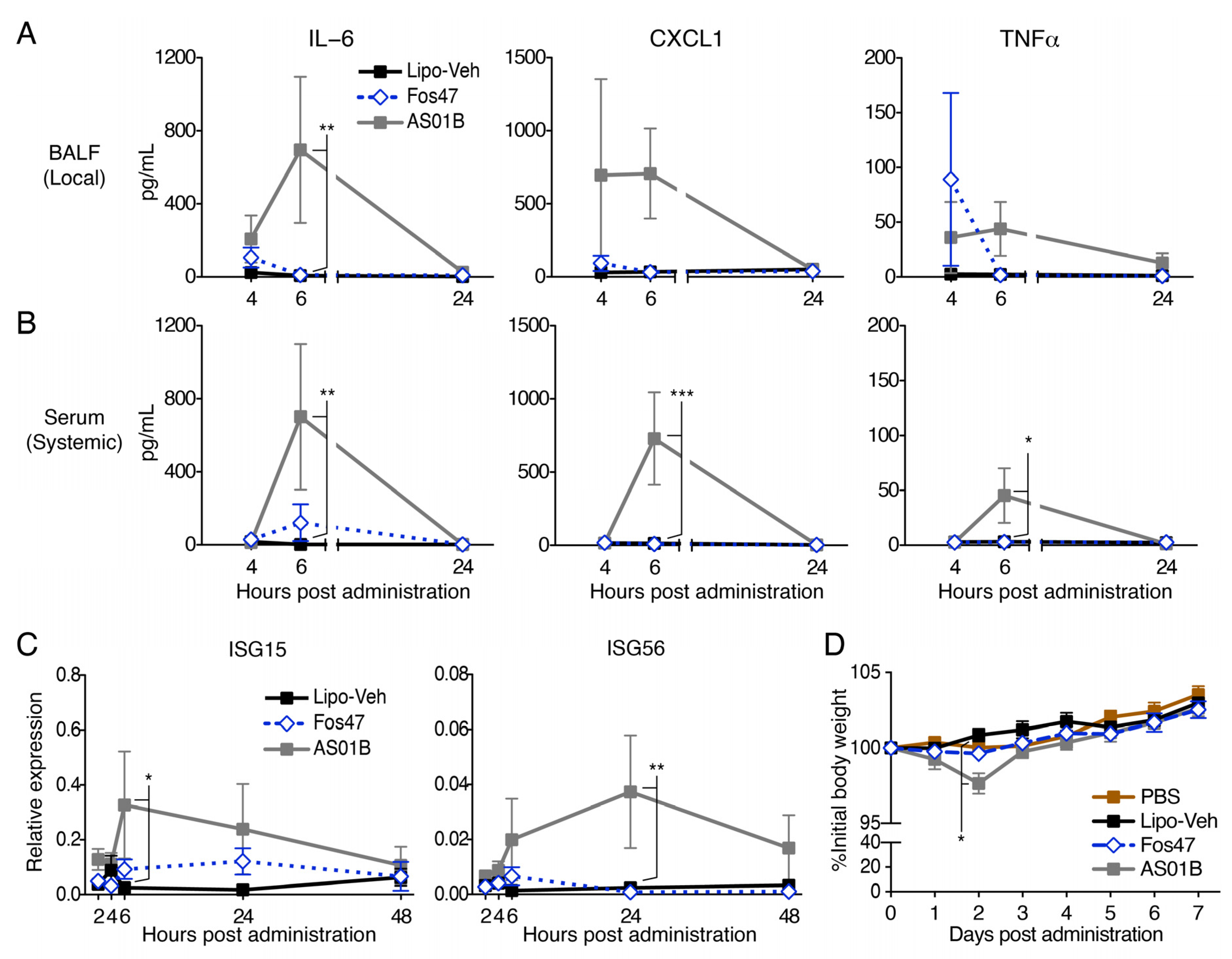
3.5. IN Administration with Fos47 Did Not Cause Local and Systemic Reactogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, E.J.; Doyle, J.D.; Uyeki, T.M. Influenza virus-related critical illness: Prevention, diagnosis, treatment. Crit. Care 2019, 23, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef] [Green Version]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E.; Randall, T.D. Scent of a vaccine. Science 2021, 373, 397–399. [Google Scholar] [CrossRef]
- Wei, C.J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-generation influenza vaccines: Opportunities and challenges. Nat. Rev. Drug Discov. 2020, 19, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Bleier, B.S.; Ramanathan, M., Jr.; Lane, A.P. COVID-19 Vaccines May Not Prevent Nasal SARS-CoV-2 Infection and Asymptomatic Transmission. Otolaryngol. Head Neck Surg. 2021, 164, 305–307. [Google Scholar] [CrossRef]
- Rubin, R. Trying to Block SARS-CoV-2 Transmission With Intranasal Vaccines. JAMA 2021, 326, 1661–1663. [Google Scholar] [CrossRef]
- Mouro, V.; Fischer, A. Dealing with a mucosal viral pandemic: Lessons from COVID-19 vaccines. Mucosal Immunol. 2022, 15, 584–594. [Google Scholar] [CrossRef]
- Mazanec, M.B.; Coudret, C.L.; Fletcher, D.R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 1995, 69, 1339–1343. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.L. Host Immune Response to Influenza A Virus Infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef]
- Iwasaki, A. Exploiting Mucosal Immunity for Antiviral Vaccines. Annu. Rev. Immunol. 2016, 34, 575–608. [Google Scholar] [CrossRef] [PubMed]
- van Riet, E.; Ainai, A.; Suzuki, T.; Hasegawa, H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine 2012, 30, 5893–5900. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Funato, H.; Hirabayashi, Y.; Suzuki, Y.; Nagamine, T.; Aizawa, C.; Kurata, T. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur. J. Immunol. 1991, 21, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Funato, H.; Hirabayashi, Y.; Kikuta, K.; Suzuki, Y.; Nagamine, T.; Aizawa, C.; Nakagawa, M.; Kurata, T. Functional role of respiratory tract haemagglutinin-specific IgA antibodies in protection against influenza. Vaccine 1990, 8, 479–485. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, S.P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccin. Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef] [Green Version]
- Tanner, A.R.; Dorey, R.B.; Brendish, N.J.; Clark, T.W. Influenza vaccination: Protecting the most vulnerable. Eur. Respir. Rev. 2021, 30, 200258. [Google Scholar] [CrossRef]
- Nguyen-Contant, P.; Sangster, M.Y.; Topham, D.J. Squalene-Based Influenza Vaccine Adjuvants and Their Impact on the Hemagglutinin-Specific B Cell Response. Pathogens 2021, 10, 355. [Google Scholar] [CrossRef]
- Nanishi, E.; Dowling, D.J.; Levy, O. Toward precision adjuvants: Optimizing science and safety. Curr. Opin. Pediatr. 2020, 32, 125–138. [Google Scholar] [CrossRef] [Green Version]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert. Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Friedland, L.R.; Hanon, E.; Didierlaurent, A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017, 47, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Harbecke, R.; Cohen, J.I.; Oxman, M.N. Herpes Zoster Vaccines. J. Infect. Dis. 2021, 224, S429–S442. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.-Y.; Couture, M.; D’Aoust, M.-A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 trial of a Candidate Recombinant Virus-Like Particle Vaccine for Covid-19 Disease Produced in Plants. medRxiv 2020. [Google Scholar] [CrossRef]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Couch, R.B. Nasal vaccination, Escherichia coli enterotoxin, and Bell’s palsy. N. Engl. J. Med. 2004, 350, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Bell Palsy Following Intranasal Influenza Vaccination. Available online: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/bell-s-palsy-following-intranasal-vaccination (accessed on 28 August 2022).
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 vaccines: From bench to bed. EBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Hayashi, T.; Kuy, C.S.; Gray, C.S.; Wu, C.C.; Corr, M.; Wrasidlo, W.; Cottam, H.B.; Carson, D.A. Synthesis and immunological characterization of toll-like receptor 7 agonistic conjugates. Bioconjug Chem. 2009, 20, 1194–1200. [Google Scholar] [CrossRef] [Green Version]
- Chan, M.; Hayashi, T.; Mathewson, R.D.; Nour, A.; Hayashi, Y.; Yao, S.; Tawatao, R.I.; Crain, B.; Tsigelny, I.F.; Kouznetsova, V.L.; et al. Identification of substituted pyrimido[5,4-b]indoles as selective Toll-like receptor 4 ligands. J. Med. Chem. 2013, 56, 4206–4223. [Google Scholar] [CrossRef]
- Goff, P.H.; Hayashi, T.; Martinez-Gil, L.; Corr, M.; Crain, B.; Yao, S.; Cottam, H.B.; Chan, M.; Ramos, I.; Eggink, D.; et al. Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses. J. Virol. 2015, 89, 3221–3235. [Google Scholar] [CrossRef] [Green Version]
- Goff, P.H.; Hayashi, T.; He, W.; Yao, S.; Cottam, H.B.; Tan, G.S.; Crain, B.; Krammer, F.; Messer, K.; Pu, M.; et al. Synthetic Toll-Like Receptor 4 (TLR4) and TLR7 Ligands Work Additively via MyD88 To Induce Protective Antiviral Immunity in Mice. J. Virol. 2017, 91, e01050-17. [Google Scholar] [CrossRef]
- Sato-Kaneko, F.; Yao, S.; Lao, F.S.; Shpigelman, J.; Messer, K.; Pu, M.; Shukla, N.M.; Cottam, H.B.; Chan, M.; Chu, P.J.; et al. A Novel Synthetic Dual Agonistic Liposomal TLR4/7 Adjuvant Promotes Broad Immune Responses in an Influenza Vaccine With Minimal Reactogenicity. Front. Immunol. 2020, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Lecrenier, N.; Beukelaers, P.; Colindres, R.; Curran, D.; De Kesel, C.; De Saegher, J.P.; Didierlaurent, A.M.; Ledent, E.Y.; Mols, J.F.; Mrkvan, T.; et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert. Rev. Vaccines 2018, 17, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Sato-Kaneko, F.; Yao, S.; Lao, F.S.; Nan, J.; Shpigelman, J.; Cheng, A.; Saito, T.; Messer, K.; Pu, M.; Shukla, N.M.; et al. Mitochondria-dependent synthetic small-molecule vaccine adjuvants for influenza virus infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2025718118. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Kumar, S.; Nei, M.; Dudley, J.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform 2008, 9, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.G.; Mayer-Barber, K.; Sung, H.; Beura, L.; James, B.R.; Taylor, J.J.; Qunaj, L.; Griffith, T.S.; Vezys, V.; Barber, D.L.; et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 2014, 9, 209–222. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.B.; McLucas, B.C.; Montoya, L.A.; Brotski, C.M.; Das, S.; Miholits, M.; Sebata, T.H. Multiplexing protein and gene level measurements on a single Luminex platform. Methods 2019, 158, 27–32. [Google Scholar] [CrossRef]
- Jang, Y.H.; Seong, B.L. The Quest for a Truly Universal Influenza Vaccine. Front. Cell Infect. Microbiol. 2019, 9, 344. [Google Scholar] [CrossRef] [Green Version]
- Amanna, I.J.; Slifka, M.K. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology 2011, 411, 206–215. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Szabo, P.A.; Miron, M.; Farber, D.L. Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol. 2019, 4, eaas9673. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines-fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Zens, K.D.; Chen, J.K.; Farber, D.L. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight 2016, 1, e85832. [Google Scholar] [CrossRef]
- Nelson, S.A.; Sant, A.J. Potentiating Lung Mucosal Immunity Through Intranasal Vaccination. Front. Immunol. 2021, 12, 808527. [Google Scholar] [CrossRef]
- Paik, D.H.; Farber, D.L. Anti-viral protective capacity of tissue resident memory T cells. Curr. Opin. Virol. 2021, 46, 20–26. [Google Scholar] [CrossRef]
- Leung, S.; Liu, X.; Fang, L.; Chen, X.; Guo, T.; Zhang, J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol. Immunol. 2010, 7, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Liew, F.Y. T(H)1 and T(H)2 cells: A historical perspective. Nat. Rev. Immunol. 2002, 2, 55–60. [Google Scholar] [CrossRef]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Trondsen, M.; Aqrawi, L.A.; Zhou, F.; Pedersen, G.; Trieu, M.C.; Zhou, P.; Cox, R.J. Induction of Local Secretory IgA and Multifunctional CD4(+) T-helper Cells Following Intranasal Immunization with a H5N1 Whole Inactivated Influenza Virus Vaccine in BALB/c Mice. Scand. J. Immunol. 2015, 81, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Allie, S.R.; Bradley, J.E.; Mudunuru, U.; Schultz, M.D.; Graf, B.A.; Lund, F.E.; Randall, T.D. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 2019, 20, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Song, E.; Moriyama, M.; Wong, P.; Zhang, S.; Jiang, R.; Strohmeier, S.; Kleinstein, S.H.; Krammer, F.; Iwasaki, A. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci. Immunol. 2021, 6, eabj5129. [Google Scholar] [CrossRef]
- Hellfritzsch, M.; Scherliess, R. Mucosal Vaccination via the Respiratory Tract. Pharmaceutics 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mettelman, R.C.; Allen, E.K.; Thomas, P.G. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 2022, 55, 749–780. [Google Scholar] [CrossRef]
- Giudice, E.L.; Campbell, J.D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 2006, 58, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.; Holmgren, J. Cholera toxin-a foe & a friend. Indian J. Med. Res. 2011, 133, 153–163. [Google Scholar]
- Kurono, Y.; Yamamoto, M.; Fujihashi, K.; Kodama, S.; Suzuki, M.; Mogi, G.; McGhee, J.R.; Kiyono, H. Nasal immunization induces Haemophilus influenzae-specific Th1 and Th2 responses with mucosal IgA and systemic IgG antibodies for protective immunity. J. Infect. Dis. 1999, 180, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Mantis, N.J.; Forbes, S.J. Secretory IgA: Arresting microbial pathogens at epithelial borders. Immunol. Invest. 2010, 39, 383–406. [Google Scholar] [CrossRef]
- Asahi, Y.; Yoshikawa, T.; Watanabe, I.; Iwasaki, T.; Hasegawa, H.; Sato, Y.; Shimada, S.; Nanno, M.; Matsuoka, Y.; Ohwaki, M.; et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J. Immunol. 2002, 168, 2930–2938. [Google Scholar] [CrossRef] [Green Version]
- Ainai, A.; Tamura, S.; Suzuki, T.; van Riet, E.; Ito, R.; Odagiri, T.; Tashiro, M.; Kurata, T.; Hasegawa, H. Intranasal vaccination with an inactivated whole influenza virus vaccine induces strong antibody responses in serum and nasal mucus of healthy adults. Hum. Vaccin. Immunother. 2013, 9, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Asahi-Ozaki, Y.; Yoshikawa, T.; Iwakura, Y.; Suzuki, Y.; Tamura, S.; Kurata, T.; Sata, T. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J. Med. Virol. 2004, 74, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Russell, S.M.; Appleyard, G.; Brand, C.M.; Beale, J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur. J. Immunol. 1984, 14, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Lapuente, D.; Fuchs, J.; Willar, J.; Vieira Antao, A.; Eberlein, V.; Uhlig, N.; Issmail, L.; Schmidt, A.; Oltmanns, F.; Peter, A.S.; et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat. Commun. 2021, 12, 6871. [Google Scholar] [CrossRef]
- Southam, D.S.; Dolovich, M.; O’Byrne, P.M.; Inman, M.D. Distribution of intranasal instillations in mice: Effects of volume, time, body position, and anesthesia. Am. J. Physiol Lung Cell Mol. Physiol. 2002, 282, L833–L839. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato-Kaneko, F.; Yao, S.; Lao, F.S.; Sako, Y.; Jin, J.; Shukla, N.M.; Cottam, H.B.; Chan, M.; Belsuzarri, M.M.; Carson, D.A.; et al. A Dual Adjuvant System for Intranasal Boosting of Local and Systemic Immunity for Influenza Vaccination. Vaccines 2022, 10, 1694. https://doi.org/10.3390/vaccines10101694
Sato-Kaneko F, Yao S, Lao FS, Sako Y, Jin J, Shukla NM, Cottam HB, Chan M, Belsuzarri MM, Carson DA, et al. A Dual Adjuvant System for Intranasal Boosting of Local and Systemic Immunity for Influenza Vaccination. Vaccines. 2022; 10(10):1694. https://doi.org/10.3390/vaccines10101694
Chicago/Turabian StyleSato-Kaneko, Fumi, Shiyin Yao, Fitzgerald S. Lao, Yukiya Sako, Jasmine Jin, Nikunj M. Shukla, Howard B. Cottam, Michael Chan, Masiel M. Belsuzarri, Dennis A. Carson, and et al. 2022. "A Dual Adjuvant System for Intranasal Boosting of Local and Systemic Immunity for Influenza Vaccination" Vaccines 10, no. 10: 1694. https://doi.org/10.3390/vaccines10101694
APA StyleSato-Kaneko, F., Yao, S., Lao, F. S., Sako, Y., Jin, J., Shukla, N. M., Cottam, H. B., Chan, M., Belsuzarri, M. M., Carson, D. A., & Hayashi, T. (2022). A Dual Adjuvant System for Intranasal Boosting of Local and Systemic Immunity for Influenza Vaccination. Vaccines, 10(10), 1694. https://doi.org/10.3390/vaccines10101694





