Comparing COVID-19 Vaccination Outcomes with Parental Values, Beliefs, Attitudes, and Hesitancy Status, 2021–2022
Abstract
:1. Introduction
2. Materials and Methods
- Modified Vaccine Hesitancy Scale (VHS): A 9-item scale (with responses on a 4-point Likert-type scale ranging from Strongly agree, Agree, Disagree, to Strongly disagree) adapted from the Vaccine Hesitancy Scale for childhood vaccines [20].
- Kaiser Family Foundation (KFF) COVID-19 Vaccine Monitor: This ongoing research project uses both surveys and qualitative research to understand, in part, parental attitudes and experiences with COVID-19. We used and adapted reported questions from this resource (Questions 23, 29, 35–36 in Supplementary Table S2 and Questions 32–33 in Supplementary Table S3) [12].
- CDC Vaccine Confidence Survey (Questions 38–40 in Supplementary Table S2 and Questions 36–38 in Supplementary Table S3) [24].
- Vaccination Attitudes Examination (VAX) Scale: A 12-item scale (with responses on a 6-point Likert-type scale ranging from (1) “strongly agree” to (6) “strongly disagree”) created to understand the following: trust/mistrust of vaccine benefit, worries over unforeseen future effects, concerns about commercial profiteering, and preference for natural immunity (Questions 6–17 in Supplementary Table S3) [25]. The first three items were reverse-coded so lower total scores reflected stronger anti-vaccination attitudes, and we summed all twelve item scores to obtain a composite score as well as a median score. The four themes above corresponded to validated subscales, which we calculated by summing the scores from the three items in each subscale.
- Coronavirus Anxiety Scale: A 5-item screening tool to identify probable cases of dysfunctional anxiety associated with the coronavirus (Questions 41–45 in Supplementary Table S3) [26].
- Media and Technology Usage and Attitudes Scale (MTUAS): The overall MTUAS was created to measure media and technology involvement of respondents [27]. We used the 9-item General Social Media Usage Subscale (Questions 46–54 in Supplementary Table S3).
3. Results
3.1. Vaccine Access, Beliefs, and Attitudes
3.1.1. Vaccine Access
3.1.2. Vaccine Reasons and Hesitancy
3.1.3. Vaccine Attitudes Examination (VAX) Scale
3.2. Coronavirus Anxiety Scale
3.3. Non-Pharmaceutical Interventions and Behaviors
3.4. School Restrictions and Behaviors
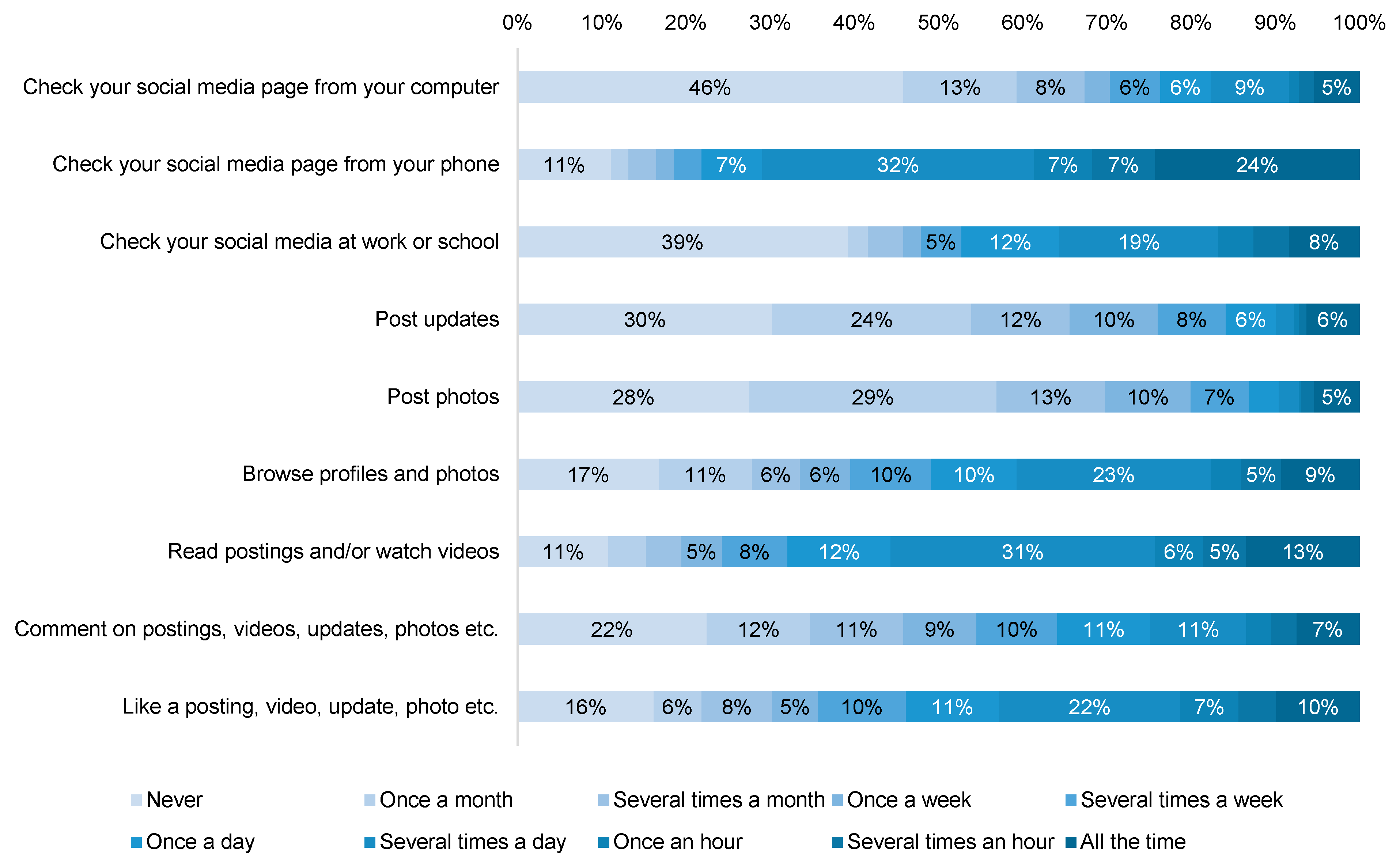
3.5. Social Media Usage
3.6. Multivariable Logistic Regression
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suthar, A.B.; Wang, J.; Seffren, V.; Wiegand, R.E.; Griffing, S.; Zell, E. Public Health Impact of Covid-19 Vaccines in the US: Observational Study. BMJ 2022, 377, e069317. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; McLaughlin, J.M.; Khan, F.; Angulo, F.J.; Anis, E.; Lipsitch, M.; Singer, S.R.; Mircus, G.; Brooks, N.; Smaja, M.; et al. Infections, Hospitalisations, and Deaths Averted via a Nationwide Vaccination Campaign Using the Pfizer–BioNTech BNT162b2 MRNA COVID-19 Vaccine in Israel: A Retrospective Surveillance Study. Lancet Infect. Dis. 2022, 22, 357–366. [Google Scholar] [CrossRef]
- McLaughlin, J.M.; Khan, F.; Pugh, S.; Swerdlow, D.L.; Jodar, L. County-Level Vaccination Coverage and Rates of COVID-19 Cases and Deaths in the United States: An Ecological Analysis. Lancet Reg. Health-Am. 2022, 9, 100191. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention COVID-19 Vaccination and Case Trends by Age Group. Available online: https://data.cdc.gov/Vaccinations/COVID-19-Vaccination-and-Case-Trends-by-Age-Group-/gxj9-t96f/data (accessed on 14 August 2022).
- Shen, A.K.; Bramer, C.A.; Kimmins, L.M.; Swanson, R.; Vranesich, P.; Orenstein, W. Vaccine Coverage Across the Life Course in Michigan During the COVID-19 Pandemic: January–September 2020. Am. J. Public Health 2021, 111, 2027–2035. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention CDC COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 21 June 2021).
- Adams, E.L.; Smith, D.; Caccavale, L.J.; Bean, M.K. Parents Are Stressed! Patterns of Parent Stress Across COVID-19. Front. Psychiatry 2021, 12, 300. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention CDC COVID Data Tracker: Multisystem Inflammatory Syndrome in Children (MIS-C). Available online: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance (accessed on 15 August 2022).
- Shen, A.K.; Browne, S.; Srivastava, T.; Michel, J.J.; Tan, A.S.L.; Kornides, M.L. Factors Influencing Parental and Individual COVID-19 Vaccine Decision Making in a Pediatric Network. Vaccines 2022, 10, 1277. [Google Scholar] [CrossRef]
- Weaver, J.L.; Swank, J.M. Parents’ Lived Experiences With the COVID-19 Pandemic. Fam. J. 2020, 29, 136–142. [Google Scholar] [CrossRef]
- Abrams, E.M.; Greenhawt, M.; Shaker, M.; Pinto, A.D.; Sinha, I.; Singer, A. The COVID-19 Pandemic: Adverse Effects on the Social Determinants of Health in Children and Families. Ann. Allergy Asthma Immunol. 2022, 128, 19–25. [Google Scholar] [CrossRef]
- Kaiser Family Foundation KFF COVID-19 Vaccine Monitor Dashboard|KFF. Available online: https://www.kff.org/coronavirus-covid-19/dashboard/kff-covid-19-vaccine-monitor-dashboard/ (accessed on 9 May 2022).
- Rane, M.S.; Robertson, M.M.; Westmoreland, D.A.; Teasdale, C.A.; Grov, C.; Nash, D. Intention to Vaccinate Children Against COVID-19 Among Vaccinated and Unvaccinated US Parents. JAMA Pediatr. 2022, 176, 201–203. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Shah, M.D.; Delgado, J.R.; Thomas, K.; Vizueta, N.; Cui, Y.; Vangala, S.; Shetgiri, R.; Kapteyn, A. Parents’ Intentions and Perceptions About COVID-19 Vaccination for Their Children: Results From a National Survey. Pediatrics 2021, 148, e2021052335. [Google Scholar] [CrossRef]
- Salomoni, M.G.; di Valerio, Z.; Gabrielli, E.; Montalti, M.; Tedesco, D.; Guaraldi, F.; Gori, D. Hesitant or Not Hesitant? A Systematic Review on Global COVID-19 Vaccine Acceptance in Different Populations. Vaccines 2021, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- The SAGE Working Group on Vaccine Hesitancy. Report of the SAGE Working Group on Vaccine Hesitancy; World Health Organization: Geneva, Switzerland, 2014.
- Aw, J.; Seng, J.J.B.; Seah, S.S.Y.; Low, L.L. COVID-19 Vaccine Hesitancy—A Scoping Review of Literature in High-Income Countries. Vaccines 2021, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.B.; Bell, R.A. Parental COVID-19 Vaccine Hesitancy in the United States. Public Health Rep. 2022, 333549221114346. [Google Scholar] [CrossRef]
- Savoia, E.; Piltch-Loeb, R.; Goldberg, B.; Miller-Idriss, C.; Hughes, B.; Montrond, A.; Kayyem, J.; Testa, M.A. Predictors of COVID-19 Vaccine Hesitancy: Socio-Demographics, Co-Morbidity, and Past Experience of Racial Discrimination. Vaccines 2021, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Helmkamp, L.J.; Szilagyi, P.G.; Zimet, G.; Saville, A.W.; Gurfinkel, D.; Albertin, C.; Breck, A.; Vangala, S.; Kempe, A. A Validated Modification of the Vaccine Hesitancy Scale for Childhood, Influenza and HPV Vaccines. Vaccine 2021, 39, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Kempe, A.; Saville, A.W.; Albertin, C.; Zimet, G.; Breck, A.; Helmkamp, L.; Vangala, S.; Dickinson, L.M.; Rand, C.; Humiston, S.; et al. Parental Hesitancy About Routine Childhood and Influenza Vaccinations: A National Survey. Pediatrics 2020, 146, e20193852. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Albertin, C.S.; Gurfinkel, D.; Saville, A.W.; Vangala, S.; Rice, J.D.; Helmkamp, L.; Zimet, G.D.; Valderrama, R.; Breck, A.; et al. Prevalence and Characteristics of HPV Vaccine Hesitancy among Parents of Adolescents across the US. Vaccine 2020, 38, 6027–6037. [Google Scholar] [CrossRef]
- Feemster, K.A.; Li, Y.; Grundmeier, R.; Localio, A.R.; Metlay, J.P. Validation of a Pediatric Primary Care Network in a US Metropolitan Region as a Community-Based Infectious Disease Surveillance System. Interdiscip. Perspect. Infect. Dis. 2011, 2011, 219859. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Vaccine Confidence Survey Question Bank. Centers for Disease Control and Prevention; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- Martin, L.R.; Petrie, K.J. Understanding the Dimensions of Anti-Vaccination Attitudes: The Vaccination Attitudes Examination (VAX) Scale. Ann. Behav. Med. 2017, 51, 652–660. [Google Scholar] [CrossRef]
- Lee, S.A. Coronavirus Anxiety Scale: A Brief Mental Health Screener for COVID-19 Related Anxiety. Death Stud. 2020, 44, 393–401. [Google Scholar] [CrossRef]
- Rosen, L.D.; Whaling, K.; Carrier, L.M.; Cheever, N.A.; Rokkum, J. The Media and Technology Usage and Attitudes Scale: An Empirical Investigation. Comput. Hum. Behav. 2013, 29, 2501–2511. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Hudson, A.; Montelpare, W.J. Predictors of Vaccine Hesitancy: Implications for COVID-19 Public Health Messaging. Int. J. Environ. Res. Public Health 2021, 18, 8054. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.; Ozkaya-Parlakay, A.; Senel, E. Evaluation of COVID-19 Vaccine Refusal in Parents. Pediatr. Infect. Dis. J. 2021, 40, E134–E136. [Google Scholar] [CrossRef]
- Kahn, K.E.; Santibanez, T.A.; Zhai, Y.; Bridges, C.B. Association between Provider Recommendation and Influenza Vaccination Status among Children. Vaccine 2018, 36, 3486. [Google Scholar] [CrossRef]
- Caldwell, A.C.; Madden, C.A.; Thompson, D.M.; Garbe, M.C.; Roberts, J.R.; Jacobson, R.M.; Darden, P.M. The Impact of Provider Recommendation on Human Papillomavirus Vaccine and Other Adolescent Vaccines. Hum. Vaccin. Immunother. 2021, 17, 1059–1067. [Google Scholar] [CrossRef]
- Mitropoulos, A.; Haslett, C. Fall COVID-19 Boosters Could Roll out Soon Pending Green Light from FDA, CDC-ABC News. Available online: https://abcnews.go.com/Health/fall-covid-19-boosters-roll-pending-green-light/story?id=88973334 (accessed on 30 August 2022).
- Guntuku, S.C.; Buttenheim, A.M.; Sherman, G.; Merchant, R.M. Twitter Discourse Reveals Geographical and Temporal Variation in Concerns about COVID-19 Vaccines in the United States. Vaccine 2021, 39, 4034–4038. [Google Scholar] [CrossRef]
- Sparks, G.; Hamel, L.; Kirzinger, A.; Stokes, M.; Brodie, M. KFF COVID-19 Vaccine Monitor: Differences in Vaccine Attitudes Between Rural, Suburban, and Urban Areas|KFF. Available online: https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-vaccine-attitudes-rural-suburban-urban/ (accessed on 14 August 2022).
- MIT Election Data and Science Lab U.S. President 1976–2020. Available online: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/42MVDX (accessed on 14 August 2022).
- Shen, A.K.; Sobczyk, E.A.; Orenstein, W.A. Building Layers of Trust Through Financial Coverage for COVID-19 Counseling/Health Affairs. Health Forefr. 2022. Available online: https://www.healthaffairs.org/do/10.1377/forefront.20220406.332885 (accessed on 14 August 2022).
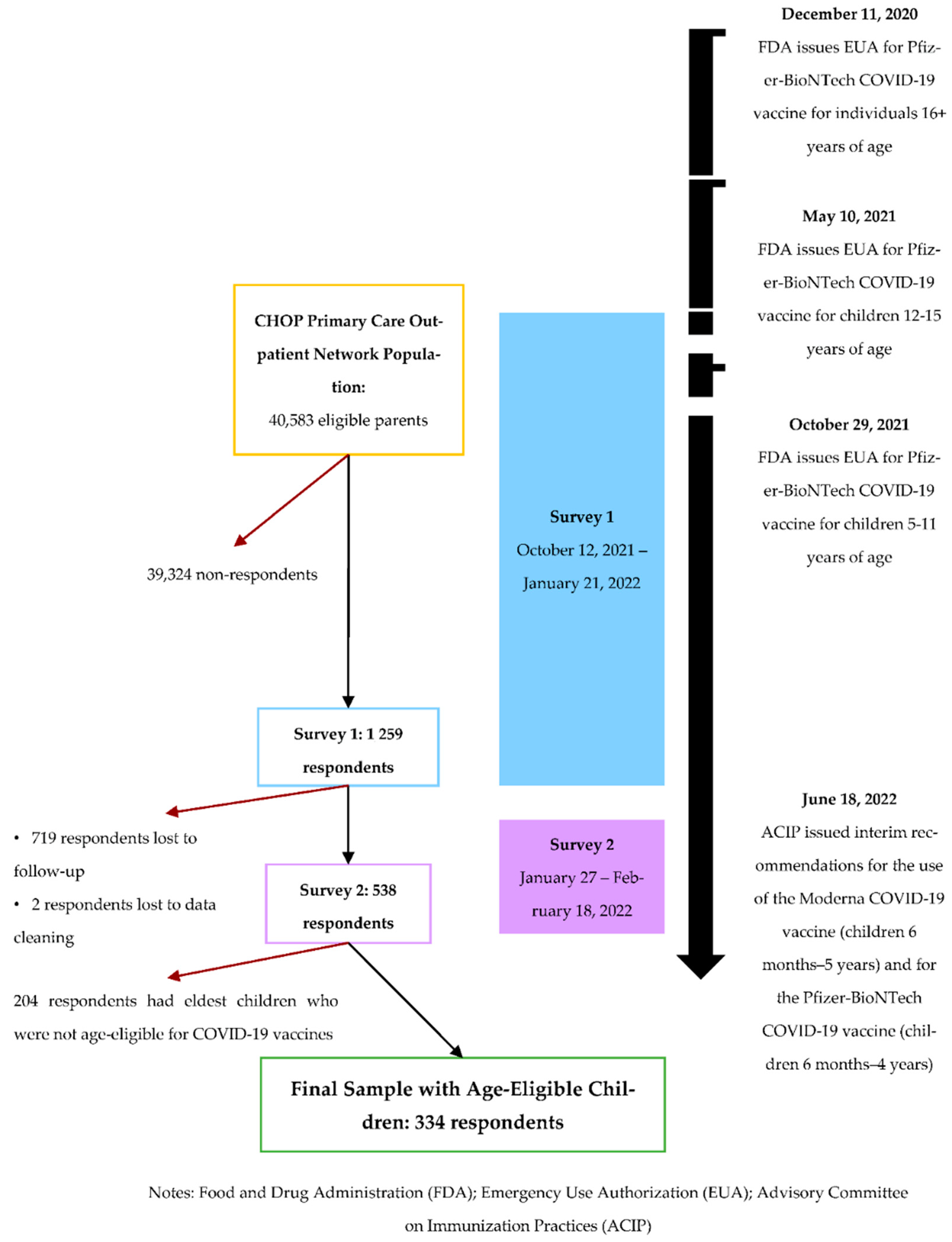

| Parental Characteristics | No, Is Age-Eligible But Did Not Receive Two Doses of COVID-19 Vaccine | Yes, Received Two Doses of COVID-19 Vaccine | Total | Χ2 p-Value |
|---|---|---|---|---|
| (n = 101) | (n = 233) | (n = 334) | ||
| Parental Age | 0.048 * | |||
| Under 35 years | 23 (22.8%) | 28 (12.0%) | 51 (15.3%) | |
| 35–38 years | 21 (20.8%) | 44 (18.9%) | 65 (19.5%) | |
| 38–43 years | 21 (20.8%) | 71 (30.5%) | 92 (27.5%) | |
| Over 43 years | 36 (35.6%) | 88 (37.8%) | 124 (37.1%) | |
| Sex (assigned at birth) | 0.745 | |||
| Male | 7 (6.9%) | 19 (8.2%) | 26 (7.8%) | |
| Female | 94 (93.1%) | 213 (91.4%) | 307 (91.9%) | |
| Gender Identity | 0.111 | |||
| Man | 7 (6.9%) | 19 (8.2%) | 26 (7.8%) | |
| Woman | 90 (89.1%) | 211 (90.6%) | 301 (90.1%) | |
| Nonbinary | 2 (2.0%) | 1 (0.4%) | 3 (0.9%) | |
| Prefer not to answer | 0 (0%) | 2 (0.9%) | 2 (0.6%) | |
| Other | 2 (2.0%) | 0 (0%) | 2 (0.6%) | |
| Sexual Orientation | 0.548 | |||
| Heterosexual or straight | 91 (90.1%) | 209 (89.7%) | 300 (89.8%) | |
| Gay or lesbian | 3 (3.0%) | 3 (1.3%) | 6 (1.8%) | |
| Bisexual | 3 (3.0%) | 9 (3.9%) | 12 (3.6%) | |
| Prefer not to answer | 2 (2.0%) | 10 (4.3%) | 12 (3.6%) | |
| Different identity | 2 (2.0%) | 2 (0.9%) | 4 (1.2%) | |
| Race | 0.962 | |||
| White or Caucasian | 71 (70.3%) | 167 (71.7%) | 238 (71.3%) | |
| Black or African American | 19 (18.8%) | 41 (17.6%) | 60 (18.0%) | |
| Other | 11 (10.9%) | 25 (10.7%) | 36 (10.8%) | |
| Hispanic Origin | 0.790 | |||
| Not Hispanic | 96 (95.0%) | 223 (95.7%) | 319 (95.5%) | |
| Hispanic | 5 (5.0%) | 10 (4.3%) | 15 (4.5%) | |
| Income | 0.936 | |||
| Under USD 150,000/year | 59 (58.4%) | 135 (57.9%) | 194 (58.1%) | |
| Over USD 150,000/year | 42 (41.6%) | 98 (42.1%) | 140 (41.9%) | |
| Education | 0.035 * | |||
| No college degree (High school diploma, GED, some college credit) | 27 (26.7%) | 39 (16.7%) | 66 (19.8%) | |
| College degree | 74 (73.3%) | 194 (83.3%) | 268 (80.2%) | |
| Insurance Type | 0.265 | |||
| Public | 32 (31.7%) | 60 (25.8%) | 92 (27.5%) | |
| Private | 69 (68.3%) | 173 (74.2%) | 242 (72.5%) | |
| Practice Type | 0.011 * | |||
| Philadelphia | 46 (45.5%) | 141 (60.5%) | 187 (56.0%) | |
| Suburban | 55 (54.5%) | 92 (39.5%) | 147 (44.0%) | |
| Number of Children | 0.968 # | |||
| Median [Min, Max] | 2 [1, 4] | 2 [1, 5] | 2 [1, 5] | |
| Child Age, Eldest Child | 0.068 # | |||
| Median [Min, Max] | 9 [1, 18] | 10 [1, 18] | 10 [1, 18] | |
| COVID-19 Vaccination Status, Parent | <0.001 * | |||
| Yes | 79 (78.2%) | 231 (99.1%) | 310 (92.8%) | |
| No | 22 (21.8%) | 2 (0.9%) | 24 (7.2%) | |
| First Dose of COVID-19 Vaccine Received, Eldest Child | <0.001 * | |||
| Yes, received | 12 (11.9%) | 233 (100%) | 245 (73.4%) | |
| No, but is eligible | 89 (88.1%) | - | 89 (26.6%) | |
| Influenza Vaccination Status, Parent | <0.001 * | |||
| Yes | 66 (65.3%) | 190 (81.5%) | 256 (76.6%) | |
| No | 35 (34.7%) | 42 (18.0%) | 77 (23.1%) | |
| Influenza Vaccination Status, Eldest Child | <0.001 * | |||
| Yes | 68 (67.3%) | 209 (89.7%) | 277 (82.9%) | |
| No | 33 (32.7%) | 24 (10.3%) | 57 (17.1%) | |
| Variables | Adjusted Odds Ratio (aOR) | [95% Confidence Interval] | p-Value | |
|---|---|---|---|---|
| Parental Age (Reference: Under 35 years) | ||||
| 35–38 years | 1.20 | 0.49 | 2.90 | 0.692 |
| 38–43 years | 1.77 | 0.73 | 4.31 | 0.206 |
| Over 43 years | 1.07 | 0.44 | 2.56 | 0.888 |
| Parental Education (Reference: No college degree) | ||||
| College degree | 2.12 | 1.04 | 4.36 | 0.039 * |
| Household Income (Reference: Under USD 150,000/year | ||||
| Over USD 150,000/year | 0.70 | 0.37 | 1.30 | 0.260 |
| Practice Location (Reference: Philadelphia) | ||||
| Suburban | 0.38 | 0.21 | 0.67 | 0.001 * |
| Child Age at Survey 1 (Reference: 16+ years) | ||||
| 12–15 years | 1.06 | 0.39 | 2.76 | 0.915 |
| 5–11 years | 0.55 | 0.20 | 1.39 | 0.216 |
| Under 5 Years | 0.32 | 0.09 | 1.04 | 0.061 |
| Child Influenza Vaccine Receipt 2021 (Reference: No) | ||||
| Yes | 4.07 | 2.08 | 8.12 | <0.001 * |
| COVID-19 Vaccine Safety Perception for Youngest Child (Reference: Not very safe) | ||||
| Very safe | 2.69 | 1.47 | 4.99 | 0.001 * |
| Child Vaccination Location Preference (Reference: Other or No Preference) | ||||
| At pediatrician/physician’s office | 1.90 | 1.09 | 3.34 | 0.024 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, T.; Shen, A.K.; Browne, S.; Michel, J.J.; Tan, A.S.L.; Kornides, M.L. Comparing COVID-19 Vaccination Outcomes with Parental Values, Beliefs, Attitudes, and Hesitancy Status, 2021–2022. Vaccines 2022, 10, 1632. https://doi.org/10.3390/vaccines10101632
Srivastava T, Shen AK, Browne S, Michel JJ, Tan ASL, Kornides ML. Comparing COVID-19 Vaccination Outcomes with Parental Values, Beliefs, Attitudes, and Hesitancy Status, 2021–2022. Vaccines. 2022; 10(10):1632. https://doi.org/10.3390/vaccines10101632
Chicago/Turabian StyleSrivastava, Tuhina, Angela K. Shen, Safa Browne, Jeremy J. Michel, Andy S. L. Tan, and Melanie L. Kornides. 2022. "Comparing COVID-19 Vaccination Outcomes with Parental Values, Beliefs, Attitudes, and Hesitancy Status, 2021–2022" Vaccines 10, no. 10: 1632. https://doi.org/10.3390/vaccines10101632
APA StyleSrivastava, T., Shen, A. K., Browne, S., Michel, J. J., Tan, A. S. L., & Kornides, M. L. (2022). Comparing COVID-19 Vaccination Outcomes with Parental Values, Beliefs, Attitudes, and Hesitancy Status, 2021–2022. Vaccines, 10(10), 1632. https://doi.org/10.3390/vaccines10101632






