Systemic and Mucosal Humoral Immune Response Induced by Three Doses of the BNT162b2 SARS-CoV-2 mRNA Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Individuals and Sample Collection
2.2. Immunological Analyses
2.3. Virological Analyses
2.4. Statistical Analysis
3. Results
3.1. Clinical Parameters
3.2. Serum SARS-CoV-2 IgG and IgA Antibody Response after Vaccination
3.3. Neutralizing Antibodies Induced by Vaccination
3.4. Salivary SARS-CoV-2 IgA Isotype Antibody Response after Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- FDA Takes Key Action in Fight against COVID-19 by Issuing Emergency Use Authorization for First COVID-19 Vaccine|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-COVID-19 (accessed on 13 July 2022).
- Approval of Vaccines in the European Union. Available online: https://vaccination-info.eu/en/vaccine-facts/approval-vaccines-european-union (accessed on 13 July 2022).
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, M.; Francavilla, B.; Fiorelli, D.; Nuccetelli, M.; Passali, F.M.; Coppeta, L.; Somma, G.; Bernardini, S.; Magrini, A.; Di Girolamo, S. Nasal and Salivary Mucosal Humoral Immune Response Elicited by mRNA BNT162b2 COVID-19 Vaccine Compared to SARS-CoV-2 Natural Infection. Vaccines 2021, 9, 1499. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Faro-Viana, J.; Bergman, M.-L.; Gonçalves, L.A.; Duarte, N.; Coutinho, T.P.; Borges, P.C.; Diwo, C.; Castro, R.; Matoso, P.; Malheiro, V.; et al. Population homogeneity for the antibody response to COVID-19 BNT162b2/Comirnaty vaccine is only reached after the second dose across all adult age ranges. Nat. Commun. 2022, 13, 140. [Google Scholar] [CrossRef]
- Governo Italiano—Report Vaccini Anti COVID-19. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 13 July 2022).
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Toraldo, D.M.; Satriano, F.; Rollo, R.; Verdastro, G.; Imbriani, G.; Rizzo, E.; Argentiero, A.; Falco, A.; Ambrosino, P.; Miani, A.; et al. COVID-19 IgG/IgM patterns, early IL-6 elevation and long-term radiological sequelae in 75 patients hospitalized due to interstitial pneumonia followed up from 3 to 12 months. PLoS ONE 2022, 17, e0262911. [Google Scholar] [CrossRef] [PubMed]
- Fill Malfertheiner, S.; Brandstetter, S.; Roth, S.; Harner, S.; Buntrock-Döpke, H.; Toncheva, A.A.; Borchers, N.; Gruber, R.; Ambrosch, A.; Kabesch, M.; et al. Immune response to SARS-CoV-2 in health care workers following a COVID-19 outbreak: A prospective longitudinal study. J. Clin. Virol. 2020, 130, 104575. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Jernbom Falk, A.; Klingström, J.; Ng, H.; Greilert-Norin, N.; Gabrielsson, L.; Salomonsson, A.-C.; Isaksson, E.; Rudberg, A.-S.; Hellström, C.; et al. SARS-CoV-2 induces a durable and antigen specific humoral immunity after asymptomatic to mild COVID-19 infection. PLoS ONE 2022, 17, e0262169. [Google Scholar] [CrossRef] [PubMed]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild COVID-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Bruni, M.; Cecatiello, V.; Diaz-Basabe, A.; Lattanzi, G.; Mileti, E.; Monzani, S.; Pirovano, L.; Rizzelli, F.; Visintin, C.; Bonizzi, G.; et al. Persistence of Anti-SARS-CoV-2 Antibodies in Non-Hospitalized COVID-19 Convalescent Health Care Workers. J. Clin. Med. 2020, 9, 3188. [Google Scholar] [CrossRef]
- Bonnet, B.; Chabrolles, H.; Archimbaud, C.; Brebion, A.; Cosme, J.; Dutheil, F.; Lambert, C.; Junda, M.; Mirand, A.; Ollier, A.; et al. Decline of Humoral and Cellular Immune Responses Against SARS-CoV-2 6 Months After Full BNT162b2 Vaccination in Hospital Healthcare Workers. Front. Immunol. 2022, 13, 842912. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Skrzat-Klapaczyńska, A.; Bieńkowski, C.; Kowalska, J.; Paciorek, M.; Puła, J.; Krogulec, D.; Stengiel, J.; Pawełczyk, A.; Perlejewski, K.; Osuch, S.; et al. The Beneficial Effect of the COVID-19 Vaccine Booster Dose among Healthcare Workers in an Infectious Diseases Center. Vaccines 2022, 10, 552. [Google Scholar] [CrossRef]
- Valcourt, E.J.; Manguiat, K.; Robinson, A.; Chen, J.C.-Y.; Dimitrova, K.; Philipson, C.; Lamoureux, L.; McLachlan, E.; Schiffman, Z.; Drebot, M.A.; et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn Microbiol Infect Dis 2021, 99, 115294. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Maurino, V.; Focosi, D.; et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine 2022, 75, 103788. [Google Scholar] [CrossRef] [PubMed]
- Darwich, A.; Pozzi, C.; Fornasa, G.; Lizier, M.; Azzolini, E.; Spadoni, I.; Carli, F.; Voza, A.; Desai, A.; Ferrero, C.; et al. BNT162b2 vaccine induces antibody release in saliva: A possible role for mucosal viral protection? EMBO Mol. Med. 2022, 14, e15326. [Google Scholar] [CrossRef] [PubMed]
- Rastawicki, W.; Juszczyk, G.; Gierczyński, R.; Zasada, A.A. Comparison of anti-SARS-CoV-2 IgG and IgA antibody responses post complete vaccination, 7 months later and after 3rd dose of the BNT162b2 vaccine in healthy adults. J. Clin. Virol. 2022, 152, 105193. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to COVID-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Bradley, T.; Grundberg, E.; Selvarangan, R.; LeMaster, C.; Fraley, E.; Banerjee, D.; Belden, B.; Louiselle, D.; Nolte, N.; Biswell, R.; et al. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1959–1961. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Lo Sasso, B.; Giglio, R.V.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.M.; Ciaccio, A.M.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef]
- Saadat, S.; Rikhtegaran Tehrani, Z.; Logue, J.; Newman, M.; Frieman, M.B.; Harris, A.D.; Sajadi, M.M. Binding and Neutralization Antibody Titers after a Single Vaccine Dose in Health Care Workers Previously Infected with SARS-CoV-2. JAMA 2021, 325, 1467. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, L.; Pezzati, L.; Oreni, L.; Kullmann, C.; Lai, A.; Gabrieli, A.; Bestetti, G.; Beschi, C.; Conti, F.; Ottomano, C.; et al. Impact of prior infection status on antibody response to the BNT162b2 mRNA COVID-19 vaccine in healthcare workers at a COVID-19 referral hospital in Milan, Italy. Hum. Vaccines Immunother. 2021, 17, 4747–4754. [Google Scholar] [CrossRef]
- Ontañón, J.; Blas, J.; de Cabo, C.; Santos, C.; Ruiz-Escribano, E.; García, A.; Marín, L.; Sáez, L.; Beato, J.L.; Rada, R.; et al. Influence of past infection with SARS-CoV-2 on the response to the BNT162b2 mRNA vaccine in health care workers: Kinetics and durability of the humoral immune response. EBioMedicine 2021, 73, 103656. [Google Scholar] [CrossRef]
- Desmecht, S.; Tashkeev, A.; El Moussaoui, M.; Marechal, N.; Perée, H.; Tokunaga, Y.; Fombellida-Lopez, C.; Polese, B.; Legrand, C.; Wéry, M.; et al. Kinetics and Persistence of the Cellular and Humoral Immune Responses to BNT162b2 mRNA Vaccine in SARS-CoV-2-Naive and -Experienced Subjects: Impact of Booster Dose and Breakthrough Infections. Front. Immunol. 2022, 13, 863554. [Google Scholar] [CrossRef]
- Fraley, E.; LeMaster, C.; Geanes, E.; Banerjee, D.; Khanal, S.; Grundberg, E.; Selvarangan, R.; Bradley, T. Humoral immune responses during SARS-CoV-2 mRNA vaccine administration in seropositive and seronegative individuals. BMC Med. 2021, 19, 169. [Google Scholar] [CrossRef]
- Fraley, E.; LeMaster, C.; Khanal, S.; Banerjee, D.; Pastinen, T.; Grundberg, E.; Selvarangan, R.; Bradley, T. The Impact of Prior Infection and Age on Antibody Persistence After Severe Acute Respiratory Syndrome Coronavirus 2 Messenger RNA Vaccine. Clin. Infect. Dis. 2022, 75, e902–e904. [Google Scholar] [CrossRef]
- Parry, H.; Bruton, R.; Stephens, C.; Bentley, C.; Brown, K.; Amirthalingam, G.; Hallis, B.; Otter, A.; Zuo, J.; Moss, P. Extended interval BNT162b2 vaccination enhances peak antibody generation. npj Vaccines 2022, 7, 14. [Google Scholar] [CrossRef]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef] [PubMed]
- Jahrsdörfer, B.; Fabricius, D.; Scholz, J.; Ludwig, C.; Grempels, A.; Lotfi, R.; Körper, S.; Adler, G.; Schrezenmeier, H. BNT162b2 Vaccination Elicits Strong Serological Immune Responses Against SARS-CoV-2 Including Variants of Concern in Elderly Convalescents. Front. Immunol. 2021, 12, 743422. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-Dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Speletas, M.; Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Mouchtouri, V.A.; Nikoulis, D.J.; Tsakona, M.; Kyritsi, M.A.; Peristeri, A.-M.; Avakian, I.; et al. Intensity and Dynamics of Anti-SARS-CoV-2 Immune Responses after BNT162b2 mRNA Vaccination: Implications for Public Health Vaccination Strategies. Vaccines 2022, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef]
- Bell, M.R.; Kutzler, M.A. An old problem with new solutions: Strategies to improve vaccine efficacy in the elderly. Adv. Drug Deliv. Rev. 2022, 183, 114175. [Google Scholar] [CrossRef]
- Gavazzi, G.; Krause, K.-H. Ageing and infection. Lancet Infect. Dis. 2002, 2, 659–666. [Google Scholar] [CrossRef]
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981. [Google Scholar] [CrossRef]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.-D.; Liacos, C.-I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef]
- Costa, C.; Migliore, E.; Galassi, C.; Scozzari, G.; Ciccone, G.; Coggiola, M.; Pira, E.; Scarmozzino, A.; La Valle, G.; Cassoni, P.; et al. Factors Influencing Level and Persistence of Anti SARS-CoV-2 IgG after BNT162b2 Vaccine: Evidence from a Large Cohort of Healthcare Workers. Vaccines 2022, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Muena, N.A.; García-Salum, T.; Pardo-Roa, C.; Avendaño, M.J.; Serrano, E.F.; Levican, J.; Almonacid, L.I.; Valenzuela, G.; Poblete, E.; Strohmeier, S.; et al. Induction of SARS-CoV-2 neutralizing antibodies by CoronaVac and BNT162b2 vaccines in naïve and previously infected individuals. EBioMedicine 2022, 78, 103972. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Sancilio, A.E.; D’Aquila, R.T.; McNally, E.M.; Velez, M.P.; Ison, M.G.; Demonbreun, A.R.; McDade, T.W. A surrogate virus neutralization test to quantify antibody-mediated inhibition of SARS-CoV-2 in finger stick dried blood spot samples. Sci. Rep. 2021, 11, 15321. [Google Scholar] [CrossRef]
- Infantino, M.; Manfredi, M.; Stacchini, L.; Cosma, C.; Grossi, V.; Lari, B.; Russo, E.; Amedei, A.; Benucci, M.; Veneziani, F.; et al. The role of neutralizing antibodies by sVNT after two doses of BNT162b2 mRNA vaccine in a cohort of Italian healthcare workers. Clin. Chem. Lab. Med. (CCLM) 2022, 60, 934–940. [Google Scholar] [CrossRef]
- Padoan, A.; Cosma, C.; Bonfante, F.; Della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall’Olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing antibody titers six months after Comirnaty vaccination: Kinetics and comparison with SARS-CoV-2 immunoassays. Clin. Chem. Lab. Med. 2022, 60, 456–463. [Google Scholar] [CrossRef]
- Malipiero, G.; D’Agaro, P.; Segat, L.; Moratto, A.; Villalta, D. Long-term decay of anti-RBD IgG titers after BNT162b2 vaccination is not mirrored by loss of neutralizing bioactivity against SARS-CoV-2. Clin. Chim. Acta 2022, 524, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; De Nitto, S.; Gianfilippi, G.; Lippi, G. The pronounced decline of anti-SARS-CoV-2 spike trimeric IgG and RBD IgG in baseline seronegative individuals six months after BNT162b2 vaccination is consistent with the need for vaccine boosters. Clin. Chem. Lab. Med. (CCLM) 2021, 60, e29–e31. [Google Scholar] [CrossRef]
- Benning, L.; Morath, C.; Bartenschlager, M.; Reineke, M.; Töllner, M.; Nusshag, C.; Kälble, F.; Reichel, P.; Schaier, M.; Klein, K.; et al. Neutralizing antibody activity against the B.1.617.2 (delta) variant 8 months after two-dose vaccination with BNT162b2 in health care workers. Clin. Microbiol. Infect. 2022, 28, 1024.e7–1024.e12. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P.; Zeng, C.; Carlin, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; Gumina, R.J.; Liu, S.-L. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 2022, 14, eabn8057. [Google Scholar] [CrossRef] [PubMed]

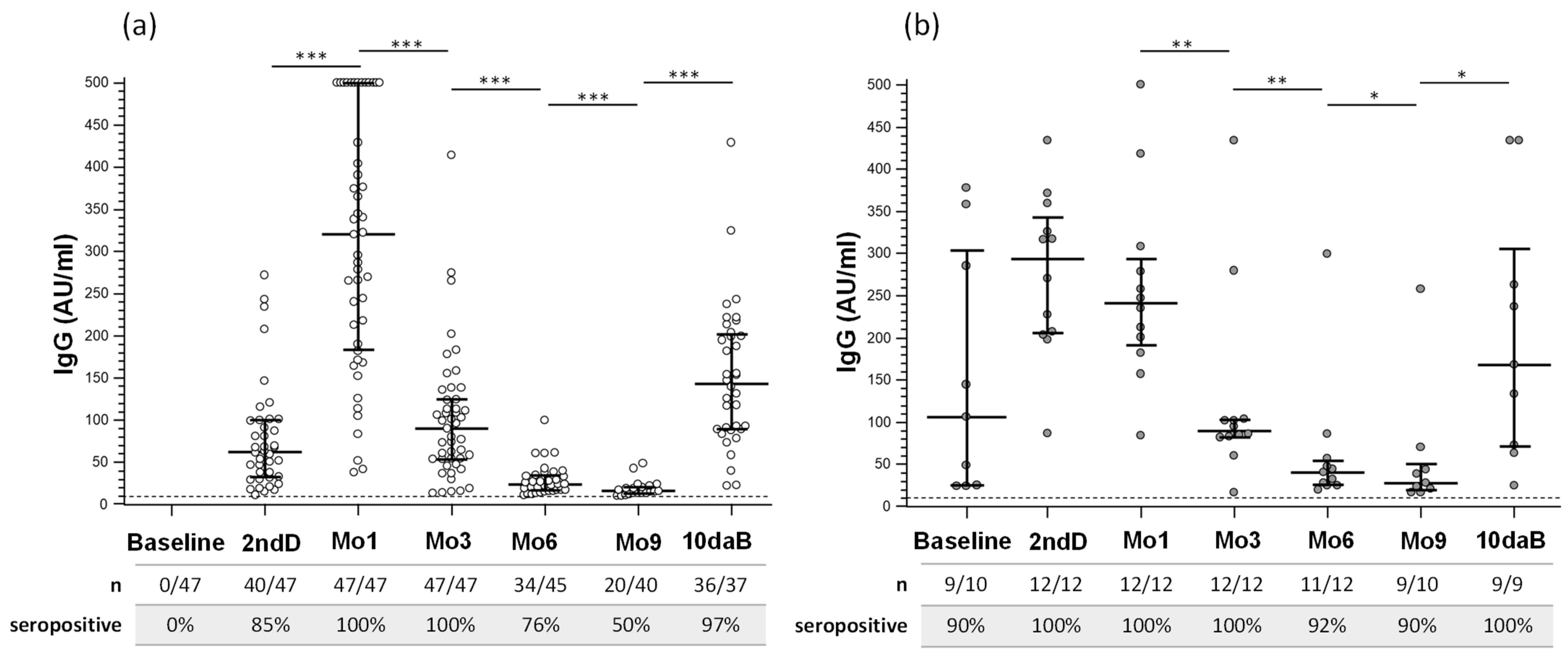
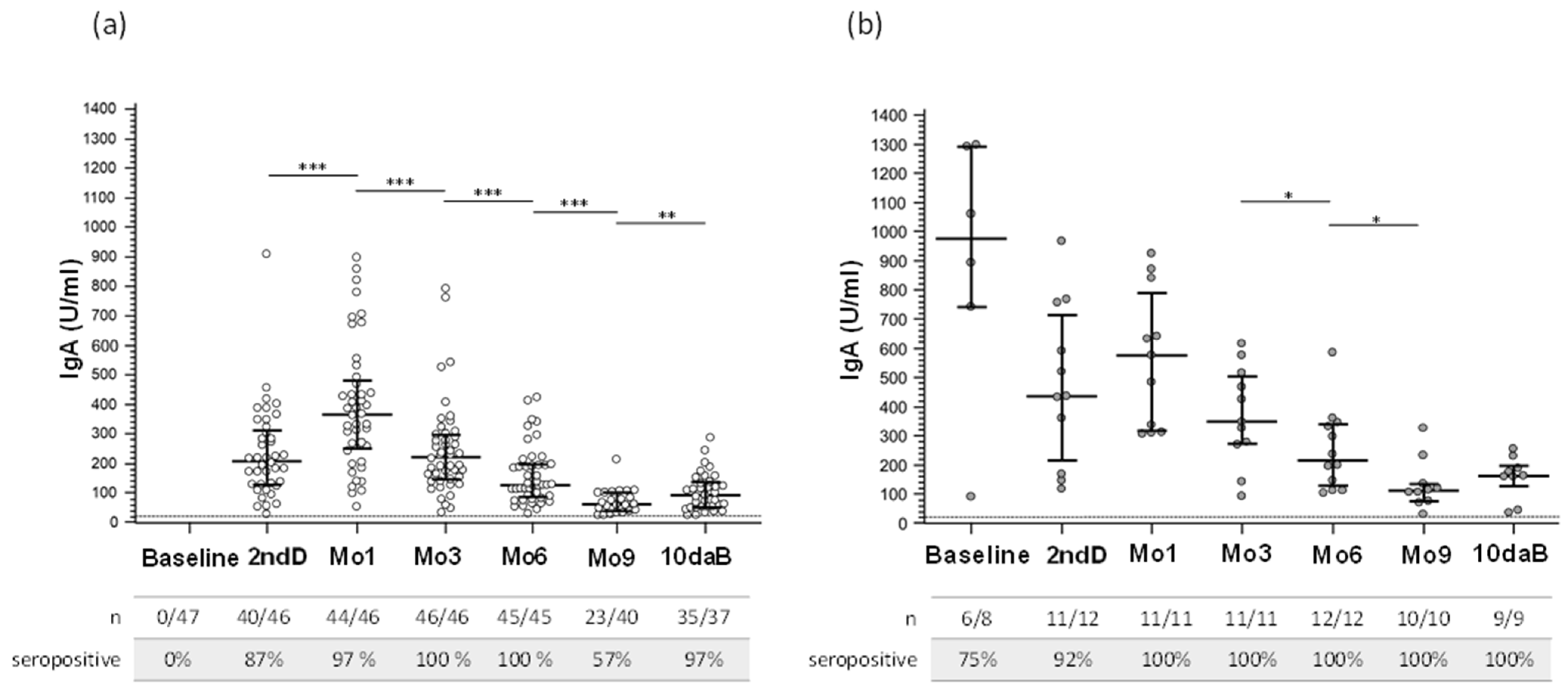
| Naïve-HCWs | Infected-HCWs | Total | |
|---|---|---|---|
| N | 47 | 12 | 59 |
| Gender (M/F) | 15/32 | 4/8 | 19/40 |
| Age, years (mean ± SD) | 44.51 ± 13.74 | 49.50 ± 8.79 | 45.53 ± 12.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, R.; Agostini, S.; Citterio, L.A.; Chiarini, D.; Santangelo, M.A.; Clerici, M. Systemic and Mucosal Humoral Immune Response Induced by Three Doses of the BNT162b2 SARS-CoV-2 mRNA Vaccines. Vaccines 2022, 10, 1649. https://doi.org/10.3390/vaccines10101649
Mancuso R, Agostini S, Citterio LA, Chiarini D, Santangelo MA, Clerici M. Systemic and Mucosal Humoral Immune Response Induced by Three Doses of the BNT162b2 SARS-CoV-2 mRNA Vaccines. Vaccines. 2022; 10(10):1649. https://doi.org/10.3390/vaccines10101649
Chicago/Turabian StyleMancuso, Roberta, Simone Agostini, Lorenzo Agostino Citterio, Debora Chiarini, Maria Antonia Santangelo, and Mario Clerici. 2022. "Systemic and Mucosal Humoral Immune Response Induced by Three Doses of the BNT162b2 SARS-CoV-2 mRNA Vaccines" Vaccines 10, no. 10: 1649. https://doi.org/10.3390/vaccines10101649
APA StyleMancuso, R., Agostini, S., Citterio, L. A., Chiarini, D., Santangelo, M. A., & Clerici, M. (2022). Systemic and Mucosal Humoral Immune Response Induced by Three Doses of the BNT162b2 SARS-CoV-2 mRNA Vaccines. Vaccines, 10(10), 1649. https://doi.org/10.3390/vaccines10101649







