Therapeutic Role of Neutralizing Antibody for the Treatment against SARS-CoV-2 and Its Emerging Variants: A Clinical and Pre-Clinical Perspective
Abstract
:1. Introduction
2. Structure of a Neutralizing Antibody
3. Types of mAbs
3.1. mAb in Treatment of COVID-19
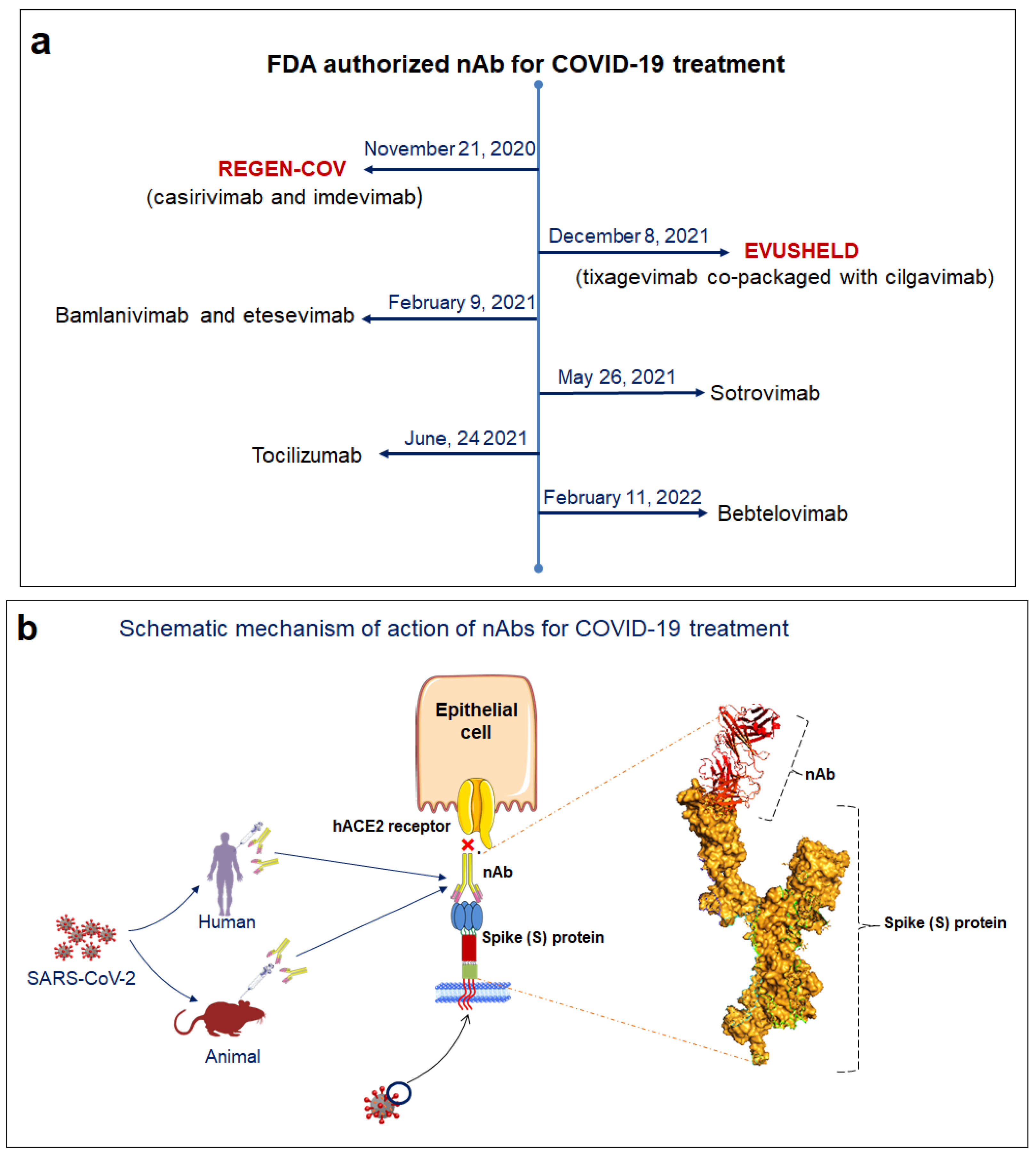
3.2. Mechanism of Action of SARS-CoV-2 nAb
4. Advantages of nAb over Vaccines and Convalescent Plasma Therapy
5. Different nAbs Employed for Treating SARS-CoV-2 That Are in Clinical Trials
5.1. JS016
5.2. MW33
5.3. CT-P59
5.4. REGEN-COV
5.5. LY3819253/LY-CoV555
5.6. VIR-7831
5.7. BGB DXP593
5.8. SCTA01
5.9. DZIF-10c
5.10. SAB-185
5.11. COR-101
5.12. Bamlanivimab and Etesevimab
6. nAbs Employed for Treating SARS-CoV-2 and Are in Pre-Clinical Trial
6.1. AR-712
6.2. IMM-BCP-01
6.3. SPKM001
7. New Emerging SARS-CoV-2 Variants and Possible Therapeutic Interventions
7.1. B.1.1.7 (Alpha)
7.2. B.1.351 (Beta)
7.3. P.1 (Gamma)
7.4. B.1.617.2 (Delta)
7.5. B.1.1.529 (Omicron)
8. Heavy Chain Antibodies (HCAbs) against SARS-CoV-2
9. Single Domain Antibody against SARS-CoV-2
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| nAb | Neutralizing Antibody |
| mAb | Monoclonal Antibody |
| RBD | Receptor binding domain |
| ACE2 | Angiotensin-converting enzyme 2 |
| S-protein | Spike glycoprotein |
| NTD | N-terminal domain |
| CPT | Convalescent plasma therapy |
| ADE | Antibody-dependent enhancement |
| HCAbs | Heavy chain antibodies |
| IL-6 | Interleukin 6 |
| CDRH | Complementarity-determining regions of heavy-chain |
| FDA | Food & Drug Administration |
| IgA | Immunoglobulin A |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| VH | Variable domain heavy chain |
| VOI | Variant of Interest |
| VOC | Variant of Concern |
References
- Bhattacharya, M.; Sharma, A.R.; Mallick, B.; Sharma, G.; Lee, S.-S.; Chakraborty, C. Immunoinformatics approach to understand molecular interaction between multi-epitopic regions of SARS-CoV-2 spike-protein with TLR4/MD-2 complex. Infect. Genet. Evol. 2020, 85, 104587. [Google Scholar] [CrossRef] [PubMed]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Sharma, G.; Lee, S.-S. Immunoinformatics approach for the identification and characterization of T cell and B cell epitopes towards the peptide-based vaccine against SARS-CoV-2. Arch. Med. Res. 2021, 52, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, S.; Adachi, Y.; Sato, T.; Tonouchi, K.; Sun, L.; Fukushi, S.; Yamada, S.; Kinoshita, H.; Nojima, K.; Kanno, T.; et al. Temporal maturation of neutralizing antibodies in COVID-19 convalescent individuals improves potency and breadth to circulating SARS-CoV-2 variants. Immunity 2021, 54, 1841–1852.e1844. [Google Scholar] [CrossRef]
- Cheedarla, N.; Hanna, L.E. Functional and Protective Role of Neutralizing Antibodies (NAbs) Against Viral Infections. In Recent Developments in Applied Microbiology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–93. [Google Scholar]
- Jiang, S.; Zhang, X.; Yang, Y.; Hotez, P.J.; Du, L. Neutralizing antibodies for the treatment of COVID-19. Nat. Biomed. Eng. 2020, 4, 1134–1139. [Google Scholar] [CrossRef]
- van der Heide, V. Neutralizing antibody response in mild COVID-19. Nat. Rev. Immunol. 2020, 20, 352. [Google Scholar] [CrossRef]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.-Y. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat. Commun. 2020, 11, 4059. [Google Scholar] [CrossRef]
- Suthar, M.S.; Zimmerman, M.G.; Kauffman, R.C.; Mantus, G.; Linderman, S.L.; Hudson, W.H.; Vanderheiden, A.; Nyhoff, L.; Davis, C.W.; Adekunle, O. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep. Med. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Woodruff, M.C.; Ramonell, R.P.; Nguyen, D.C.; Cashman, K.S.; Saini, A.S.; Haddad, N.S.; Ley, A.M.; Kyu, S.; Howell, J.C.; Ozturk, T. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020, 21, 1506–1516. [Google Scholar] [CrossRef]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef]
- Hurt, A.C.; Wheatley, A.K. Neutralizing Antibody Therapeutics for COVID-19. Viruses 2021, 13, 628. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S. A Detailed Overview of Immune Escape, Antibody Escape, Partial Vaccine Escape of SARS-CoV-2 and Their Emerging Variants With Escape Mutations. Front. Immunol. 2022, 13, 801522. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.P.; Sepkowitz, K.A. The myth of the medical breakthrough: Smallpox, vaccination, and Jenner reconsidered. Int. J. Infect. Dis. 1998, 3, 54–60. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, X.; Du, L. Therapeutic antibodies and fusion inhibitors targeting the spike protein of SARS-CoV-2. Expert Opin. Ther. Targets 2021, 25, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. Bayl. Univ. Med. Cent. Proc. 2005, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H. Remembering Emil von Behring: From tetanus treatment to antibody cooperation with phagocytes. MBio 2017, 8, 00117-17. [Google Scholar] [CrossRef]
- Bordon, Y. Milestone 2: The many sides of Paul Ehrlich. Nat. Milest. Antib. 2016, S, 6. [Google Scholar]
- Kugelberg, E. Searching for the antibody producers. Nat. Immunol. 2016, 17 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Ribatti, D. Edelman’s view on the discovery of antibodies. Immunol. Lett. 2015, 164, 72–75. [Google Scholar] [CrossRef]
- Inbar, D.; Hochman, J.; Givol, D. Localization of antibody-combining sites within the variable portions of heavy and light chains. Proc. Natl. Acad. Sci. USA 1972, 69, 2659–2662. [Google Scholar] [CrossRef]
- Plückthun, A. Antibody engineering. Curr. Opin. Biotechnol. 1991, 2, 238–246. [Google Scholar] [CrossRef]
- Demarest, S.J.; Hariharan, K.; Dong, J. Emerging antibody combinations in oncology. MAbs 2011, 3, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Glass, T.R.; Ohmura, N.; Saiki, H.; Sawadaishi, K.; Kataoka, C.; Takagi, Y.; Ohiwa, T. Development and characterization of new monoclonal antibodies specific for coplanar polychlorinated biphenyls. Anal. Chim. Acta 2004, 517, 161–168. [Google Scholar] [CrossRef]
- Qiu, H.; Yuan, X.Y.; Cabral, T.; Manguiat, K.; Robinson, A.; Wood, H.; Grant, C.; McQueen, P.; Westmacott, G.; Beniac, D.R.; et al. Development and characterization of SARS-CoV-2 variant-neutralizing monoclonal antibodies. Antivir. Res. 2021, 196, 105206. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.-T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R. Emerging mutations in the SARS-CoV-2 variants and their role in antibody escape to small molecule-based therapeutic resistance. Curr. Opin. Pharmacol. 2022, 62, 64–73. [Google Scholar] [CrossRef]
- Liu, L.D.; Lian, C.; Yeap, L.-S.; Meng, F.-L. The development of neutralizing antibodies against SARS-CoV-2 and their common features. J. Mol. Cell Biol. 2020, 12, 980–986. [Google Scholar] [CrossRef]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Chatterjee, S.; Sharma, A.R.; Agoramoorthy, G.; Chakraborty, C. D614G mutation and SARS-CoV-2: Impact on S-protein structure, function, infectivity, and immunity. Appl. Microbiol. Biotechnol. 2021, 105, 9035–9045. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.; Werbner, M.; Alter, J.; Safra, M.; Chomsky, E.; Lee, J.C.; Hada-Neeman, S.; Polonsky, K.; Nowell, C.J.; Clark, A.E. Multi-clonal SARS-CoV-2 neutralization by antibodies isolated from severe COVID-19 convalescent donors. PLoS Pathog. 2021, 17, e1009165. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Nambulli, S.; Xiao, Z.; Liu, H.; Sang, Z.; Duprex, W.P.; Schneidman-Duhovny, D.; Zhang, C.; Shi, Y. Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2. Science 2020, 370, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Boonnak, K.; Dambach, K.M.; Donofrio, G.C.; Tassaneetrithep, B.; Marovich, M.A. Cell type specificity and host genetic polymorphisms influence antibody-dependent enhancement of dengue virus infection. J. Virol. 2011, 85, 1671–1683. [Google Scholar] [CrossRef]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.-M.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sharma, A.R.; Patra, P.; Ghosh, P.; Sharma, G.; Patra, B.C.; Lee, S.S.; Chakraborty, C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J. Med. Virol. 2020, 92, 618–631. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sharma, A.R.; Patra, P.; Ghosh, P.; Sharma, G.; Patra, B.C.; Saha, R.P.; Lee, S.-S.; Chakraborty, C. A SARS-CoV-2 vaccine candidate: In-silico cloning and validation. Inform. Med. Unlocked 2020, 20, 100394. [Google Scholar] [CrossRef]
- Chen, J.; Gao, K.; Wang, R.; Nguyen, D.D.; Wei, G.-W. Review of COVID-19 antibody therapies. Annu. Rev. Biophys. 2021, 50, 1–30. [Google Scholar] [CrossRef]
- Jin, D.; Wei, J.; Sun, J. Analysis of the molecular mechanism of SARS-CoV-2 antibodies. Biochem. Biophys. Res. Commun. 2021, 566, 45–52. [Google Scholar] [CrossRef]
- Hussain, A.; Hasan, A.; Babadaei, M.M.N.; Bloukh, S.H.; Chowdhury, M.E.; Sharifi, M.; Haghighat, S.; Falahati, M. Targeting SARS-CoV2 spike protein receptor binding domain by therapeutic antibodies. Biomed. Pharmacother. 2020, 130, 110559. [Google Scholar] [CrossRef] [PubMed]
- Gavor, E.; Choong, Y.K.; Er, S.Y.; Sivaraman, H.; Sivaraman, J. Structural basis of SARS-CoV-2 and SARS-CoV–antibody interactions. Trends Immunol. 2020, 41, 1006–1022. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Ku, Z.; Xie, X.; Davidson, E.; Ye, X.; Su, H.; Menachery, V.D.; Li, Y.; Yuan, Z.; Zhang, X.; Muruato, A.E. Molecular determinants and mechanism for antibody cocktail preventing SARS-CoV-2 escape. Nat. Commun. 2021, 12, 469. [Google Scholar] [CrossRef]
- Harvala, H.; Robb, M.L.; Watkins, N.; Ijaz, S.; Dicks, S.; Patel, M.; Supasa, P.; Wanwisa, D.; Liu, C.; Mongkolsapaya, J. Convalescent plasma therapy for the treatment of patients with COVID-19: Assessment of methods available for antibody detection and their correlation with neutralising antibody levels. Transfus. Med. 2021, 31, 167–175. [Google Scholar] [CrossRef]
- Wu, X.; Li, N.; Wang, G.; Liu, W.; Yu, J.; Cao, G.; Wang, J.; Chen, Y.; Ma, J.; Wu, J. Tolerability, Safety, Pharmacokinetics, and Immunogenicity of a Novel SARS-CoV-2 Neutralizing Antibody, Etesevimab in Chinese Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, First-In-Human Phase 1 Study. Antimicrob. Agents Chemother. 2021, 65, e00350-21. [Google Scholar] [CrossRef]
- Meng, X.; Wang, P.; Xiong, Y.; Wu, Y.; Lin, X.; Lu, S.; Li, R.; Zhao, B.; Liu, J.; Zeng, S. Safety, tolerability, pharmacokinetic characteristics, and immunogenicity of MW33: A Phase 1 clinical study of the SARS-CoV-2 RBD-targeting monoclonal antibody. Emerg. Microbes Infect. 2021, 10, 1638–1648. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jang, Y.R.; Hong, J.H.; Jung, J.G.; Park, J.-H.; Streinu-Cercel, A.; Streinu-Cercel, A.; Săndulescu, O.; Lee, S.J.; Kim, S.H. Safety, Virologic Efficacy, and Pharmacokinetics of CT-P59, a Neutralizing Monoclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Protein: Two Randomized, Placebo-Controlled, Phase I Studies in Healthy Individuals and Patients With Mild SARS-CoV-2 Infection. Clin. Ther. 2021, 43, 1706–1727. [Google Scholar]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Xiao, J.; Hooper, A.T.; Hamilton, J.D.; Musser, B.J. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N. Engl. J. Med. 2021, 385, e81. [Google Scholar] [CrossRef]
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 229–237. [Google Scholar] [CrossRef]
- Tuccori, M.; Ferraro, S.; Convertino, I.; Cappello, E.; Valdiserra, G.; Blandizzi, C.; Maggi, F.; Focosi, D. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: Clinical pipeline. In MAbs; Taylor & Francis: Abingdon, UK, 2020; Volume 12, p. 1854149. [Google Scholar]
- Li, Y.; Qi, L.; Bai, H.; Sun, C.; Xu, S.; Wang, Y.; Han, C.; Li, Y.; Liu, L.; Cheng, X. Safety, tolerability, pharmacokinetics and immunogenicity of a monoclonal antibody (SCTA01) targeting SARS-CoV-2 in healthy adults: A randomized, double-blind, placebo-controlled, phase I study. Antimicrob. Agents Chemother. 2021, 65, e01063-21. [Google Scholar] [CrossRef]
- Halwe, S.; Kupke, A.; Vanshylla, K.; Liberta, F.; Gruell, H.; Zehner, M.; Rohde, C.; Krähling, V.; Gellhorn-Serra, M.; Kreer, C. Intranasal administration of a monoclonal neutralizing antibody protects mice against SARS-CoV-2 infection. Viruses 2021, 13, 1498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, H.; Egland, K.A.; Gilliland, T.C.; Dunn, M.D.; Luke, T.C.; Sullivan, E.J.; Klimstra, W.B.; Bausch, C.L.; Whelan, S.P. Human immunoglobulin from transchromosomic bovines hyperimmunized with SARS-CoV-2 spike antigen efficiently neutralizes viral variants. Hum. Vaccines Immunother. 2022, 18, 1940652. [Google Scholar] [CrossRef] [PubMed]
- Bertoglio, F.; Fühner, V.; Ruschig, M.; Heine, P.A.; Abassi, L.; Klünemann, T.; Rand, U.; Meier, D.; Langreder, N.; Steinke, S. A SARS-CoV-2 neutralizing antibody selected from COVID-19 patients binds to the ACE2-RBD interface and is tolerant to most known RBD mutations. Cell Rep. 2021, 36, 109433. [Google Scholar] [CrossRef] [PubMed]
- The Antibody Society. COVID-19 Biologics Tracker. 2021. Available online: https://www.antibodysociety.org/covid-19-biologics-tracker/ (accessed on 13 July 2022).
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.B.; Tedla, N.; Bull, R.A. Broadly-Neutralizing Antibodies Against Emerging SARS-CoV-2 Variants. Front. Immunol. 2021, 4025. [Google Scholar] [CrossRef]
- Geng, J.; Chen, L.; Yuan, Y.; Wang, K.; Wang, Y.; Qin, C.; Wu, G.; Chen, R.; Zhang, Z.; Wei, D. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma. Signal Transduct. Target. Ther. 2021, 6, 347. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2022, 399, 665–676. [Google Scholar] [CrossRef]
- Lee, S.; Jang, S.; Kang, J.; Park, S.B.; Han, Y.W.; Nam, H.; Kim, M.; Lee, J.; Cho, K.J.; Kim, J. MG1141A as a Highly Potent Monoclonal Neutralizing Antibody Against SARS-CoV-2 Variants. Front. Immunol. 2021, 12, 778829. [Google Scholar] [CrossRef]
- Boschi, C.; Colson, P.; Bancod, A.; Moal, V.; La Scola, B. Omicron variant escapes therapeutic mAbs including recently released Evusheld®, contrary to eight prior main VOC. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, e534–e535. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Valoriani, B.; Barbieri, C.; Occhineri, S.; Mazzetti, P.; Vatteroni, M.L.; Suardi, L.R.; Riccardi, N.; Pistello, M. Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern. Infect. Dis. Ther. 2021, 10, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Evans, J.P.; Chakravarthy, K.; Qu, P.; Reisinger, S.; Song, N.J.; Rubinstein, M.P.; Shields, P.G.; Li, Z.; Liu, S.L. COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 Omicron variant in patients with cancer. Cancer Cell. 2022, 40, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Murugan, N.A.; Srivastava, V. Improved binding affinity of omicron’s spike protein for the human angiotensin-converting enzyme 2 receptor is the key behind its increased virulence. Int. J. Mol. Sci. 2022, 23, 3409. [Google Scholar] [CrossRef] [PubMed]
- Bayani, F.; Hashkavaei, N.S.; Uversky, V.N.; Mozaffari-Jovin, S.; Sefidbakht, Y. Insights into the structural peculiarities of the N-terminal and receptor binding domains of the spike protein from the SARS-CoV-2 Omicron variant. Comput. Biol. Med. 2022, 147, 105735. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sharma, A.R.; Dhama, K.; Agoramoorthy, G.; Chakraborty, C. Omicron variant (B. 1.1. 529) of SARS-CoV-2: Understanding mutations in the genome, S-glycoprotein, and antibody-binding regions. GeroScience 2022, 44, 619–637. [Google Scholar] [CrossRef]
- Kozlov, M. Omicron overpowers key COVID antibody treatments in early tests. Nature 2021, 10, 20211221. [Google Scholar] [CrossRef]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.S.; Winkler, M.S.; et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 185, 447–456. [Google Scholar] [CrossRef]
- Tada, T.; Zhou, H.; Dcosta, B.M.; Samanovic, M.I.; Chivukula, V.; Herati, R.S.; Hubbard, S.R.; Mulligan, M.J.; Landau, N.R. Increased resistance of SARS-CoV-2 Omicron variant to neutralization by vaccine-elicited and therapeutic antibodies. EBioMedicine 2022, 78, 103944. [Google Scholar] [CrossRef]
- Kannan, S.R.; Spratt, A.N.; Sharma, K.; Chand, H.S.; Byrareddy, S.N.; Singh, K. Omicron SARS-CoV-2 variant: Unique features and their impact on pre-existing antibodies. J. Autoimmun. 2022, 126, 102779. [Google Scholar] [CrossRef]
- Huang, D.T.; McCreary, E.K.; Bariola, J.R.; Minnier, T.E.; Wadas, R.J.; Shovel, J.A.; Albin, D.; Marroquin, O.C.; Kip, K.E.; Collins, K. Effectiveness of casirivimab and imdevimab, and sotrovimab during Delta variant surge: A prospective cohort study and comparative effectiveness randomized trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Shafer, S.L.; Simoneaux, R. Omicron Therapeutics. ASA Monitor 2022, 86, 21–37. [Google Scholar] [CrossRef]
- Czajka, T.F.; Vance, D.J.; Mantis, N.J. Slaying SARS-CoV-2 one (single-domain) antibody at a time. Trends Microbiol. 2021, 29, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.C.; Ma, B.; Trinklein, N.D.; Schellenberger, U.; Osborn, M.J.; Ouisse, L.-H.; Boudreau, A.; Davison, L.M.; Harris, K.E.; Ugamraj, H.S. Multispecific antibody development platform based on human heavy chain antibodies. Front. Immunol. 2019, 9, 3037. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Aghamollaei, H.; Hosseindokht, M.; Heiat, M.; Razei, A.; Bakherad, H. Nanobodies, the potent agents to detect and treat the Coronavirus infections: A systematic review. Mol. Cell. Probes 2021, 55, 101692. [Google Scholar] [CrossRef]
- Chi, X.; Liu, X.; Wang, C.; Zhang, X.; Li, X.; Hou, J.; Ren, L.; Jin, Q.; Wang, J.; Yang, W. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting the spike receptor binding domain. Nat. Commun. 2020, 11, 4528. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Zhang, T.; Xu, J.; Shang, S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am. J. Physiol. -Gastrointest. Liver Physiol. 2020, 319, G245–G252. [Google Scholar] [CrossRef]
- Haga, K.; Takai-Todaka, R.; Matsumura, Y.; Song, C.; Takano, T.; Tojo, T.; Nagami, A.; Ishida, Y.; Masaki, H.; Tsuchiya, M. Nasal delivery of single-domain antibody improves symptoms of SARS-CoV-2 infection in an animal model. PLoS Pathog. 2021, 17, e1009542. [Google Scholar] [CrossRef]
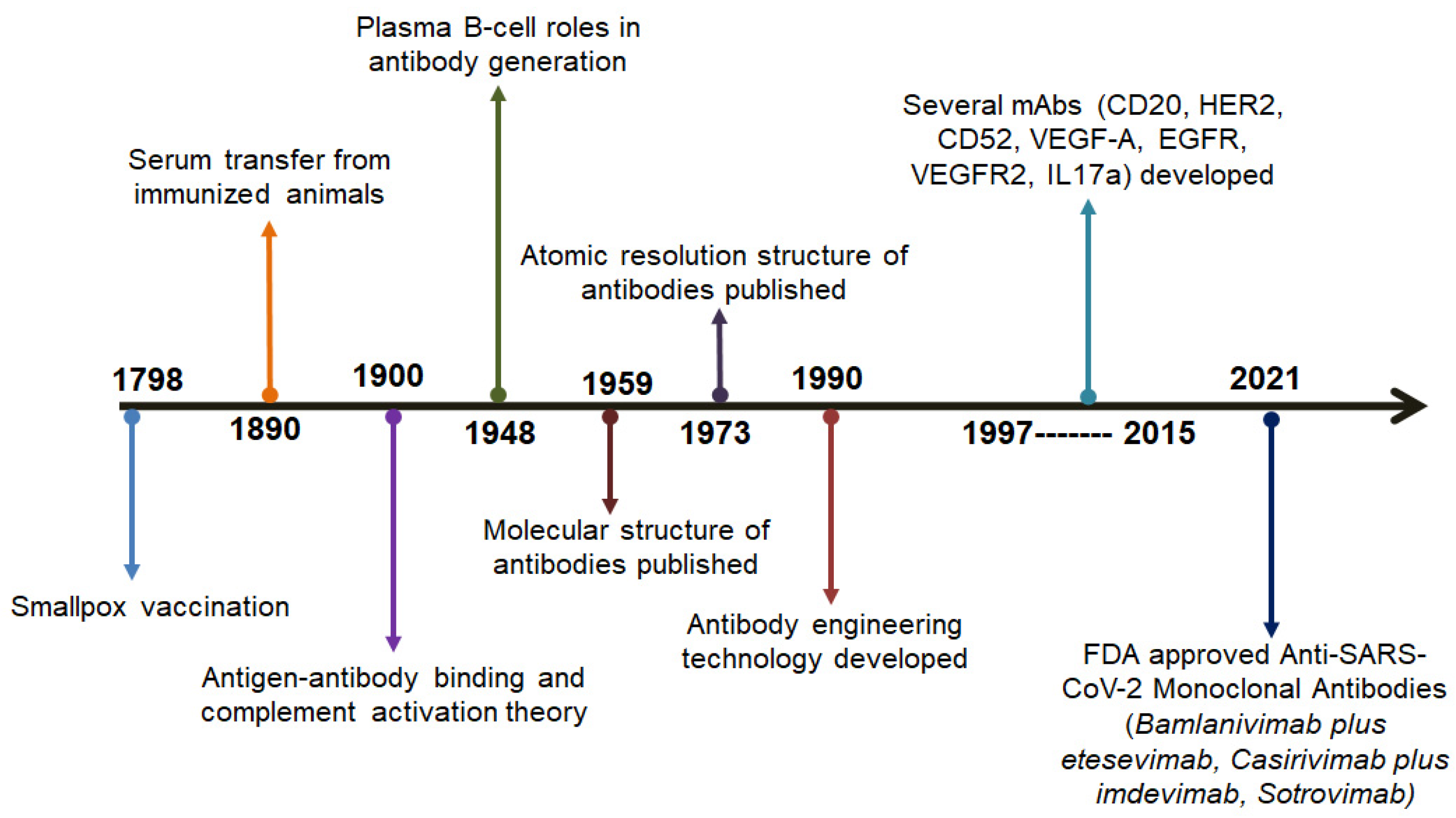








| Sl. No. | Year | Scientists Involved | Progress in the Development of Antibodies | Reference |
|---|---|---|---|---|
| 1. | 1798 | Edward Jenner | The breakthrough of the smallpox vaccine | [16] |
| 2. | 1890 | Emil von Behring and Shibasabura Kitasato | The transfer of serum to cure diphtheria taken from immunized animal | [17] |
| 3. | 1900 | Paul Ehrlich | The advancements of several concepts such as antigen-antibody binding, side-chain theory, and complement activation | [18] |
| 4. | 1948 | Astrid Fagraeus | Elucidated the importance of B cells | [19] |
| 5. | 1959 | Gerald Edelman and Rodney R. Porter | The publication of the molecular tructures of various antibodies | [20] |
| 6. | 1973 | D Inbar, J Hochman, and D Givol | The publication of the molecular tructures of the antibodies fragment | [21] |
| 7. | 1990 | A Plückthun | Antibody engineering | [22] |
| 8. | 1997–2015 | - | Development of various antibodies such as CD20, HER2, CD52, VEGF-A, EGFR, VEGFR2, and IL17A | [23,24] |
| 9. | 2021 | - | Development of anti-SARS-CoV-2 antibodies such as Bamlanivimab plus etesevimab, Casirivimab plus imdevimab, and Sotrovimab | [25] |
| Sl. No. | nAb | Trial No. | Status | Recruitment | No. of Participants | Sponsor | Country | Allocation | Remarks |
|---|---|---|---|---|---|---|---|---|---|
| 1. | JS016 | NCT04441918 | Phase II | Recruiting | 40 | Shanghai Junshi Bioscience Co., Ltd. | China | Randomized | A randomized, placebo-controlled study reporting its safety, pharmacokinetics, and immunogenicity administered in healthy subjects. |
| 2. | LY3832479 | NCT04441931 | Phase II | Completed | 26 | Eli Lilly and Company | United States | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of the mAb in healthy adult volunteers. |
| 3. | LY-CoV016 | NCT04427501 | Phase II | Active but not recruiting | 3290 | Eli Lilly and Company | United States | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 4. | TY027 | NCT04429529 | Phase III | Completed | 32 | Tychan Pte Ltd. | Singapore | Randomized Randomized | A randomized, placebo-controlled, time-lagged study conducted in healthy subjects. |
| NCT04649515 | Recruiting | 1305 | A randomised, placebo controlled study of TY027 aimed for treating COVID-19 patients. | ||||||
| 5. | BRII-196 | NCT04479631 | Phase III | Completed | 16 | Brii Biosciences Limited | China | Randomized | A randomized, placebo-controlled study of BRIL-196 monoclonal antibodies reporting its safety, tolerability, and pharmacokinetics. |
| 6. | BRII-198 | NCT04479644 | Phase III | Completed | 17 | Brii Biosciences Limited | China | Randomized | A randomized, placebo-controlled study of BRIL-196 monoclonal antibodies reporting its safety, tolerability, and pharmacokinetics. |
| 7. | CT-P63 | NCT05017168 | Phase I pending | Not yet recruiting | 24 | Celltrion | Poland | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 8. | XVR011 | NCT04884295 | Phase I | Recruiting | 279 | ExeVir Bio BV | Belgium and Italy | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 9. | ABBV-47D11 | NCT04644120 | Phase I | Completed | 25 | AbbVie | United States | Randomized | A randomized, placebo-controlled study of ABBV-47D11 and ABBV-2B04 monoclonal antibodies reporting its safety, pharmacodynamics, and pharmacokinetics. |
| 10. | HFB30132A | NCT04590430 | Phase I | Active but not recruiting | 24 | HiFiBiO Therapeutics | United States | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of the mAb in healthy adult volunteers. |
| 11. | ADM03820 | NCT04592549 | Phase I | Recruiting | 40 | Ology Bioservices | United States | Randomized | A randomized, placebo-controlled study reporting its safety, pharmacokinetics, and immunogenicity. |
| 12. | DXP604 | NCT04669262 | Phase I | Completed | 25 | BeiGene | Australia | Randomized | A randomized, placebo-controlled study reporting its safety, pharmacokinetics, and immunogenicity in healthy volunteers. |
| 13. | HLX70 | NCT04561076 | Phase I | Not yet | 24 | Hengenix Biotech Inc | United States | Randomized | A randomized, placebo-controlled study reporting its safety and pharmacokinetics. |
| 14. | COR-101 | NCT04674566 | Phase I and Phase II | Recruiting | 45 | Corat Therapeutics Gmbh | Germany | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics and immunogenicity of COR-101 in hospitalized COVID patients. |
| 15. | VIR-7832 | NCT04746183 | Phase I and Phase II | Recruiting | 600 | University of Liverpool | United Kingdom | Randomized | A randomized, placebo-controlled trial aimed to evaluate the efficacy of the drug in treating COVID-19 patients. |
| 16. | LY-CoV1404, LY3853113 | NCT04634409 | Phase II | Active but not recruiting | 1782 | Eli Lilly and Company | United States | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 17. | COVI-AMG (STI-2020) | NCT04734860 | Phase II | Recruiting | 500 | Sorrento Therapeutics, Inc. | United States | Randomized | A randomized, placebo-controlled study aimed to evaluate the safety and efficacy of the nAb in patients having mild COVID-19 symptoms. |
| 18. | DXP593 | NCT04532294 | Phase II | Completed | 18 | BeiGene | Australia | Randomized | A randomized, placebo-controlled study reporting its safety, pharmacokinetics, and immunogenicity in healthy volunteers. |
| NCT04551898 | 181 | United States | A randomized, placebo-controlled study highlighting the neutralizing efficiency of the BGBDXP593 mAb in COVID-19 patients having mild and moderate symptoms. | ||||||
| 19. | MW33 | NCT04533048 | Phase II | Completed | 42 | Mabwell (Shanghai) Bioscience Co., Ltd. | China | Randomized | A clinical study to evaluate the safety, pharmacokinetics, and immunogenicity of MW33 in normal, healthy volunteers. |
| NCT04627584 | Recruiting | 150 | A randomized, placebo-controlled study reporting the efficiency and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. | ||||||
| 20. | MAD0004J08 | NCT04932850 | Phase II and Phase III | Active but not recruiting | 30 | Toscana Life Sciences Sviluppo s.r.l. | Italy | Randomized | A randomized study to evaluate the safety, pharmacokinetics, and immunogenicity of MAD0004J08 in normal, healthy volunteers. |
| NCT04952805 | Recruiting | 800 | A randomized, placebo-controlled study aimed to evaluate the safety and efficacy profile of the antibody in adult COVID-19 volunteers who were asymptomatic, or had moderately severe symptoms. | ||||||
| 21. | C144-LS and C-135-LS | NCT04700163 | Phase II | Active but not recruiting | 23 | Rockefeller University | United States | Randomized | A randomized study to evaluate the safety, pharmacokinetics of two antibodies in normal, healthy volunteers. |
| 22. | SCTA01 | NCT04483375 | Phase II and Phase III | Completed | 33 | Sinocelltech Ltd. | China | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of SCTA01 in healthy adult volunteers. |
| NCT04644185 | Recruiting | 795 | United States | A randomized, placebo-controlled study employed for examining the efficiency of SCTA01 in COVID-19 affected subjects having severe symptoms. | |||||
| 23. | ADG20 | NCT04805671 | Phase II and Phase III | Recruiting | 1084 | Adagio Therapeutics, Inc. | Germany, Greece, Brazil, Argentina, Poland, Ukraine | Randomized | A randomized, placebo-controlled study reporting the efficacy of the ADG20 mAb in healthcare workers having mild to moderate symptoms. |
| NCT04859517 | 6412 | United States | A randomized, placebo controlled trial for evaluating the safety profile of the antibody in preventing SARS-CoV-2 infection. | ||||||
| 24. | AZD7442 (AZD8895 + AZD1061) | NCT04507256 | Phase III | Active but not recruiting | 60 | AstraZeneca | United Kingdom | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of AZD7442 in healthy adult volunteers. |
| NCT04625725 | 5197 | United States | A randomized, placebo-controlled study employed for evaluating the efficiency of AZD7442 in subjects who are not yet encountered by the SARS-CoV-2 virus. | ||||||
| NCT04625972 | 1121 | United States | A randomized, placebo-controlled study employed for evaluating the efficiency of AZD7442 in subjects who are already infected by the SARS-CoV-2 virus. | ||||||
| 25. | CT-P59 | NCT04525079 | EUA | Recruiting | 32 | Celltrion | Republic of Korea | Randomized | A randomized, placebo-controlled study reporting its safety and pharmacokinetics of CT-P59 in healthy volunteers. |
| NCT04593641 | Active but not recruiting | 18 | A randomized, Double-placebo-controlled, study reporting the viral nature, safety, and tolerability of the nAb in patients having mild symptoms. | ||||||
| NCT04602000 | Recruiting | 1020 | A randomized, placebo-controlled study employed for examining the efficiency of CT-P59 in COVID-19 affected subjects having severe symptoms. | ||||||
| 26. | VIR-7831 | NCT04545060 | EUA | Completed | 1057 | Vir Biotechnology, Inc. | United States | Randomized | A randomized, placebo-controlled study employed for evaluating the safety and efficiency of the nAb for treating COVID-19 patients who did not require any hospital support. |
| 27. | REGN-COV2 | NCT04425629 | EUA | Recruiting | 6420 | Regeneron Pharmaceuticals | United States | Randomized | A master protocol study reporting the safety and efficacy of the anti-spike mAbs in healthcare works affected with SARS-CoV-2 virus. |
| NCT04426695 | Completed | 2252 | A master protocol study reporting the safety and efficacy of the anti-spike mAbs in COVID-19 positive patients who required hospital support. | ||||||
| NCT04452318 | Active but not recruiting | 3750 | A randomized, placebo-controlled study evaluating the efficacy and the safety of the mAb in the household contacts to prevent SARS-CoV-2 infection. | ||||||
| 28. | LY-CoV555 (LY3819253); combination of LY-CoV555 with LY-CoV016 (LY3832479) | NCT04411628 | Phase I | Completed | 24 | Eli Lilly and Company | United States | Randomized | A randomized, placebo-controlled study reporting its safety, tolerability, and pharmacokinetics of LY3819253 in hospitalized COVID-19 patients. |
| NCT04427501 | Phase II | Recruiting | 3290 | A randomized, placebo-controlled study highlighting the neutralizing efficiency of the mAb in COVID-19 patients having mild and moderate symptoms. | |||||
| NCT04497987 | Phase III | Completed | 1374 | A randomized, placebo-controlled trial highlighting the safety and efficacy of the mAb alone and in combination to evaluate the immune response in nursing staffs to prevent SARS-CoV-2 infection. | |||||
| NCT04501978 | Phase III | Recruiting | 10,000 | University of Minnesota | A randomized, blinded controlled trial reporting the safety and efficacy of the COVID-19 positive patients who required hospital support. | ||||
| NCT04518410 | Phase II and Phase III | Recruiting | 8797 | National Institute of Allergy and Infectious Diseases (NIAID) | A randomized study evaluating the efficacy of LY3819253 in COVID-19 patients who did not require hospital support. | ||||
| 29. | Anti-SARS-CoV-2 mAb | NCT04748588 | Phase IV | Recruiting | 648 | University of Calgary | Canada | Randomized | A final trial aiming to evaluate the efficacy and safety of the antibody in the nosocomial COVID-19 patients in Canada. |
| 30. | BI 767551 | NCT04822701 | Phase II and Phase III | Active but not recruiting | 5 | Boehringer Ingelheim | United States | Randomized | A randomized, placebo-controlled study reporting the tolerability, efficiency, and safety profile of the antibody in COVID-19 patients with mild to moderate symptoms. |
| 31. | SAB- 185 | NCT04469179 | Phase I | Active but not recruiting | 21 | SAb Biotherap eutics, Inc. | United States | Randomized | A randomized study evaluating the efficacy of SAB-185 in COVID-19 patients who did not require hospital support. |
| 32. | Bamlanivimab | NCT04796402 | Phase IV | Active but not recruiting | 576 | Fraser Health | Canada | Randomized | A Phase IV study implicated for the emergency use of Bamlanivimab during the pandemic. |
| Sl. No. | nAb | International Nonpropreitary Name (INN) | Source | Type |
|---|---|---|---|---|
| 1. | LY-CoV555 | Bamlanivimab | Human B cells | mAb human IgG1 |
| 2. | JS016 | Etesevimab + Bamlanivimab | Human B cells | mAb human, combination of 2 mAb |
| 3. | LY-CoV016 | Etesevimab + Bamlanivimab | Human B cells | mAb human, combination of 2 mAb |
| 4. | LY3832479 | Etesevimab + Bamlanivimab | Human B cells | mAb human, combination of 2 mAb |
| 5. | REGN-COV2 | Casirivimab + Imdevimab | Convalescent sources and immunization | mAb human |
| 6. | TY027 | - | - | mAb |
| 7. | BRII-196 | - | Human B cells | mAb human |
| 8. | BRII-198 | - | Human B cells | mAb human |
| 9. | CT-P59 | Regdanvimab | Human B cells | mAb human |
| 10. | SCTA01 | - | - | mAb humanized |
| 11. | SAB- 185 | - | Immunization | Polyclonal recombinant human Ab |
| 12. | MW33 | - | - | mAb human |
| 13. | AZD7442 | Tixagevimab + Cilgavimab | Human B cells | mAb human |
| 14. | VIR-7831 | Sotrovimab | Human B cells | mAb human |
| 15. | DXP-593 | - | Human B cells | mAb |
| 16. | Anti-SARS-CoV-2 mAb | - | - | mAb, chicken IgY |
| 17. | ABBV-47D11 | - | Immunization | mAb human IgG1 |
| 18. | DXP604 | - | Human B cells | mAb |
| 19. | COVI-AMG (STI-2020) | - | In vitro libraries | mAb human |
| 20. | C144-LS and C-135-LS | - | - | Mixture of 2 mAb |
| 21. | ADG20 | - | Human B cells | mAb human |
| 22. | COR-101 | - | In vitro libraries and human B cells | mAb human |
| Sl. No. | Name of the Variant | Effective nAb against the SARS-CoV-2 Variants | Reference |
|---|---|---|---|
| 1. | B.1.1.7 (Alpha) | CD147 (Meplazumab), COVOX-222, COVOX-253, A23-58.1, MG1141A, Sotrovimab, Casirivimab + Imdevimab, Bamlanivimab + Etesevimab, Tixagevimab + Cilgavimeb | [59,60,61] |
| 2. | B.1.351 (Beta) | CD147 (Meplazumab), MG1141A, Casirivimab + Imdevimab, Sotrovimab, Tixagevimab + Cilgavimeb | [59,60,62] |
| 3. | P.1 (Gamma) | CD147 (Meplazumab), COVOX-222, COVOX-253, A23-58.1, Sotrovimab, Casirivimab + Imdevimab, MG1141A, Tixagevimab + Cilgavimeb | [59,60,62] |
| 4. | B.1.617.2 (Delta) | CD147 (Meplazumab), A23-58.1, Sotrovimab, Casirivimab + Imdevimab, Bamlanivimab + Etesevimab, Tixagevimab + Cilgavimeb | [60,62,67] |
| 5. | B.1.1.529 (Omicron) | Sotrovimab, Paxlovid, molnupiravir | [73,74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, M.; Chatterjee, S.; Mallik, B.; Sharma, A.R.; Chakraborty, C. Therapeutic Role of Neutralizing Antibody for the Treatment against SARS-CoV-2 and Its Emerging Variants: A Clinical and Pre-Clinical Perspective. Vaccines 2022, 10, 1612. https://doi.org/10.3390/vaccines10101612
Bhattacharya M, Chatterjee S, Mallik B, Sharma AR, Chakraborty C. Therapeutic Role of Neutralizing Antibody for the Treatment against SARS-CoV-2 and Its Emerging Variants: A Clinical and Pre-Clinical Perspective. Vaccines. 2022; 10(10):1612. https://doi.org/10.3390/vaccines10101612
Chicago/Turabian StyleBhattacharya, Manojit, Srijan Chatterjee, Bidyut Mallik, Ashish Ranjan Sharma, and Chiranjib Chakraborty. 2022. "Therapeutic Role of Neutralizing Antibody for the Treatment against SARS-CoV-2 and Its Emerging Variants: A Clinical and Pre-Clinical Perspective" Vaccines 10, no. 10: 1612. https://doi.org/10.3390/vaccines10101612
APA StyleBhattacharya, M., Chatterjee, S., Mallik, B., Sharma, A. R., & Chakraborty, C. (2022). Therapeutic Role of Neutralizing Antibody for the Treatment against SARS-CoV-2 and Its Emerging Variants: A Clinical and Pre-Clinical Perspective. Vaccines, 10(10), 1612. https://doi.org/10.3390/vaccines10101612










