Cost-Effectiveness Analysis of Cell Versus Egg-Based Seasonal Influenza Vaccination in Children and Adults in Argentina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Intervention and Comparators
2.2. Target Population
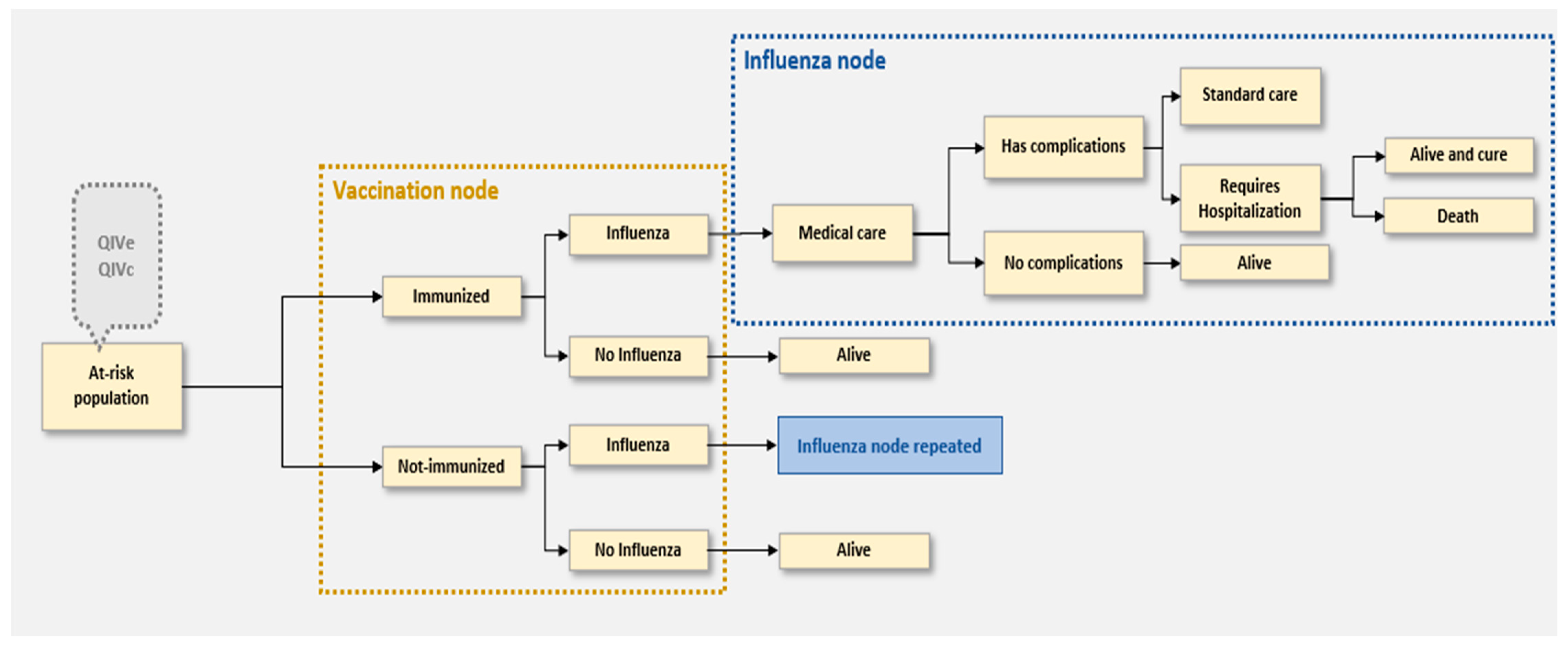
2.3. Model Structure
2.4. High Egg-Adaptation Scenario
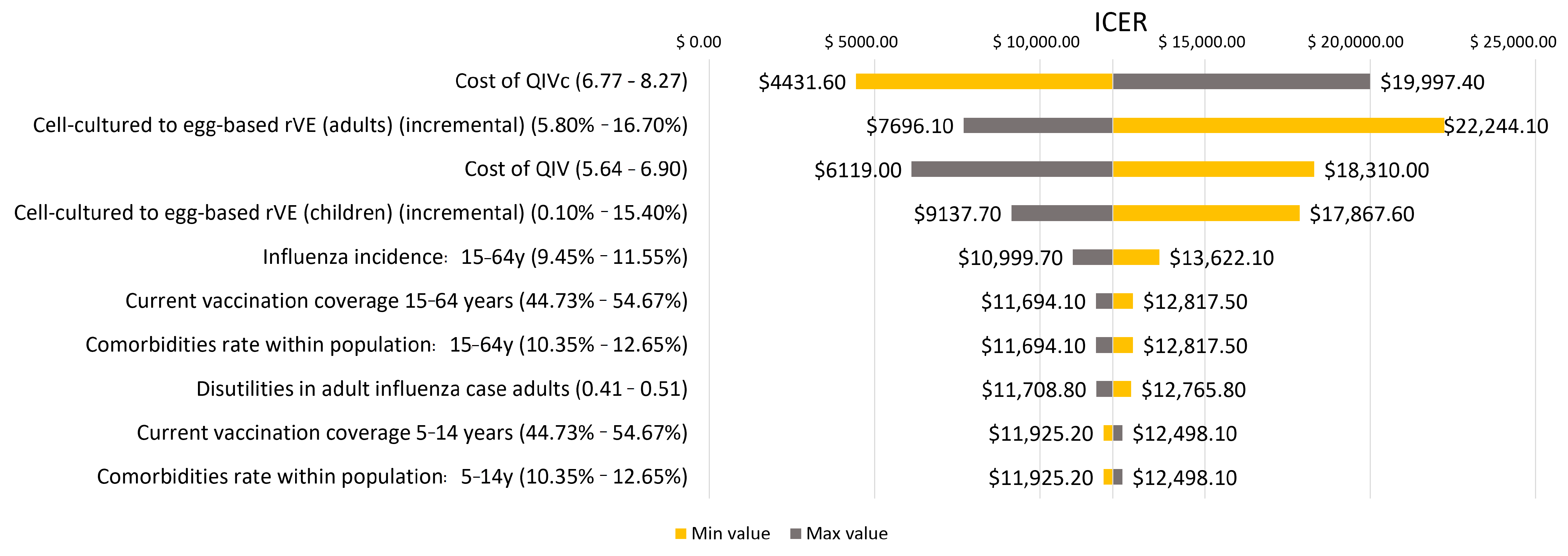
2.5. Sensitivity Analysis
| Parameter (Reference) | 6–23 Months (95% CI) | 2–4 Years Old (95% CI) | 5–14 Years Old (95% CI) | 15–64 Years Old (95% CI) |
|---|---|---|---|---|
| Estimated number of individuals at risk, based on 2021 Argentinean population [27] | 1,123,252 | 252,524 | 848,590 | 3,378,426 |
| Vaccination coverage (%) † [29] | 74.6% | 83.0% | 49.7% | 49.7% |
| Probability of influenza (influenza incidence) in unvaccinated subjects [4] | 5.7% (5.1–6.3%) | 7.5% (6.8–8.3%) | 8.6% (7.7–9.5%) | 10.5% (9.5–11.6%) |
| Probability of complication in subjects with comorbidities [43] | 18.29% (16.2–19.8%) | 18.29% (16.20–19.8%) | 18.29% (16.20–19.80%) | 12.45% (11.21–13.7%) |
| Probability of complication in subjects without comorbidities [25] | 14.1% (12.7–15.5%) | - | - | - |
| Probability of hospitalization in subjects with comorbidities [25,36] | 3.4% (3.0–3.7%) | 3.4% (3.0–3.7%) | 15.8% (14.2–17.4%) | 15.8% (14.2–17.4%) |
| Probability of hospitalization in subjects without comorbidities [25,36] | 3.4% (3.0–3.7%) | - | - | - |
| QIVe Absolute effectiveness against A strains [32] | 59.0% (53.1–64.9%) | 59.0% (53.1–64.9%) | 59.0% (53.1–64.9%) | 61.0% (9.5–67.1%) |
| QIVe Absolute effectiveness against B strains [32] | 66.0% (59.4–72.6%) | 66.0% (59.4–72.6%) | 77.0% (69.3–84.7%) | 76.0% (68.4–83.6%) |
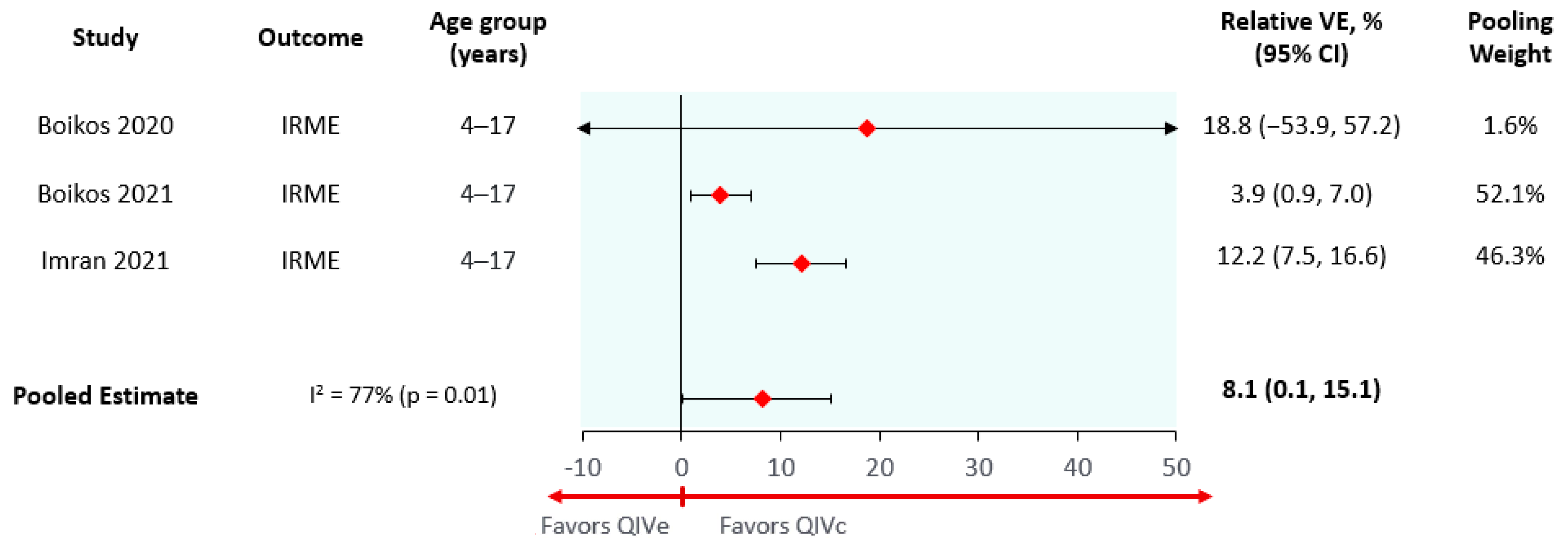
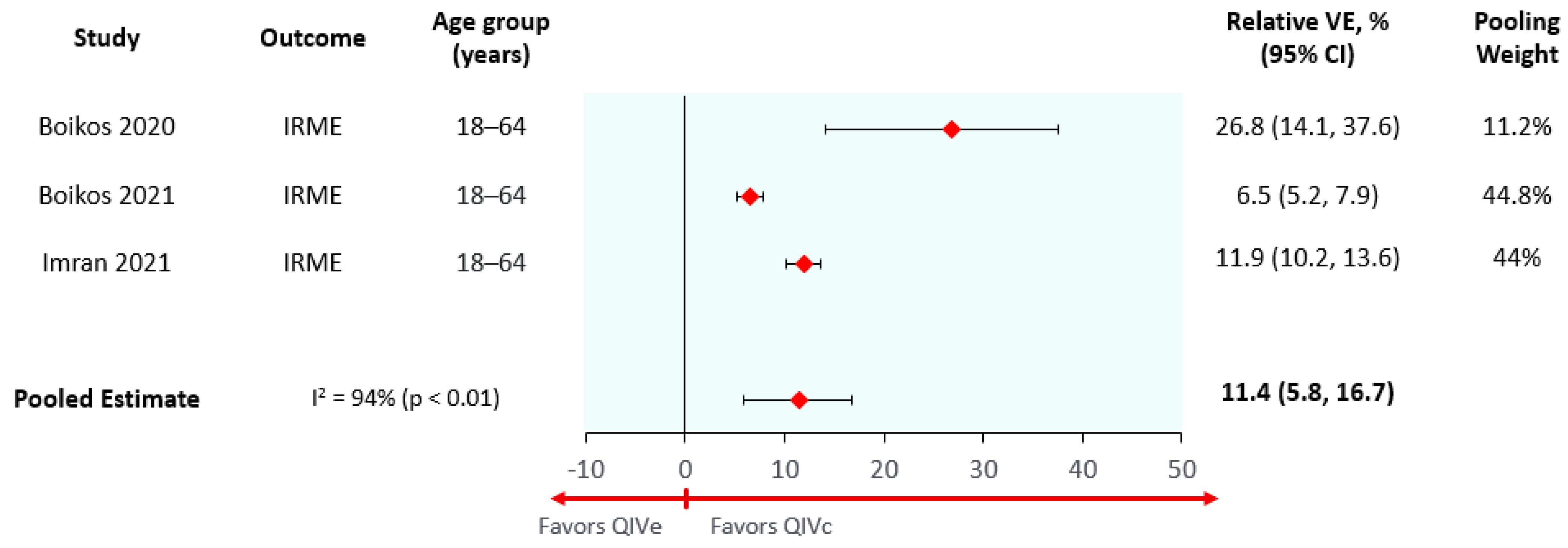
| Cell-based to egg-based relative vaccine effectiveness (average season) * | 8.1% (0.1–15.4%) | 8.1% (0.1–15.4%) | 8.1% (0.1–15.4%) | 11.4% (5.8–16.7%) |
| Cell-based to egg-based relative vaccine effectiveness (high egg-adaptation season) [34] | 18.8% (16.9–20.7%) | 18.8% (16.9–20.7%) | 18.8% (16.9–20.7%) | 26.8% (24.1–29.5%) |
| Daily disutility of an influenza case † [9] | 0.41 | 0.41 | 0.41 | 0.46 |
| Prevalence of comorbidities [44] | 11.5% | 11.5% | 11.5% | 11.5% |
| Daily disutility of influenza complication † [9] | 0.54 | 0.54 | 0.54 | 0.60 |
| Attack rate–Influenza A † [31] | 12.27% | 12.27% | 2.32% | 2.32% |
| Attack rate–Influenza B † [31] | 5.50% | 5.50% | 0.59% | 0.59% |
| Mortality on hospitalized influenza patients with complications † [36] | 1.66% | 4.00% | 4.00% | 4.00% |
| Mortality on hospitalized influenza patients without complications † [36] | 0.04% | 0.04% | 0.60% | 0.60% |
| Parameter (Reference) | Cost (95% CI) USD |
|---|---|
| QIVc Acquisition cost (per dose) * | 7.52 (6.77–8.27) |
| QIVe Acquisition Cost (per dose) [44] | 6.27 (5.64–6.90) |
| Average Hospitalization Cost (per event) [4] ** | 1970 (1773–2134) |
| Average Ambulatory influenza Costs–pediatric subjects without complications (per event) [45] ** | 17.56 (15.80–19.32) |
| Average Ambulatory influenza Costs–adult subjects without complications (per event) [46] ** | 51.79 (46.61–56.97) |
| Average Ambulatory influenza Costs–subjects with complications (per event) [46] | 53.50 (48.15–58.85) |
| Premature death costs—indirect costs (per year) [40] ** | 9734 (8760–10,707) |
| Daily absenteeism indirect costs [40] ** | 40.00 (36.00–44.00) |
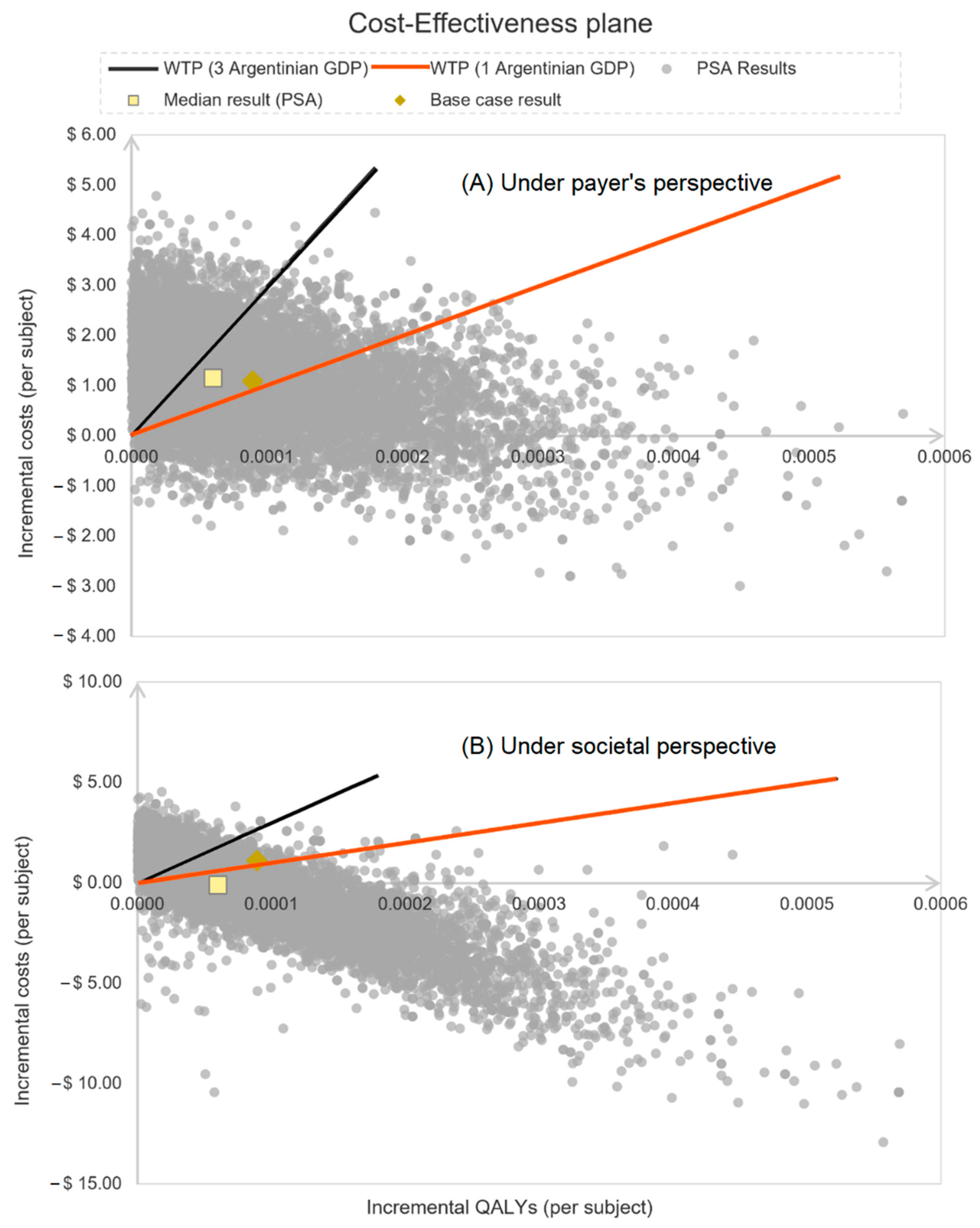
3. Results
High Egg-Adaptation Scenario
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Influenza (Seasonal)—WHO Fact Sheet on Influenza. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 6 February 2022).
- McElhaney, J.E. The Unmet Need in the Elderly: Designing New Influenza Vaccines for Older Adults. Vaccine 2005, 23, S10–S25. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of Myocardial Infarction and Stroke after Acute Infection or Vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Urueña, A.; Micone, P.; Magneres, C.; Mould-Quevedo, J.; Giglio, N. Cost-Effectiveness Analysis of Switching from Trivalent to Quadrivalent Seasonal Influenza Vaccine in Argentina. Vaccines 2021, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- National Ministry of Health Integrated Surveillance Bulletin for Years 2015–2019. Available online: http://www.argentina.gob.ar/salud/epidemiologia/boletines2019 (accessed on 4 May 2020).
- Kostova, D.; Reed, C.; Finelli, L.; Cheng, P.-Y.; Gargiullo, P.M.; Shay, D.K.; Singleton, J.A.; Meltzer, M.I.; Lu, P.; Bresee, J.S. Influenza Illness and Hospitalizations Averted by Influenza Vaccination in the United States, 2005–2011. PLoS ONE 2013, 8, e66312. [Google Scholar] [CrossRef] [PubMed]
- Rota, P.A.; Wallis, T.R.; Harmon, M.W.; Rota, J.S.; Kendal, A.P.; Nerome, K. Cocirculation of Two Distinct Evolutionary Lineages of Influenza Type B Virus since 1983. Virology 1990, 175, 59–68. [Google Scholar] [CrossRef]
- De Boer, P.T.; van Maanen, B.M.; Damm, O.; Ultsch, B.; Dolk, F.C.K.; Crépey, P.; Pitman, R.; Wilschut, J.C.; Postma, M.J. A Systematic Review of the Health Economic Consequences of Quadrivalent Influenza Vaccination. Expert Rev. Pharm. Outcomes Res. 2017, 17, 249–265. [Google Scholar] [CrossRef]
- García, A.; de Lejarazu, R.O.; Reina, J.; Callejo, D.; Cuervo, J.; Larragueta, R.M. Cost-Effectiveness Analysis of Quadrivalent Influenza Vaccine in Spain. Hum. Vaccines Immunother. 2016, 12, 2269–2277. [Google Scholar] [CrossRef]
- WHO. Recommended Composition of Influenza Virus Vaccines for Use in the 2013 Southern Hemisphere Influenza Season. Wkly. Epidemiol. Rec. 2012, 87, 389–400. [Google Scholar]
- Wu, N.C.; Zost, S.J.; Thompson, A.J.; Oyen, D.; Nycholat, C.M.; McBride, R.; Paulson, J.C.; Hensley, S.E.; Wilson, I.A. A Structural Explanation for the Low Effectiveness of the Seasonal Influenza H3N2 Vaccine. PLoS Pathog. 2017, 13, e1006682. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Janjua, N.Z.; De Serres, G.; Sabaiduc, S.; Eshaghi, A.; Dickinson, J.A.; Fonseca, K.; Winter, A.-L.; Gubbay, J.B.; Krajden, M.; et al. Low 2012-13 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3N2 Vaccine Strain Not Antigenic Drift in Circulating Viruses. PLoS ONE 2014, 9, e92153. [Google Scholar] [CrossRef]
- Rajaram, S.; Suphaphiphat, P.; van Boxmeer, J.; Haag, M.; Leav, B.; Iheanacho, I.; Kistler, K.; de Lejarazu, R.O. Retrospective Assessment of the Antigenic Similarity of Egg-Propagated and Cell Culture-Propagated Reference Influenza Viruses as Compared with Circulating Viruses across Influenza Seasons 2002–2003 to 2017–2018. Int. J. Environ. Res. Public Health 2020, 17, 5423. [Google Scholar] [CrossRef] [PubMed]
- Cell-Based Flu Vaccines|CDC—Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/flu/prevent/cell-based.htm (accessed on 6 February 2022).
- De Lejarazu-Leonardo, R.O.; Montomoli, E.; Wojcik, R.; Christopher, S.; Mosnier, A.; Pariani, E.; Garcia, A.T.; Fickenscher, H.; Gärtner, B.C.; Jandhyala, R.; et al. Estimation of Reduction in Influenza Vaccine Effectiveness due to Egg-Adaptation Changes-Systematic Literature Review and Expert Consensus. Vaccines 2021, 9, 1255. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Hilsky, Y.; Mould-Quevedo, J. The Epidemiological and Economic Impact of a Cell-Based Quadrivalent Influenza Vaccine in Adults in the US: A Dynamic Modeling Approach. Vaccines 2021, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Divino, V.; Anupindi, V.R.; DeKoven, M.; Mould-Quevedo, J.; Pelton, S.I.; Postma, M.J.; Levin, M.J. A Real-World Clinical and Economic Analysis of Cell-Derived Quadrivalent Influenza Vaccine Compared to Standard Egg-Derived Quadrivalent Influenza Vaccines During the 2019-2020 Influenza Season in the United States. Open Forum Infect. Dis. 2022, 9, ofab604. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aragón, J.; Gani, R.; Márquez, S.; Alvarez, P. Estimated Cost-Effectiveness and Burden of Disease Associated with Quadrivalent Cell-Based and Egg-Based Influenza Vaccines in Spain. Hum. Vaccines Immunother. 2020, 16, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Ballalai, I.; Toniolo, J.; Kfouri, R.; Vespa, G.; Magneres, C.; Mould-Quevedo, J.; Pires, B.; Angerami, R. Custo-efetividade da vacina contra influenza quadrivalente baseada em células comparada com a trivalente baseada em ovo do Programa Nacional de Imunizações brasileiro. JBES Braz. J. Health Econ. 2021, 13, 136–144. [Google Scholar] [CrossRef]
- Maschio, M.; Kohli, M.A.; Ashraf, M.; Drummond, M.F.; Weinstein, M.C.; Mould-Quevedo, J.F. An Economic Comparison of Influenza Vaccines Recommended for Use in Eligible Adults under 65 Years in the United Kingdom. Vaccines 2022, 10, 599. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Gerlier, L.; Eichner, M.; Schwehm, M.; Rajaram, S.; Mould-Quevedo, J.; Lamotte, M. Cost-Effectiveness of the Cell-Based Quadrivalent versus the Standard Egg-Based Quadrivalent Influenza Vaccine in Germany. J. Med. Econ. 2021, 24, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Divino, V.; Krishnarajah, G.; Pelton, S.I.; Mould-Quevedo, J.; Anupindi, V.R.; DeKoven, M.; Postma, M.J. A Real-World Study Evaluating the Relative Vaccine Effectiveness of a Cell-Based Quadrivalent Influenza Vaccine Compared to Egg-Based Quadrivalent Influenza Vaccine in the US during the 2017–2018 Influenza Season. Vaccine 2020, 38, 6334–6343. [Google Scholar] [CrossRef] [PubMed]
- Krishnarajah, G.; Divino, V.; Postma, M.J.; Pelton, S.I.; Anupindi, V.R.; DeKoven, M.; Mould-Quevedo, J. Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018-19 Influenza Season in the United States. Vaccines 2021, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de la Salud—Argentina Resolución 35/11—MS—Incorpórase Al Programa Nacional de Control de Enfermedades Inmunoprevenibles, Con Carácter Gratuito y Obligatorio, La Inmunización Con La Vacuna Antigripal Al Personal de Salud e Intégrase La Misma Al Calendario Nacional de Inmuni. Available online: http://servicios.infoleg.gob.ar/infolegInternet/anexos/175000-179999/178182/norma.htm (accessed on 7 January 2022).
- Van Bellinghen, L.-A.; Marijam, A.; de Araujo, G.T.B.; Gomez, J.; Van Vlaenderen, I. Cost-Utility of Quadrivalent versus Trivalent Influenza Vaccine in Brazil—Comparison of Outcomes from Different Static Model Types. Braz. J. Infect. Dis. 2018, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bianculli, P.M.; Bellier, L.; Mangado, I.O.; Pérez, C.G.; Mieres, G.; Lazarov, L.; Petitjean, A.; Dibarboure, H.; Lopez, J.G. Switching from Trivalent to Quadrivalent Inactivated Influenza Vaccines in Uruguay: A Cost-Effectiveness Analysis. Hum. Vaccines Immunother. 2022, 18, 2050653. [Google Scholar] [CrossRef]
- INDEC: Instituto Nacional de Estadística y Censos de la República Argentina Proyecciones Nacionales. Available online: https://www.indec.gob.ar/indec/web/Nivel4-Tema-2-24-84 (accessed on 9 March 2022).
- Ministerio de Salud de la Nación—Argentina Tercera Encuesta Nacional Para Factores de Riesgo de Enfermedades No Transmisibles. Available online: http://www.msal.gob.ar/images/stories/bes/graficos/0000000544cnt-2015_09_04_encuesta_nacional_factores_riesgo.pdf (accessed on 22 March 2022).
- Ministerio de la Salud—Argentina Coberturas de Vacunación. Available online: https://www.argentina.gob.ar/salud/inmunoprevenibles/coberturas-de-vacunacion (accessed on 6 January 2022).
- Banco Central de la Republica Argentina Tipos de Cambio. Available online: http://www.bcra.gov.ar/PublicacionesEstadisticas/Tipos_de_cambios.asp (accessed on 6 January 2022).
- Jayasundara, K.; Soobiah, C.; Thommes, E.; Tricco, A.C.; Chit, A. Natural Attack Rate of Influenza in Unvaccinated Children and Adults: A Meta-Regression Analysis. BMC Infect. Dis. 2014, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing Influenza Vaccine Efficacy against Mismatched and Matched Strains: A Systematic Review and Meta-Analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ortiz, J.R.; McLean, H.Q.; Fisher, L.; O’Brien, D.; Bonafede, M.; Mansi, J.A.; Boikos, C. Relative Effectiveness of Cell-Based versus Egg-Based Quadrivalent Influenza Vaccines in Children and Adolescents in the United States during the 2019-2020 Influenza Season. Pediatr. Infect. Dis. J. 2022, 41, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Boikos, C.; Sylvester, G.C.; Sampalis, J.S.; Mansi, J.A. Relative Effectiveness of the Cell-Cultured Quadrivalent Influenza Vaccine Compared to Standard, Egg-Derived Quadrivalent Influenza Vaccines in Preventing Influenza-like Illness in 2017–2018. Clin. Infect. Dis. 2020, 71, e665–e671. [Google Scholar] [CrossRef]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative Effectiveness of the Cell-Derived Inactivated Quadrivalent Influenza Vaccine Versus Egg-Derived Inactivated Quadrivalent Influenza Vaccines in Preventing Influenza-Related Medical Encounters During the 2018-2019 Influenza Season in the United Sta. Clin. Infect. Dis. 2021, 73, e692–e698. [Google Scholar] [CrossRef]
- Cromer, D.; van Hoek, A.J.; Jit, M.; Edmunds, W.J.; Fleming, D.; Miller, E. The Burden of Influenza in England by Age and Clinical Risk Group: A Statistical Analysis to Inform Vaccine Policy. J. Infect. 2014, 68, 363–371. [Google Scholar] [CrossRef]
- Hollmann, M.; Garin, O.; Galante, M.; Ferrer, M.; Dominguez, A.; Alonso, J. Impact of Influenza on Health-Related Quality of Life among Confirmed (H1N1)2009 Patients. PLoS ONE 2013, 8, e60477. [Google Scholar] [CrossRef]
- Bonvehí, P.; Querci, M.; Rüttimann, R.; Vidal, G.; Giglio, N. Impacto de Los Costos Médicos Directos Relacionados Con El Virus de Influenza Durante El Período 2006 a 2009 En Adultos En Un Hospital Universitario de Buenos Aires, Argentina (Abstract #28923). XXII Congreso de la Sociedad Argentina de Infectología. 2009. Available online: https://drive.google.com/file/d/1S52YFHsN-nuEo1HG37NzjmQ_p8lBe1y_/view?usp=sharing (accessed on 11 August 2022).
- Indec Índice de Precios Al Consumidor. Available online: https://www.indec.gob.ar/indec/web/Nivel4-Tema-3-5-31 (accessed on 6 January 2022).
- Boletín de Estadísticas Laborales (BEL)|Argentina.Gob.Ar. Available online: https://www.trabajo.gob.ar/estadisticas/bel/index.asp (accessed on 10 March 2022).
- Baltussen, R.M.P.M.; Adam, T.; Edejer, T.T.-T.; Hutubessy, R.C.W.; Acharya, A.; Evans, D.B.; Murray, C.J.L. (Eds.) WHO-CHOICE Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Levine, M.Z.; Martin, E.T.; Petrie, J.G.; Lauring, A.S.; Holiday, C.; Jefferson, S.; Fitzsimmons, W.J.; Johnson, E.; Ferdinands, J.M.; Monto, A.S. Antibodies Against Egg- and Cell-Grown Influenza A(H3N2) Viruses in Adults Hospitalized During the 2017–2018 Influenza Season. J. Infect. Dis. 2019, 219, 1904–1912. [Google Scholar] [CrossRef]
- Van Bellinghen, L.-A.; Meier, G.; Van Vlaenderen, I. The Potential Cost-Effectiveness of Quadrivalent versus Trivalent Influenza Vaccine in Elderly People and Clinical Risk Groups in the UK: A Lifetime Multi-Cohort Model. PLoS ONE 2014, 9, e98437. [Google Scholar] [CrossRef] [PubMed]
- PAHO Revolving Fund Vaccine Prices for 2020—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/documents/paho-revolving-fund-vaccine-prices-2020 (accessed on 10 March 2022).
- Castellano, V.; Giglio, N.; Gentile, A. Cost-Effectiveness of Influenza Vaccine in Healthy Children. In Proceedings of the 12th National Meeting Pediatric Research, Buenos Aires, Argentina, 2010. [Google Scholar]
- Nguyen, V.H.; Vizzotti, C.; Uruena, A.; Giglio, N.; Magneres, C.; Richmond, H. Cost-Effectiveness of Introducing an MF59-Adjuvanted Trivalent Influenza Vaccine for Older Adults in Argentina. Vaccine 2020, 38, 3682–3689. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Vesikari, T.; Szymczakiewicz-Multanowska, A.; Lattanzi, M.; Izu, A.; Groth, N.; Holmes, S. Clinical Efficacy of Cell Culture–Derived and Egg-derived Inactivated Subunit Influenza Vaccines in Healthy Adults. Clin. Infect. Dis. 2010, 51, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz Perez, S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 Influenza Viruses Have a Glycosylation Site That Alters Binding of Antibodies Elicited by Egg-Adapted Vaccine Strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [PubMed]
- Marseille, E.; Larson, B.; Kazi, D.S.; Kahn, J.G.; Rosen, S. Thresholds for the Cost–Effectiveness of Interventions: Alternative Approaches. Bull. World Health Organ. 2015, 93, 118–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Outcome | QIVc | QIVe | Difference (QIVc − QIVe) |
|---|---|---|---|
| Number of GP visits † | 312,010 | 329,552 | −17,541 |
| Number of complications *† | 43,478 | 45,896 | −2418 |
| Number of influenza-related hospitalizations | 6349 | 6664 | −316 |
| Number of influenza-related deaths | 248 | 261 | −12 |
| QALYs | 33,357,208 | 33,356,927 | 282 |
| Vaccination costs | USD 23,746,747.82 | USD 18,884,851.69 | USD 4,861,896.14 |
| Medical care costs | USD 27,272,877.34 | USD 28,696,051.82 | USD −1,423,174.48 |
| Absenteeism costs | USD 38,060,335.60 | USD 40,198,038.48 | USD −2,137,702.89 |
| Early death costs ** | USD 58,763,713.66 | USD 61,618,116.82 | USD −2,854,403.16 |
| Total direct costs | USD 51,019,625.16 | USD 47,580,903.51 | USD 3,438,721.65 |
| Total costs (both direct and indirect) | USD 147,843,674.42 | USD 149,397,058.81 | USD −1,553,384.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urueña, A.; Micone, P.; Magneres, M.C.; McGovern, I.; Mould-Quevedo, J.; Sarmento, T.T.R.; Giglio, N. Cost-Effectiveness Analysis of Cell Versus Egg-Based Seasonal Influenza Vaccination in Children and Adults in Argentina. Vaccines 2022, 10, 1627. https://doi.org/10.3390/vaccines10101627
Urueña A, Micone P, Magneres MC, McGovern I, Mould-Quevedo J, Sarmento TTR, Giglio N. Cost-Effectiveness Analysis of Cell Versus Egg-Based Seasonal Influenza Vaccination in Children and Adults in Argentina. Vaccines. 2022; 10(10):1627. https://doi.org/10.3390/vaccines10101627
Chicago/Turabian StyleUrueña, Analía, Paula Micone, María Cecilia Magneres, Ian McGovern, Joaquin Mould-Quevedo, Túlio Tadeu Rocha Sarmento, and Norberto Giglio. 2022. "Cost-Effectiveness Analysis of Cell Versus Egg-Based Seasonal Influenza Vaccination in Children and Adults in Argentina" Vaccines 10, no. 10: 1627. https://doi.org/10.3390/vaccines10101627
APA StyleUrueña, A., Micone, P., Magneres, M. C., McGovern, I., Mould-Quevedo, J., Sarmento, T. T. R., & Giglio, N. (2022). Cost-Effectiveness Analysis of Cell Versus Egg-Based Seasonal Influenza Vaccination in Children and Adults in Argentina. Vaccines, 10(10), 1627. https://doi.org/10.3390/vaccines10101627








