Benefits of Switching Mycophenolic Acid to Sirolimus on Serological Response after a SARS-CoV-2 Booster Dose among Kidney Transplant Recipients: A Pilot Study
Abstract
:1. Introduction
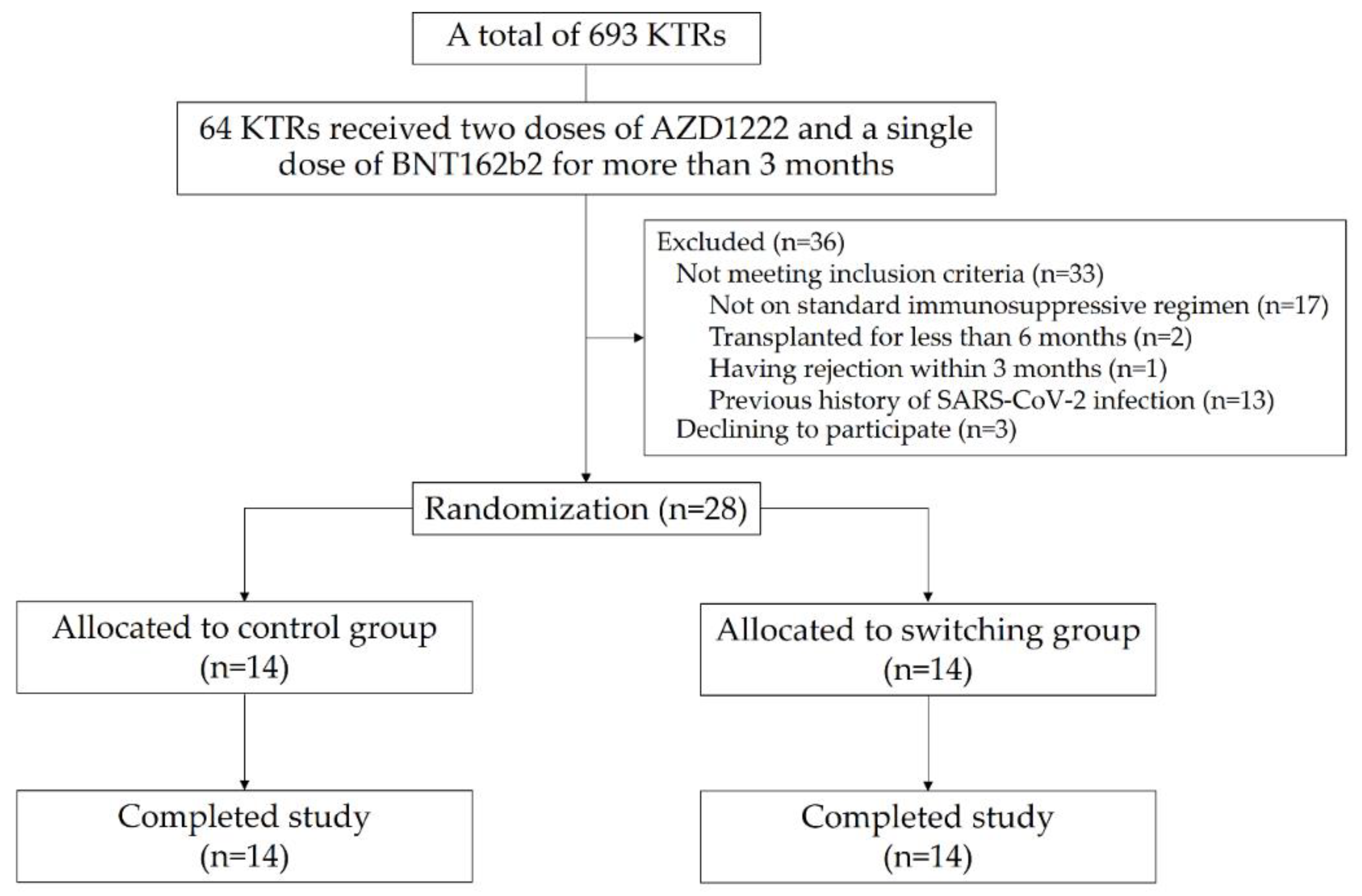
2. Materials and Methods
2.1. Study Design
2.2. Immunosuppressive Regimens and Vaccination
2.3. Outcome Measurements
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Participants
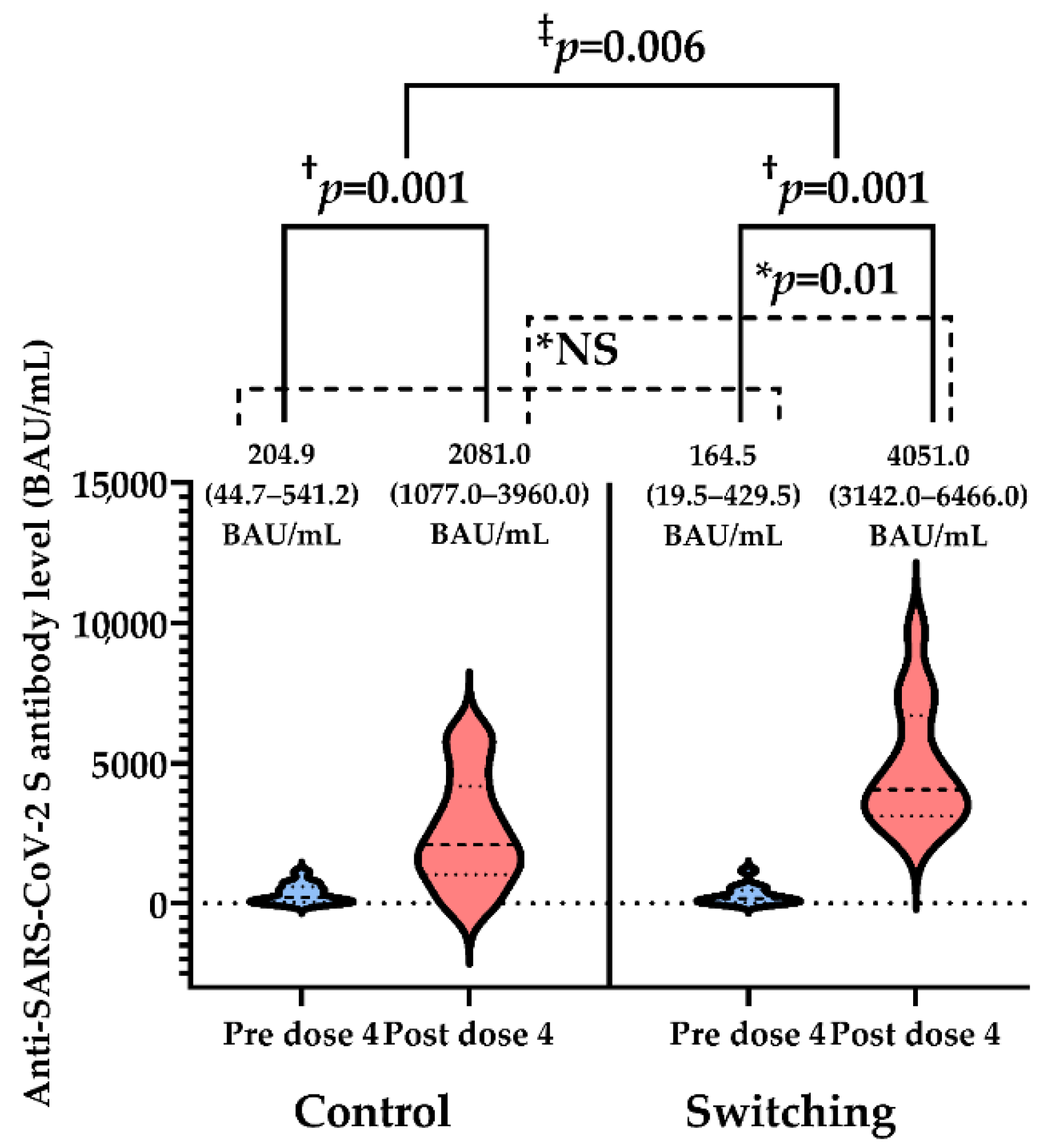
3.2. Post-Vaccination Anti-SARS-CoV-2 S Antibody Level and Seroconversion Rate
3.3. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okumura, K.; Nishida, S.; Dhand, A. Trends in COVID-19 mortality among solid organ transplant recipients: Implications for prevention. Transplantation 2022, 106, e380–e381. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody response to 2-Dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (accessed on 15 September 2022).
- Ekberg, H.; Tedesco-Silva, H.; Demirbas, A.; Vitko, S.; Nashan, B.; Gurkan, A.; Margreiter, R.; Hugo, C.; Grinyo, J.M.; Frei, U.; et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N. Engl. J. Med. 2007, 357, 2562–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. 3), S1–S155. [CrossRef] [PubMed]
- Tedesco-Silva, H.; Pascual, J.; Viklicky, O.; Basic-Jukic, N.; Cassuto, E.; Kim, D.Y.; Cruzado, J.M.; Sommerer, C.; Adel Bakr, M.; Garcia, V.D.; et al. Safety of everolimus with reduced calcineurin inhibitor exposure in de novo kidney transplants: An analysis from the randomized TRANSFORM study. Transplantation 2019, 103, 1953–1963. [Google Scholar] [CrossRef]
- Sommerer, C.; Suwelack, B.; Dragun, D.; Schenker, P.; Hauser, I.A.; Witzke, O.; Hugo, C.; Kamar, N.; Merville, P.; Junge, M.; et al. An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int. 2019, 96, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Pascual, J.; Berger, S.P.; Witzke, O.; Tedesco, H.; Mulgaonkar, S.; Qazi, Y.; Chadban, S.; Oppenheimer, F.; Sommerer, C.; Oberbauer, R.; et al. Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J. Am. Soc. Nephrol. 2018, 29, 1979–1991. [Google Scholar] [CrossRef] [Green Version]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Kantauskaite, M.; Muller, L.; Kolb, T.; Fischer, S.; Hillebrandt, J.; Ivens, K.; Andree, M.; Luedde, T.; Orth, H.M.; Adams, O.; et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am. J. Transplant. 2022, 22, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021, 325, 1784–1786. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Gautier, G.; Perrin, P.; Olagne, J.; Cognard, N.; Fafi-Kremer, S.; Caillard, S. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 2021, 326, 1063–1065. [Google Scholar] [CrossRef]
- Rozen-Zvi, B.; Yahav, D.; Agur, T.; Zingerman, B.; Ben-Zvi, H.; Atamna, A.; Tau, N.; Mashraki, T.; Nesher, E.; Rahamimov, R. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin. Microbiol. Infect. 2021, 27, 1173.e1171–1173.e1174. [Google Scholar] [CrossRef]
- Bruminhent, J.; Setthaudom, C.; Chaumdee, P.; Boongird, S.; Kiertiburanakul, S.; Malathum, K.; Nongnuch, A.; Phuphuakrat, A.; Jirasiritham, S.; Janphram, C.; et al. SARS-CoV-2-specific humoral and cell-mediated immune responses after immunization with inactivated COVID-19 vaccine in kidney transplant recipients (CVIM 1 study). Am. J. Transplant. 2022, 22, 813–822. [Google Scholar] [CrossRef]
- Egli, A.; Humar, A.; Widmer, L.A.; Lisboa, L.F.; Santer, D.M.; Mueller, T.; Stelling, J.; Baluch, A.; O’Shea, D.; Houghton, M.; et al. Effect of Immunosuppression on T-Helper 2 and B-Cell Responses to Influenza Vaccination. J. Infect. Dis. 2015, 212, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Osmanodja, B.; Ronicke, S.; Budde, K.; Jens, A.; Hammett, C.; Koch, N.; Seelow, E.; Waiser, J.; Zukunft, B.; Bachmann, F.; et al. Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. J. Clin. Med. 2022, 11, 2565. [Google Scholar] [CrossRef]
- Netti, G.S.; Infante, B.; Troise, D.; Mercuri, S.; Panico, M.; Spadaccino, F.; Catalano, V.; Gigante, M.; Simone, S.; Pontrelli, P.; et al. mTOR inhibitors improve both humoral and cellular response to SARS-CoV-2 messenger RNA BNT16b2 vaccine in kidney transplant recipients. Am. J. Transplant. 2022, 22, 1475–1482. [Google Scholar] [CrossRef]
- De Boer, S.E.; Berger, S.P.; van Leer–Buter, C.C.; Kroesen, B.-J.; van Baarle, D.; Sanders, J.-S.F. Enhanced humoral immune response after COVID-19 vaccination in elderly kidney transplant recipients on everolimus versus mycophenolate mofetil–containing immunosuppressive regimens. Transplantation 2022, 106, 1615–1621. [Google Scholar] [CrossRef]
- Qin, C.X.; Moore, L.W.; Anjan, S.; Rahamimov, R.; Sifri, C.D.; Ali, N.M.; Morales, M.K.; Tsapepas, D.S.; Basic-Jukic, N.; Miller, R.A.; et al. Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation 2021, 105, e265–e266. [Google Scholar] [CrossRef]
- Lee, A.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of covid-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021, 21, 2727–2739. [Google Scholar] [CrossRef]
- Sood, A.; Tran, M.; Murthy, V.; Gonzalez, E. Immunogenicity and Safety of SARS-CoV-2 Vaccination in Patients With Rheumatic Diseases: A Systematic Review and Meta-analysis. J. Clin. Rheumatol. 2022. Epub ahead of print: 3 June 2022. [Google Scholar] [CrossRef]
- Keshtkar-Jahromi, M.; Argani, H.; Rahnavardi, M.; Mirchi, E.; Atabak, S.; Tara, S.A.; Gachkar, L.; Noori-Froothghe, A.; Mokhtari-Azad, T. Antibody response to influenza immunization in kidney transplant recipients receiving either azathioprine or mycophenolate: A controlled trial. Am. J. Nephrol. 2008, 28, 654–660. [Google Scholar] [CrossRef]
- Prasoppokakorn, T.; Vanichanan, J.; Chaiteerakij, R.; Jutivorakool, K.; Udomkarnjananun, S.; Pongpirul, K.; Taesombat, W.; Wattanatorn, S.; Avihingsanon, Y.; Tungsanga, K.; et al. A randomized controlled trial of comparative effectiveness between the 2 dose and 3 dose regimens of hepatitis a vaccine in kidney transplant recipients. Sci. Rep. 2021, 11, 50. [Google Scholar] [CrossRef]
- Girerd, S.; Schikowski, J.; Girerd, N.; Duarte, K.; Busby, H.; Gambier, N.; Ladrière, M.; Kessler, M.; Frimat, L.; Aarnink, A. Impact of reduced exposure to calcineurin inhibitors on the development of de novo DSA: A cohort of non-immunized first kidney graft recipients between 2007 and 2014. BMC Nephrol. 2018, 19, 232. [Google Scholar] [CrossRef]
- Choi, B.S.; Shin, M.J.; Shin, S.J.; Kim, Y.S.; Choi, Y.J.; Kim, Y.S.; Moon, I.S.; Kim, S.Y.; Koh, Y.B.; Bang, B.K.; et al. Clinical significance of an early protocol biopsy in living-donor renal transplantation: Ten-year experience at a single center. Am. J. Transplant. 2005, 5, 1354–1360. [Google Scholar] [CrossRef]
- Karnell, J.L.; Karnell, F.G., 3rd; Stephens, G.L.; Rajan, B.; Morehouse, C.; Li, Y.; Swerdlow, B.; Wilson, M.; Goldbach-Mansky, R.; Groves, C.; et al. Mycophenolic acid differentially impacts B cell function depending on the stage of differentiation. J. Immunol. 2011, 187, 3603–3612. [Google Scholar] [CrossRef] [Green Version]
- Araki, K.; Turner, A.P.; Shaffer, V.O.; Gangappa, S.; Keller, S.A.; Bachmann, M.F.; Larsen, C.P.; Ahmed, R. mTOR regulates memory CD8 T-cell differentiation. Nature 2009, 460, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Lee, J.; Xu, L.; Mohammed, A.U.; Li, W.; Hale, J.S.; Tan, W.G.; Wu, T.; Davis, C.W.; Ahmed, R.; et al. mTOR promotes antiviral humoral immunity by differentially regulating CD4 Helper T cell and B cell responses. J. Virol. 2017, 91, e01653-16. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Zang, A.; Du, M.; Ma, D.; Yuan, C.; Zhou, C.; Mu, J.; Shi, H.; Li, D.; Huang, X.; et al. mTOR regulates TLR-induced c-fos and Th1 responses to HBV and HCV vaccines. Virol. Sin. 2015, 30, 174–189. [Google Scholar] [CrossRef]
- Regele, F.; Heinzel, A.; Hu, K.; Raab, L.; Eskandary, F.; Faé, I.; Zelzer, S.; Böhmig, G.A.; Bond, G.; Fischer, G.; et al. Stopping of mycophenolic acid in kidney transplant recipients for 2 weeks peri-vaccination does not increase response to SARS-CoV-2 vaccination-A non-randomized, controlled pilot study. Front. Med. 2022, 9, 914424. [Google Scholar] [CrossRef]
- Llinas-Mallol, L.; Redondo-Pachon, D.; Perez-Saez, M.J.; Raich-Regue, D.; Mir, M.; Yelamos, J.; Lopez-Botet, M.; Pascual, J.; Crespo, M. Peripheral blood lymphocyte subsets change after steroid withdrawal in renal allograft recipients: A prospective study. Sci. Rep. 2019, 9, 7453. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Dolscheid-Pommerich, R.; Bartok, E.; Renn, M.; Kümmerer, B.M.; Schulte, B.; Schmithausen, R.M.; Stoffel-Wagner, B.; Streeck, H.; Saschenbrecker, S.; Steinhagen, K.; et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J. Med. Virol. 2022, 94, 388–392. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total (n = 28) | Control Group (n = 14) | Switching Group (n = 14) | p |
|---|---|---|---|---|
| Age, years; mean ± SD | 51.5 ± 8.7 | 50.4 ± 9.2 | 52.6 ± 8.4 | 0.51 |
| Male, n (%) | 15 (53.6%) | 9 (64.3%) | 6 (42.9%) | 0.45 |
| DKT, n (%) | 17 (60.7%) | 6 (42.9%) | 11 (78.6%) | 0.12 |
| Transplant vintage, years; median (IQR) | 3.3 (1.6–7.5) | 3.2 (1.5–14.4) | 3.3 (1.8–7.3) | 0.85 |
| Baseline serum creatinine, mg/dL; mean ± SD | 1.34 ± 0.48 | 1.40 ± 0.59 | 1.27 ± 0.37 | 0.51 |
| White blood cells, cells/μL; median (IQR) | 5725 (5110–7110) | 5710 (5280–6930) | 5985 (4680–7290) | 0.95 |
| Neutrophil, cells/μL; median (IQR) | 3720 (3145–4800) | 3525 (3180–4270) | 4055 (3120–5100) | 0.73 |
| Lymphocyte, cells/μL; median (IQR) | 1645 (1290–2085) | 1755 (1540–2100) | 1455 (1180–2070) | 0.43 |
| Dosage of MMF, mg/day; mean ± SD | 1179 ± 69 | 1250 ± 126 | 1107 ± 57 | 0.31 |
| Tacrolimus trough level, ng/mL; mean ± SD | 4.8 ± 1.0 | 4.7 ± 1.2 | 5.0 ± 0.8 | 0.59 |
| Duration between first and second vaccinations, months; median (IQR) | 2.8 (2.8–2.8) | 2.8 (2.8–2.8) | 2.8 (2.8–2.8) | 0.14 |
| Duration between second and third vaccinations, months; median (IQR) | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) | 1.3 (1.2–1.4) | 0.72 |
| Time since the last BNT162b2, months; median (IQR) | 4.9 (4.6–5.3) | 4.9 (4.6–5.3) | 5.0 (4.8–5.2) | 0.66 |
| Baseline anti-SARS-CoV-2 S antibody, BAU/mL; median (IQR) | 170.2 (36.0–510.3) | 204.9 (44.7–541.2) | 164.5 (19.5–429.5) | 0.61 |
| Adverse Events | Control Group (n = 14) | Switching Group (n = 14) |
|---|---|---|
| Immunosuppressant-related | ||
| Oral ulcers, n (%) | 0 (0%) | 2 (1.4%) |
| Edema, n (%) | 0 (0%) | 0(0%) |
| Diarrhea, n (%) | 0 (0%) | 0 (0%) |
| Pneumonitis, n (%) | 0 (0%) | 0 (0%) |
| Rejection, n (%) | 0 (0%) | 0 (0%) |
| Vaccine-related | ||
| Myalgia, n (%) | 11 (78.6%) | 13 (92.9%) |
| Fever, n (%) | 3 (2.1%) | 2 (1.4%) |
| Bleeding, n (%) | 0 (0%) | 0 (0%) |
| Chest discomfort, n (%) | 0 (0%) | 0 (0%) |
| Severe headache, n (%) | 0 (0%) | 0 (0%) |
| Vomiting, n (%) | 0 (0%) | 0 (0%) |
| Seizure, n (%) | 0 (0%) | 0 (0%) |
| Stroke-like symptoms, n (%) | 0 (0%) | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banjongjit, A.; Phirom, S.; Phannajit, J.; Jantarabenjakul, W.; Paitoonpong, L.; Kittanamongkolchai, W.; Wattanatorn, S.; Prasithsirikul, W.; Eiam-Ong, S.; Avihingsanon, Y.; et al. Benefits of Switching Mycophenolic Acid to Sirolimus on Serological Response after a SARS-CoV-2 Booster Dose among Kidney Transplant Recipients: A Pilot Study. Vaccines 2022, 10, 1685. https://doi.org/10.3390/vaccines10101685
Banjongjit A, Phirom S, Phannajit J, Jantarabenjakul W, Paitoonpong L, Kittanamongkolchai W, Wattanatorn S, Prasithsirikul W, Eiam-Ong S, Avihingsanon Y, et al. Benefits of Switching Mycophenolic Acid to Sirolimus on Serological Response after a SARS-CoV-2 Booster Dose among Kidney Transplant Recipients: A Pilot Study. Vaccines. 2022; 10(10):1685. https://doi.org/10.3390/vaccines10101685
Chicago/Turabian StyleBanjongjit, Athiphat, Supitchaya Phirom, Jeerath Phannajit, Watsamon Jantarabenjakul, Leilani Paitoonpong, Wonngarm Kittanamongkolchai, Salin Wattanatorn, Wisit Prasithsirikul, Somchai Eiam-Ong, Yingyos Avihingsanon, and et al. 2022. "Benefits of Switching Mycophenolic Acid to Sirolimus on Serological Response after a SARS-CoV-2 Booster Dose among Kidney Transplant Recipients: A Pilot Study" Vaccines 10, no. 10: 1685. https://doi.org/10.3390/vaccines10101685
APA StyleBanjongjit, A., Phirom, S., Phannajit, J., Jantarabenjakul, W., Paitoonpong, L., Kittanamongkolchai, W., Wattanatorn, S., Prasithsirikul, W., Eiam-Ong, S., Avihingsanon, Y., Hansasuta, P., Vanichanan, J., & Townamchai, N. (2022). Benefits of Switching Mycophenolic Acid to Sirolimus on Serological Response after a SARS-CoV-2 Booster Dose among Kidney Transplant Recipients: A Pilot Study. Vaccines, 10(10), 1685. https://doi.org/10.3390/vaccines10101685






