Gas Plasma Protein Oxidation Increases Immunogenicity and Human Antigen-Presenting Cell Maturation and Activation
Abstract
1. Introduction
2. Experimental Section
2.1. Monocyte Isolation and Cell Culture
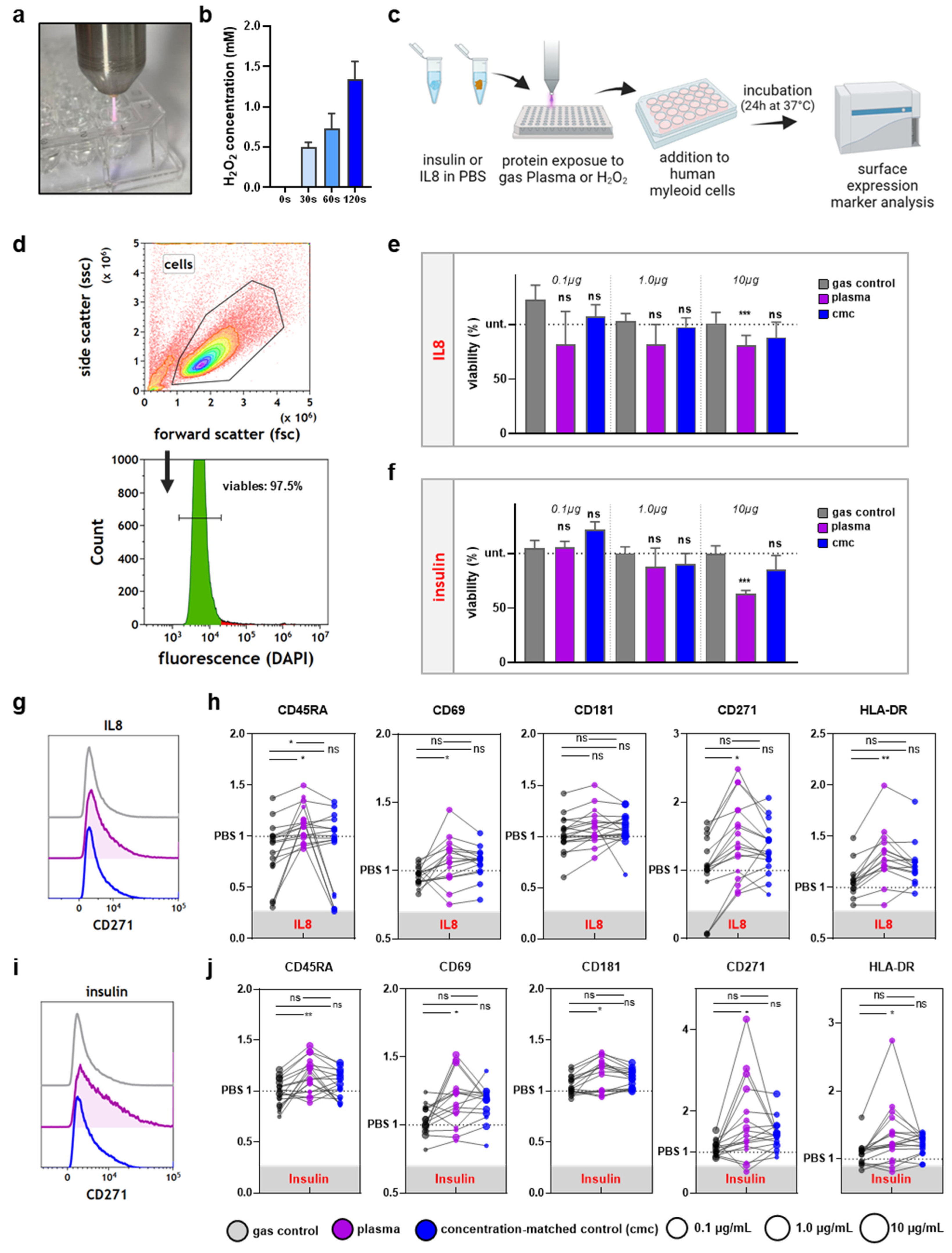
2.2. Proteins and ROS Treatment
2.3. H2O2 Quantification
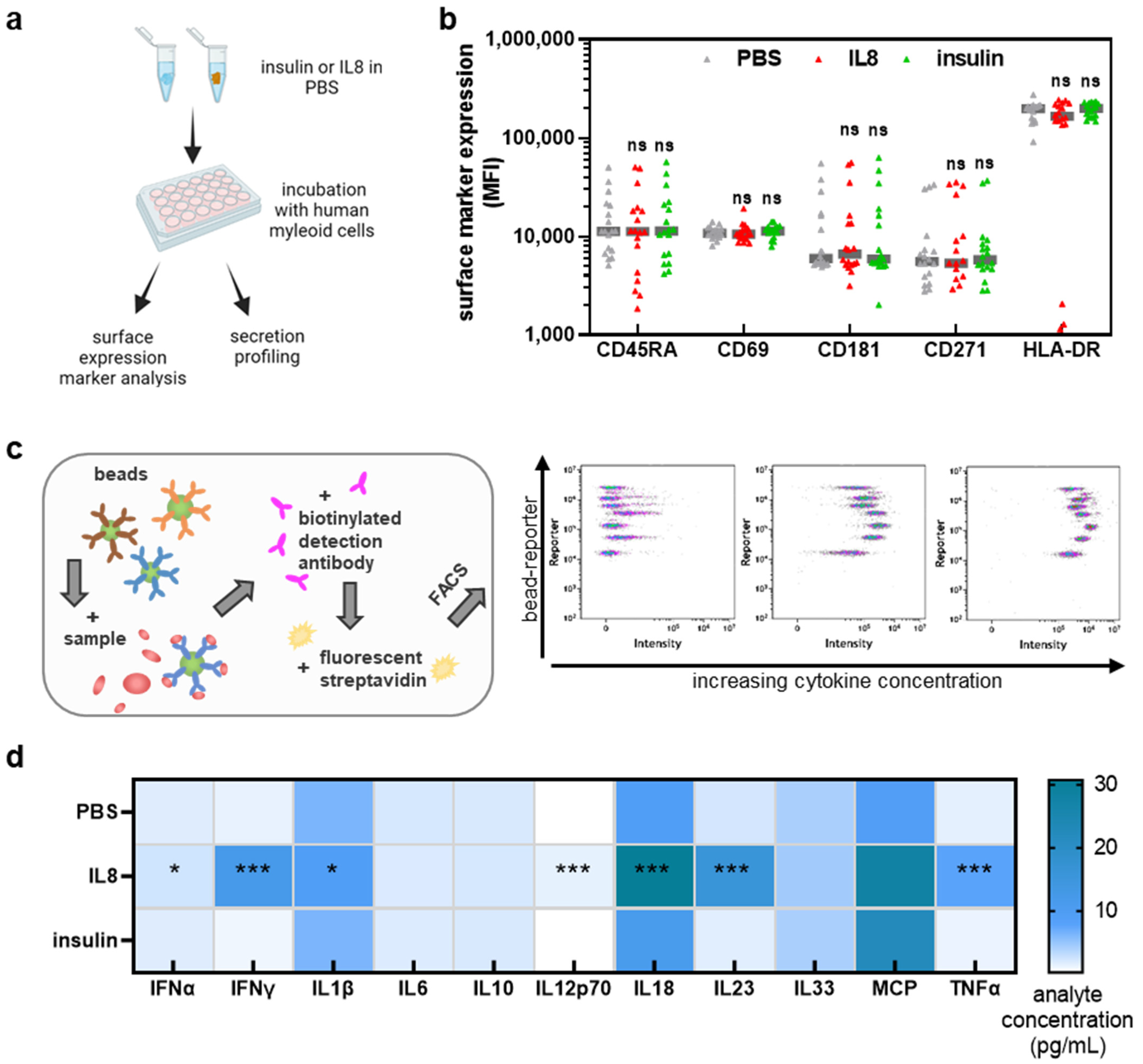
2.4. Flow Cytometry
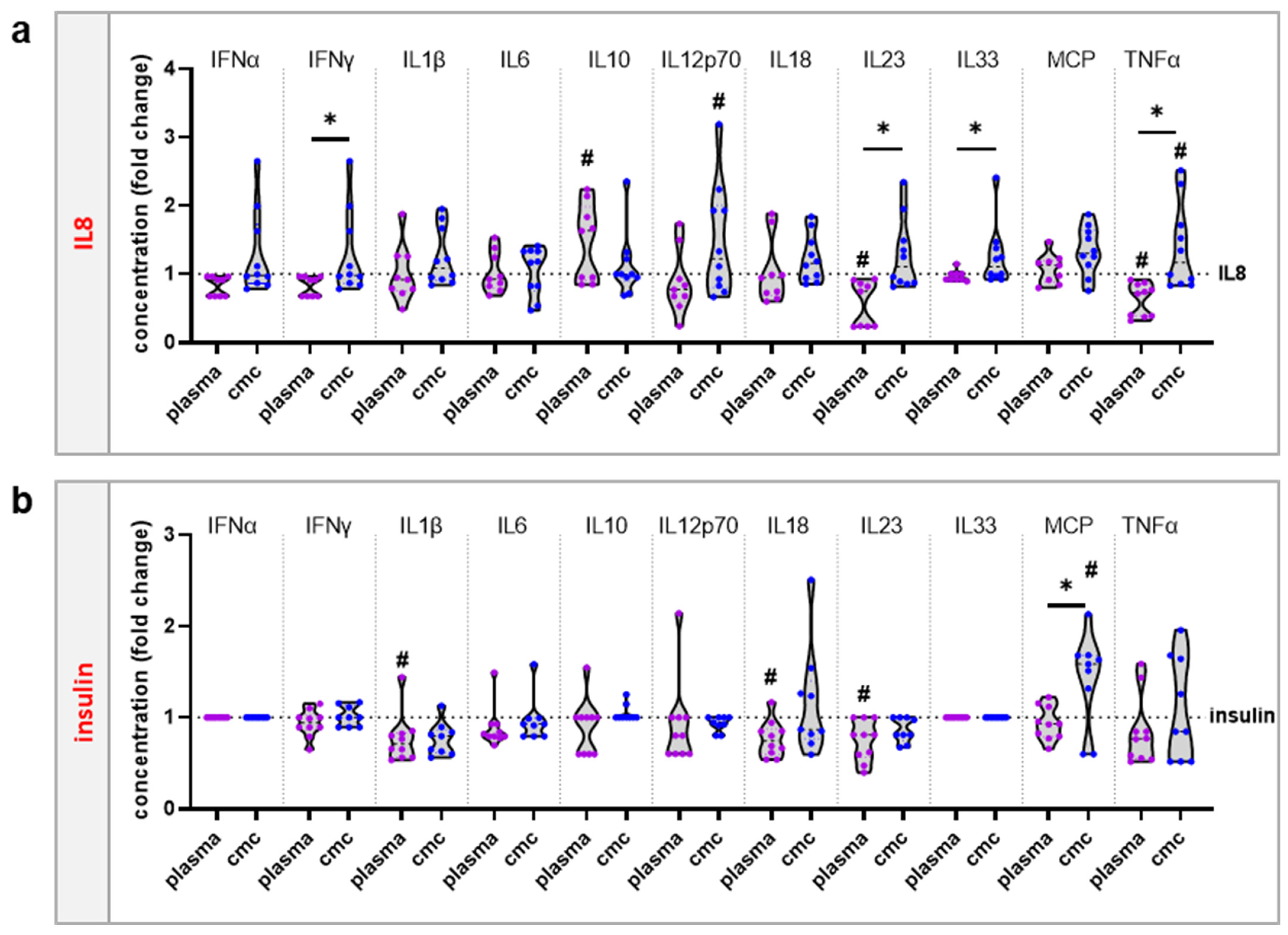
2.5. Chemokine and Cytokine Measurements
2.6. Statistical Analysis
3. Results
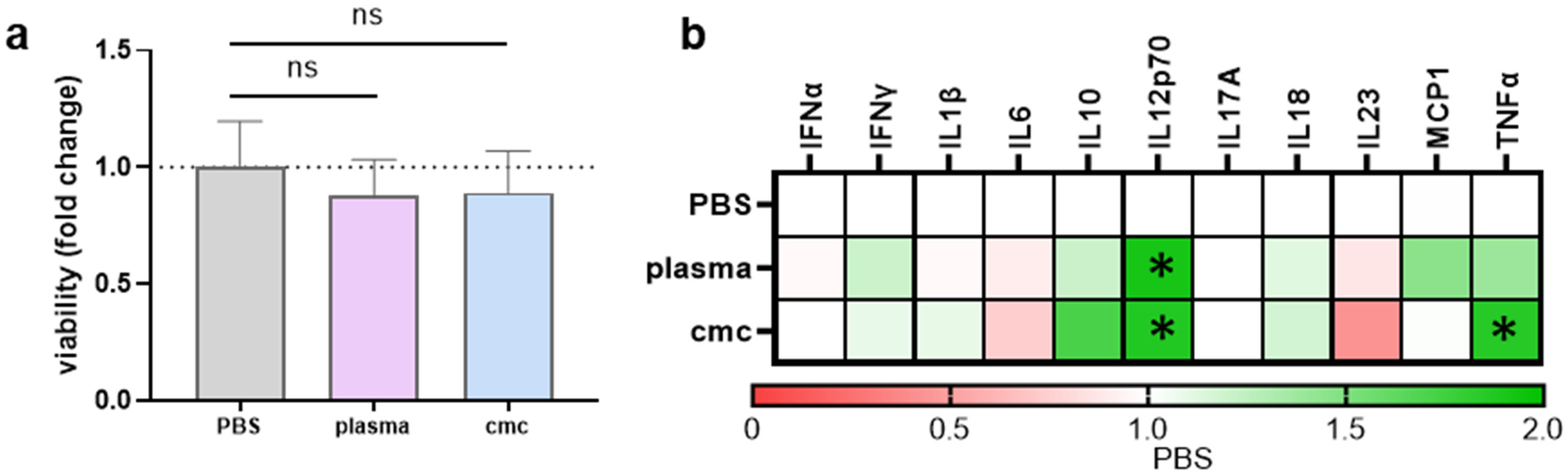
3.1. IL8, but Not Insulin, Triggered Pro-Inflammatory Cytokines Secretion in THP-1 Cells
3.2. THP-1 Cell Incubation with Gas Plasma- but Not H2O2-Oxidized Proteins Increased Activation-Related Surface Marker Expression
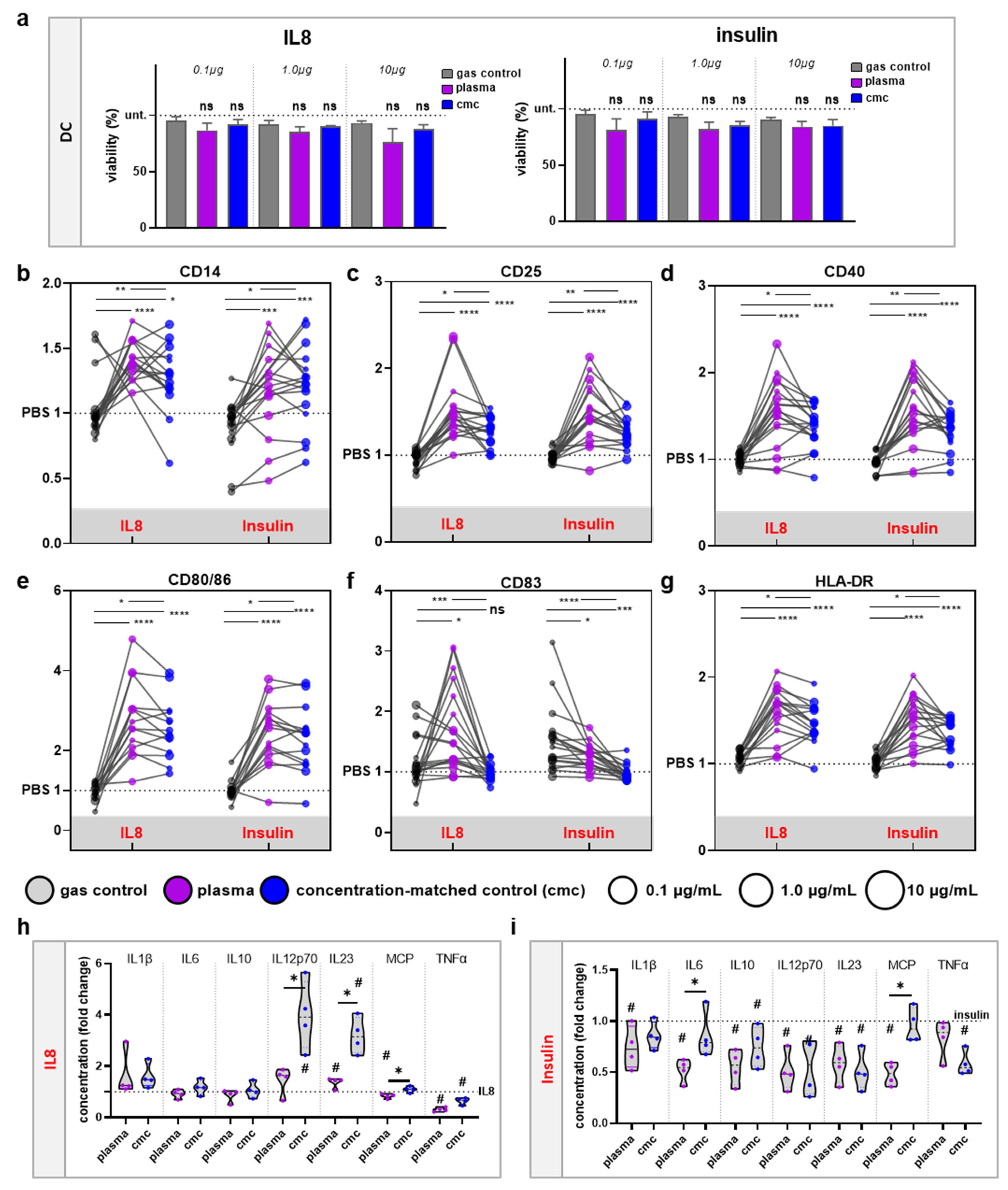
3.3. moDCs Activation and Secretion Profiles Were Profoundly Affected by Gas Plasma-Mediated Protein Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Ulfig, A.; Leichert, L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell Mol. Life Sci. 2021, 78, 385–414. [Google Scholar] [CrossRef]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef]
- Lammermann, T.; Afonso, P.V.; Angermann, B.R.; Wang, J.M.; Kastenmuller, W.; Parent, C.A.; Germain, R.N. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013, 498, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Selmeci, L. Advanced oxidation protein products (AOPP): Novel uremic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome? Free Radic. Res. 2011, 45, 1115–1123. [Google Scholar] [CrossRef]
- Strollo, R.; Vinci, C.; Arshad, M.H.; Perrett, D.; Tiberti, C.; Chiarelli, F.; Napoli, N.; Pozzilli, P.; Nissim, A. Antibodies to post-translationally modified insulin in type 1 diabetes. Diabetologia 2015, 58, 2851–2860. [Google Scholar] [CrossRef]
- Biedron, R.; Konopinski, M.K.; Marcinkiewicz, J.; Jozefowski, S. Oxidation by neutrophils-derived HOCl increases immunogenicity of proteins by converting them into ligands of several endocytic receptors involved in antigen uptake by dendritic cells and macrophages. PLoS ONE 2015, 10, e0123293. [Google Scholar] [CrossRef]
- Prokopowicz, Z.M.; Arce, F.; Biedron, R.; Chiang, C.L.; Ciszek, M.; Katz, D.R.; Nowakowska, M.; Zapotoczny, S.; Marcinkiewicz, J.; Chain, B.M. Hypochlorous acid: A natural adjuvant that facilitates antigen processing, cross-priming, and the induction of adaptive immunity. J. Immunol. 2010, 184, 824–835. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Bobek, M.; Mak, M.; Biedroń, R. Immunogenic properties of collagen and ovalbumin modified by chlorination. Cent. Eur. J. Immunol. 2004, 28, 160–166. [Google Scholar]
- Nybo, T.; Dieterich, S.; Gamon, L.F.; Chuang, C.Y.; Hammer, A.; Hoefler, G.; Malle, E.; Rogowska-Wrzesinska, A.; Davies, M.J. Chlorination and oxidation of the extracellular matrix protein laminin and basement membrane extracts by hypochlorous acid and myeloperoxidase. Redox Biol. 2019, 20, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Nasri, Z.; Memari, S.; Wenske, S.; Clemen, R.; Martens, U.; Delcea, M.; Bekeschus, S.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Singlet Oxygen-Induced Phospholipase A2 Inhibition: A Major Role for Interfacial Tryptophan Dioxidation. Chemistry 2021, 27, 14702–14710. [Google Scholar] [CrossRef] [PubMed]
- Wenske, S.; Lackmann, J.W.; Bekeschus, S.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Nonenzymatic post-translational modifications in peptides by cold plasma-derived reactive oxygen and nitrogen species. Biointerphases 2020, 15, 061008. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridman, A.; Lu, X.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V.; et al. Low-Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Trans. Radiat. Plasma Med. Sci. 2022, 6, 127–157. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Min, B.; Choi, K.H.; Hong, Y.J.; Miller, V.; Fridman, A.; Choi, E.H. Cytotoxic macrophage-released tumour necrosis factor-alpha (TNF-alpha) as a killing mechanism for cancer cell death after cold plasma activation. J. Phys. D Appl. Phys. 2016, 49, 084001. [Google Scholar] [CrossRef]
- Bekeschus, S.; Meyer, D.; Arlt, K.; von Woedtke, T.; Miebach, L.; Freund, E.; Clemen, R. Argon Plasma Exposure Augments Costimulatory Ligands and Cytokine Release in Human Monocyte-Derived Dendritic Cells. Int. J. Mol. Sci. 2021, 22, 3790. [Google Scholar] [CrossRef]
- Freund, E.; Moritz, J.; Stope, M.; Seebauer, C.; Schmidt, A.; Bekeschus, S. Plasma-Derived Reactive Species Shape a Differentiation Profile in Human Monocytes. Appl. Sci. 2019, 9, 2530. [Google Scholar] [CrossRef]
- Crestale, L.; Laurita, R.; Liguori, A.; Stancampiano, A.; Talmon, M.; Bisag, A.; Gherardi, M.; Amoruso, A.; Colombo, V.; Fresu, L. Cold Atmospheric Pressure Plasma Treatment Modulates Human Monocytes/Macrophages Responsiveness. Plasma 2018, 1, 261–276. [Google Scholar] [CrossRef]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemiere, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-Thermal Plasma as a Unique Delivery System of Short-Lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Clemen, R.; Niessner, F.; Sagwal, S.K.; Freund, E.; Schmidt, A. Medical Gas Plasma Jet Technology Targets Murine Melanoma in an Immunogenic Fashion. Adv. Sci. 2020, 7, 1903438. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.G.; Xiang, B.; Merlino, D.J.; Baybutt, T.R.; Sahu, J.; Fridman, A.; Snook, A.E.; Miller, V. Non-thermal plasma induces immunogenic cell death in vivo in murine CT26 colorectal tumors. Oncoimmunology 2018, 7, e1484978. [Google Scholar] [CrossRef] [PubMed]
- Tomic, S.; Petrovic, A.; Puac, N.; Skoro, N.; Bekic, M.; Petrovic, Z.L.; Colic, M. Plasma-Activated Medium Potentiates the Immunogenicity of Tumor Cell Lysates for Dendritic Cell-Based Cancer Vaccines. Cancers 2021, 13, 1626. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kINPen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Bekeschus, S.; Masur, K.; Kolata, J.; Wende, K.; Schmidt, A.; Bundscherer, L.; Barton, A.; Kramer, A.; Bröker, B.; Weltmann, K.-D. Human Mononuclear Cell Survival and Proliferation is Modulated by Cold Atmospheric Plasma Jet. Plasma Process. Polym. 2013, 10, 706–713. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; Gebbe, R.; Heidecke, A.K.; Partecke, L.-I.; Bekeschus, S. In Vitro Anticancer Efficacy of Six Different Clinically Approved Types of Liquids Exposed to Physical Plasma. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 588–596. [Google Scholar] [CrossRef]
- Bekeschus, S.; Freund, E.; Wende, K.; Gandhirajan, R.K.; Schmidt, A. Hmox1 Upregulation Is a Mutual Marker in Human Tumor Cells Exposed to Physical Plasma-Derived Oxidants. Antioxidants 2018, 7, 151. [Google Scholar] [CrossRef]
- Bosshart, H.; Heinzelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 438. [Google Scholar] [CrossRef]
- Estevez, M.; Xiong, Y. Intake of Oxidized Proteins and Amino Acids and Causative Oxidative Stress and Disease: Recent Scientific Evidences and Hypotheses. J. Food Sci. 2019, 84, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Clemen, R.; Bekeschus, S. Oxidatively Modified Proteins: Cause and Control of Diseases. Appl. Sci. 2020, 10, 6419. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Winyard, P.G.; Ryan, B.; Eggleton, P.; Nissim, A.; Taylor, E.; Lo Faro, M.L.; Burkholz, T.; Szabo-Taylor, K.E.; Fox, B.; Viner, N.; et al. Measurement and meaning of markers of reactive species of oxygen, nitrogen and sulfur in healthy human subjects and patients with inflammatory joint disease. Biochem. Soc. Trans. 2011, 39, 1226–1232. [Google Scholar] [CrossRef]
- Ryan, B.J.; Nissim, A.; Winyard, P.G. Oxidative post-translational modifications and their involvement in the pathogenesis of autoimmune diseases. Redox Biol. 2014, 2, 715–724. [Google Scholar] [CrossRef] [PubMed]
- van der Vliet, A.; Janssen-Heininger, Y.M. Hydrogen peroxide as a damage signal in tissue injury and inflammation: Murderer, mediator, or messenger? J. Cell. Biochem. 2014, 115, 427–435. [Google Scholar] [CrossRef]
- Kennel, K.B.; Greten, F.R. Immune cell—Produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021, 42, 101891. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zhang, Q.; Zeh, H.J., 3rd; Lotze, M.T.; Tang, D. HMGB1 in cancer: Good, bad, or both? Clin. Cancer Res. 2013, 19, 4046–4057. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Mattei, V.; Siracusano, A.; Ortona, E.; Margutti, P.; Salvati, B.; Sorice, M.; Rigano, R. Oxidized beta2-glycoprotein I induces human dendritic cell maturation and promotes a T helper type 1 response. Blood 2005, 106, 3880–3887. [Google Scholar] [CrossRef]
- Ulfig, A.; Bader, V.; Varatnitskaya, M.; Lupilov, N.; Winklhofer, K.F.; Leichert, L.I. Hypochlorous acid-modified human serum albumin suppresses MHC class II—Dependent antigen presentation in pro-inflammatory macrophages. Redox Biol. 2021, 43, 101981. [Google Scholar] [CrossRef]
- Clemen, R.; Freund, E.; Mrochen, D.; Miebach, L.; Schmidt, A.; Rauch, B.H.; Lackmann, J.W.; Martens, U.; Wende, K.; Lalk, M.; et al. Gas Plasma Technology Augments Ovalbumin Immunogenicity and OT-II T Cell Activation Conferring Tumor Protection in Mice. Adv. Sci. 2021, 8, 2003395. [Google Scholar] [CrossRef] [PubMed]
- Chechushkov, A.V.; Kozhin, P.M.; Zaitseva, N.S.; Lemza, A.E.; Men’shchikova, E.B.; Troitskii, A.V.; Shkurupy, V.A. Oxidized Dextran Enhances Alternative Activation of Macrophages in Mice of Opposite Lines. Bull Exp. Biol. Med. 2016, 160, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Hitzler, M.; Bergert, A.; Luch, A.; Peiser, M. Evaluation of selected biomarkers for the detection of chemical sensitization in human skin: A comparative study applying THP-1, MUTZ-3 and primary dendritic cells in culture. Toxicol. In Vitro 2013, 27, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Tietze, C.; Blomeke, B. Sensitization assays: Monocyte-derived dendritic cells versus a monocytic cell line (THP-1). J. Toxicol. Environ. Health A 2008, 71, 965–968. [Google Scholar] [CrossRef]
- Larsson, K.; Lindstedt, M.; Borrebaeck, C.A. Functional and transcriptional profiling of MUTZ-3, a myeloid cell line acting as a model for dendritic cells. Immunology 2006, 117, 156–166. [Google Scholar] [CrossRef]
- Santegoets, S.J.; van den Eertwegh, A.J.; van de Loosdrecht, A.A.; Scheper, R.J.; de Gruijl, T.D. Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J. Leukoc. Biol. 2008, 84, 1364–1373. [Google Scholar] [CrossRef]
- Miebach, L.; Freund, E.; Clemen, R.; Weltmann, K.D.; Metelmann, H.R.; von Woedtke, T.; Gerling, T.; Wende, K.; Bekeschus, S. Conductivity augments ROS and RNS delivery and tumor toxicity of an argon plasma jet. Free Radic. Biol. Med. 2022, 180, 210–219. [Google Scholar] [CrossRef]
| Ligand | Fluorescent Label | Clone | Supplier |
|---|---|---|---|
| CD11C | PE-Cy7 | S-HCL-3 | BioLegend |
| CD14 | BV650 | M5E2 | BioLegend |
| CD25 | AF488 | BC96 | BioLegend |
| CD40 | APC | HB14 | BioLegend |
| CD45RA | PerCP/Cy5.5 | HI100 | BD Biosciences |
| CD69 | AF488 | FN50 | BioLegend |
| CD80 | PE | 2D10 | BioLegend |
| CD83 | PerCP/Cy5.5 | HB15 | BioLegend |
| CD86 | PE | BU63 | BioLegend |
| CD181 | PE | 8F1/CXCR1 | BioLegend |
| CD271 | PE-Cy7 | C40-1457 | BD Biosciences |
| HLA-A,B,C | AF700 | W6/32 | BioLegend |
| HLA-DR | APC-Cy7 | L246 | BioLegend |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemen, R.; Arlt, K.; von Woedtke, T.; Bekeschus, S. Gas Plasma Protein Oxidation Increases Immunogenicity and Human Antigen-Presenting Cell Maturation and Activation. Vaccines 2022, 10, 1814. https://doi.org/10.3390/vaccines10111814
Clemen R, Arlt K, von Woedtke T, Bekeschus S. Gas Plasma Protein Oxidation Increases Immunogenicity and Human Antigen-Presenting Cell Maturation and Activation. Vaccines. 2022; 10(11):1814. https://doi.org/10.3390/vaccines10111814
Chicago/Turabian StyleClemen, Ramona, Kevin Arlt, Thomas von Woedtke, and Sander Bekeschus. 2022. "Gas Plasma Protein Oxidation Increases Immunogenicity and Human Antigen-Presenting Cell Maturation and Activation" Vaccines 10, no. 11: 1814. https://doi.org/10.3390/vaccines10111814
APA StyleClemen, R., Arlt, K., von Woedtke, T., & Bekeschus, S. (2022). Gas Plasma Protein Oxidation Increases Immunogenicity and Human Antigen-Presenting Cell Maturation and Activation. Vaccines, 10(11), 1814. https://doi.org/10.3390/vaccines10111814







