Relationship between Humoral Response in COVID-19 and Seasonal Influenza Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Serum Samples
2.2. Determination of Anti-SARS-CoV-2-Specific IgG Antibodies
2.3. Statistical Analyses
3. Results
3.1. Demographic Characteristics
3.2. Prevalence of SARS-CoV-2-Specific IgG Antibodies
3.3. Titers of SARS-CoV-2-Specific IgG Antibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fink, A.L.; Klein, S.L. The Evolution of Greater Humoral Immunity in Females than Males: Implications for Vaccine Efficacy. Curr. Opin. Physiol. 2018, 6, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.B.; Varga, S.M. Effects of Aging on the Adaptive Immune Response to Respiratory Virus Infections. Aging Health 2009, 5, 775. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front Physiol. 2020, 11, 571416. [Google Scholar] [CrossRef]
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Hofmann Bowman, M.A. Impact of the Influenza Vaccine on COVID-19 Infection Rates and Severity. Am. J. Infect. Control. 2021, 49, 694–700. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Islam, N.; Dambha-Miller, H. Association between Influenza Vaccination and Hospitalisation or All-Cause Mortality in People with COVID-19: A Retrospective Cohort Study. BMJ Open Respir. Res. 2021, 8, e000857. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wang, H.; Sun, C.; Li, N.; Guo, X.; Song, Q.; Liang, Q.; Liang, M.; Ding, X.; Sun, Y. The Association between Previous Influenza Vaccination and COVID-19 Infection Risk and Severity: A Systematic Review and Meta-Analysis. Am. J. Prev. Med. 2022, 63, 121–130. [Google Scholar] [CrossRef]
- Escobar, L.E.; Molina-Cruz, A.; Barillas-Mury, C. BCG Vaccine Protection from Severe Coronavirus Disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA 2020, 117, 17720–17726. [Google Scholar] [CrossRef]
- Kovačić, D.; Gajić, A.A.; Latinović, D.; Softić, A. Hypothetical Immunological and Immunogenetic Model of Heterogenous Effects of BCG Vaccination in SARS-CoV-2 Infections: BCG-Induced Trained and Heterologous Immunity. J. Med. Sci. 2021, 90, e551. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin Induces NOD2-Dependent Nonspecific Protection from Reinfection via Epigenetic Reprogramming of Monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Moorlag, S.J.C.F.M.; Novakovic, B.; Li, Y.; Wang, S.-Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100e5. [Google Scholar] [CrossRef] [PubMed]
- Puro, V.; Castilletti, C.; Agrati, C.; Goletti, D.; Leone, S.; Agresta, A.; Cimini, E.; Tartaglia, E.; Casetti, R.; Colavita, F.; et al. Impact of Prior Influenza and Pneumoccocal Vaccines on Humoral and Cellular Response to SARS-CoV-2 BNT162b2 Vaccination. Vaccines 2021, 9, 615. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Cucci, F.; Portulano, P.; Lazzari, R.A.; Caldararo, C.; Sicuro, F.; Catanese, C.; Lobreglio, G. Effects of Influenza Vaccination on the Response to BNT162b2 Messenger RNA COVID-19 Vaccine in Healthcare Workers. J. Clin. Med. Res. 2021, 13, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Brydak, L.B.; Szymański, K.; Kondratiuk, K.; Poznańska, A.; Kolondra, A.; Hallmann, E. Importance of Influenza Anti-Hemagglutinin Antibodies during the SARS-CoV-2 Pandemic in the 2019/2020 Epidemic Season in Poland. Med. Sci. Monit. 2022, 28, e936495. [Google Scholar] [CrossRef] [PubMed]
- Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused Globally since Pandemic Start. Available online: https://nextstrain.org/ncov/gisaid/global/ (accessed on 22 September 2022).
- Hoang, V.-T.; Colson, P.; Levasseur, A.; Delerce, J.; Lagier, J.-C.; Parola, P.; Million, M.; Fournier, P.-E.; Raoult, D.; Gautret, P. Clinical Outcomes in Patients Infected with Different SARS-CoV-2 Variants at One Hospital during Three Phases of the COVID-19 Epidemic in Marseille, France. Infect. Genet. Evol. 2021, 95, 105092. [Google Scholar] [CrossRef]
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Rogalska, M.; Rorat, M.; Czupryna, P.; Lorenc, B.; Ciechanowski, P.; Kozielewicz, D.; Piekarska, A.; et al. Demographic and Clinical Overview of Hospitalized COVID-19 Patients during the First 17 Months of the Pandemic in Poland. J. Clin. Med. 2021, 11, 117. [Google Scholar] [CrossRef]
- Chen, X.; Pan, Z.; Yue, S.; Yu, F.; Zhang, J.; Yang, Y.; Li, R.; Liu, B.; Yang, X.; Gao, L.; et al. Disease Severity Dictates SARS-CoV-2-Specific Neutralizing Antibody Responses in COVID-Signal. Transduct. Target Ther. 2020, 5, 180. [Google Scholar] [CrossRef]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Montesinos, I.; Dahma, H.; Wolff, F.; Dauby, N.; Delaunoy, S.; Wuyts, M.; Detemmerman, C.; Duterme, C.; Vandenberg, O.; Martin, C.; et al. Neutralizing Antibody Responses Following Natural SARS-CoV-2 Infection: Dynamics and Correlation with Commercial Serologic Tests. J. Clin. Virol. 2021, 144, 104988. [Google Scholar] [CrossRef]
- Kubale, J.; Gleason, C.; Carreño, J.M.; Srivastava, K.; Singh, G.; PARIS Study Team; Gordon, A.; Krammer, F.; Simon, V. SARS-CoV-2 Spike-Binding Antibody Longevity and Protection from Reinfection with Antigenically Similar SARS-CoV-2 Variants. MBio 2022, e0178422. [Google Scholar] [CrossRef]
- Sasson, J.M.; Campo, J.J.; Carpenter, R.M.; Young, M.K.; Randall, A.Z.; Trappl-Kimmons, K.; Oberai, A.; Hung, C.; Edgar, J.; Teng, A.A.; et al. Diverse Humoral Immune Responses in Younger and Older Adult COVID-19 Patients. MBio 2021, 12, e0122921. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-H.; Zhao, R.; Zhou, J.-B.; Wang, F.; Kong, D.-G.; Sun, J.-B.; Ruan, Q.-F.; Liu, M.-Q. Serologic Response to SARS-CoV-2 in COVID-19 Patients with Different Severity. Virol. Sin. 2020, 35, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.M.; Faustini, S.E.; Perez-Toledo, M.; Jossi, S.; Allen, J.D.; Al-Taei, S.; Backhouse, C.; Dunbar, L.A.; Ebanks, D.; Emmanuel, B.; et al. Serological Responses to SARS-CoV-2 Following Non-Hospitalised Infection: Clinical and Ethnodemographic Features Associated with the Magnitude of the Antibody Response. BMJ Open Respir. Res. 2021, 8, e000872. [Google Scholar] [CrossRef] [PubMed]
- Fafi-Kremer, S.; Bruel, T.; Madec, Y.; Grant, R.; Tondeur, L.; Grzelak, L.; Staropoli, I.; Anna, F.; Souque, P.; Fernandes-Pellerin, S.; et al. Serologic Responses to SARS-CoV-2 Infection among Hospital Staff with Mild Disease in Eastern France. EBioMedicine 2020, 59, 102915. [Google Scholar] [CrossRef]
- Behrouzi, B.; Campoverde, M.V.A.; Liang, K.; Talbot, H.K.; Bogoch, I.I.; McGeer, A.; Fröbert, O.; Loeb, M.; Vardeny, O.; Solomon, S.D.; et al. Influenza Vaccination to Reduce Cardiovascular Morbidity and Mortality in Patients with COVID-19: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1777–1794. [Google Scholar] [CrossRef]
- Bujani, Z.M.; Behnampour, M.; Rahimi, N.; Safari, T.; Feizabad, K.A.; Sarbazi, A.H.; Baniasadi, M.; Rezaei, N.; Moghaddam, A.A. The Effect of Influenza Vaccination on COVID-19 Morbidity, Severity and Mortality: Systematic Review and Meta-Analysis. Malays. J. Med. Sci. 2021, 28, 20–31. [Google Scholar]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. B-Cell Activation by Armed Helper T Cells; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Debisarun, P.A.; Gössling, K.L.; Bulut, O.; Kilic, G.; Zoodsma, M.; Liu, Z.; Oldenburg, M.; Rüchel, N.; Zhang, B.; Xu, C.-J.; et al. Induction of Trained Immunity by Influenza Vaccination-Impact on COVID-19. PLoS Pathog. 2021, 17, e1009928. [Google Scholar] [CrossRef]
- Wagstaffe, H.R.; Pickering, H.; Houghton, J.; Mooney, J.P.; Wolf, A.-S.; Prevatt, N.; Behrens, R.H.; Holland, M.J.; Riley, E.M.; Goodier, M.R. Influenza Vaccination Primes Human Myeloid Cell Cytokine Secretion and NK Cell Function. J. Immunol. 2019, 203, 1609–1618. [Google Scholar] [CrossRef]
- Geckin, B.; Föhse, F.K.; Domínguez-Andrés, J.; Netea, M.G. Trained Immunity: Implications for Vaccination. Curr. Opin. Immunol. 2022, 77, 102190. [Google Scholar] [CrossRef]
- Lumley, S.F.; Wei, J.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; et al. The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers. Clin. Infect. Dis. 2021, 73, e699–e709. [Google Scholar] [CrossRef]
- Alfego, D.; Sullivan, A.; Poirier, B.; Williams, J.; Adcock, D.; Letovsky, S. A Population-Based Analysis of the Longevity of SARS-CoV-2 Antibody Seropositivity in the United States. EClinicalMedicine 2021, 36, 100902. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.-X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of Immune Responses to SARS-CoV-2 in Mild-Moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.K.; Yadav, R.; Chaudhary, P.K.; Maurya, A.; Kant, N.; Rugaie, O.A.; Haokip, H.R.; Yadav, D.; Roshan, R.; Prasad, R.; et al. Insights into COVID-19 Vaccine Development Based on Immunogenic Structural Proteins of SARS-CoV-2, Host Immune Responses, and Herd Immunity. Cells 2021, 10, 2949. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhuang, Z.; Zheng, J.; Li, K.; Wong, R.L.-Y.; Liu, D.; Huang, J.; He, J.; Zhu, A.; Zhao, J.; et al. Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment. Cell 2020, 182, 734–743.e5. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Bhatnagar, N.; Jeeva, S.; Oh, J.; Park, B.R.; Shin, C.H.; Wang, B.-Z.; Kang, S.-M. Immunogenicity and Neutralizing Activity Comparison of SARS-CoV-2 Spike Full-Length and Subunit Domain Proteins in Young Adult and Old-Aged Mice. Vaccines 2021, 9, 316. [Google Scholar] [CrossRef]
- Khare, S.; Azevedo, M.; Parajuli, P.; Gokulan, K. Conformational Changes of the Receptor Binding Domain of SARS-CoV-2 Spike Protein and Prediction of a B-Cell Antigenic Epitope Using Structural Data. Front Artif. Intell. 2021, 4, 630955. [Google Scholar] [CrossRef]
- Coppola, A.; Buonerba, C.; Cardinale, D.; Conte, G.L.; Sansone, D.; Rofrano, G.; Vita, S.D.; Morgante, M.; Triassi, M.; Atripaldi, L.; et al. Durability of Humoral Immune Responses to SARS-CoV-2 in Citizens of Ariano Irpino (Campania, Italy): A Longitudinal Observational Study with an 11.5-Month Follow-Up. Front Public Health 2021, 9, 801609. [Google Scholar] [CrossRef]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef]
- Planchais, C.; Fernández, I.; Bruel, T.; de Melo, G.D.; Prot, M.; Beretta, M.; Guardado-Calvo, P.; Dufloo, J.; Molinos-Albert, L.M.; Backovic, M.; et al. Potent Human Broadly SARS-CoV-2-Neutralizing IgA and IgG Antibodies Effective against Omicron BA.1 and BA.2. J. Exp. Med. 2022, 219, e20220638. [Google Scholar] [CrossRef]
- Zhou, P.; Song, G.; He, W.-T.; Beutler, N.; Tse, L.V.; Martinez, D.R.; Schäfer, A.; Anzanello, F.; Yong, P.; Peng, L.; et al. Broadly Neutralizing Anti-S2 Antibodies Protect against All Three Human Betacoronaviruses That Cause Severe Disease. bioRxiv 2022. [Google Scholar] [CrossRef]
- Pinto, D.; Sauer, M.M.; Czudnochowski, N.; Low, J.S.; Tortorici, M.A.; Housley, M.P.; Noack, J.; Walls, A.C.; Bowen, J.E.; Guarino, B.; et al. Broad Betacoronavirus Neutralization by a Stem Helix-Specific Human Antibody. Science 2021, 373, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yuan, M.; Song, G.; Beutler, N.; Shaabani, N.; Huang, D.; He, W.-T.; Zhu, X.; Callaghan, S.; Yong, P.; et al. A Human Antibody Reveals a Conserved Site on Beta-Coronavirus Spike Proteins and Confers Protection against SARS-CoV-2 Infection. Sci. Transl. Med. 2022, 14, eabi9215. [Google Scholar] [CrossRef] [PubMed]
- Budziar, W.; Gembara, K.; Harhala, M.; Szymczak, A.; Jędruchniewicz, N.; Baniecki, K.; Pikies, A.; Nahorecki, A.; Hoffmann, A.; Kardaś, A.; et al. Hidden Fraction of Polish Population Immune to SARS-CoV-2 in May 2021. PLoS ONE 2022, 17, e0253638. [Google Scholar] [CrossRef]
- Du, L.; He, Y.; Jiang, S.; Zheng, B.-J. Development of Subunit Vaccines against Severe Acute Respiratory Syndrome. Drugs Today 2008, 44, 63–73. [Google Scholar]
- Schoeman, D.; Fielding, B.C. Coronavirus Envelope Protein: Current Knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef]
- Venkatagopalan, P.; Daskalova, S.M.; Lopez, L.A.; Dolezal, K.A.; Hogue, B.G. Coronavirus Envelope (E) Protein Remains at the Site of Assembly. Virology 2015, 478, 75–85. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, M.; Liang, Z.; Zhang, L.; Wu, X.; Yang, C.; An, Y.; Tong, J.; Liu, S.; Li, T.; et al. Antigenicity Comparison of SARS-CoV-2 Omicron Sublineages with Other Variants Contained Multiple Mutations in RBD. MedComm 2022, 3, e130. [Google Scholar] [CrossRef]
- Rahmah, L.; Abarikwu, S.O.; Arero, A.G.; Essouma, M.; Jibril, A.T.; Fal, A.; Flisiak, R.; Makuku, R.; Marquez, L.; Mohamed, K.; et al. Oral Antiviral Treatments for COVID-19: Opportunities and Challenges. Pharmacol. Rep. 2022. [Google Scholar] [CrossRef]
- Flisiak, R.; Parczewski, M.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Pawłowska, M.; Piekarska, A.; Simon, K.; Tomasiewicz, K.; Zarębska-Michaluk, D. Management of SARS-CoV-2 Infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists. Annex No. 2 as of October 13, 2020. Pol. Arch. Intern. Med. 2020, 130, 915–918. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Jiang, D.; Zhou, P.; Cheng, L.; Li, Y.; Ma, X.; Jin, T. Serum IgA, IgM, and IgG Responses in COVID-Cell. Mol. Immunol. 2020, 17, 773–775. [Google Scholar] [CrossRef]
- Thompson, M.G.; Cowling, B.J. How Repeated Influenza Vaccination Effects Might Apply to COVID-19 Vaccines. Lancet Respir. Med. 2022, 10, 636–638. [Google Scholar] [CrossRef]
- Örtqvist, Å.; Brytting, M.; Leval, A.; Hergens, M.-P. Impact of Repeated Influenza Vaccinations in Persons over 65 Years of Age: A Large Population-Based Cohort Study of Severe Influenza over Six Consecutive Seasons, 2011/12-2016/17. Vaccine 2018, 36, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Murchu, E.O.; Byrne, P.; Carty, P.G.; Gascun, C.D.; Keogan, M.; O’Neill, M.; Harrington, P.; Ryan, M. Quantifying the Risk of SARS-CoV-2 Reinfection over Time. Rev. Med. Virol. 2022, 32, e2260. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Ali, K.M.; Fatah, M.H.; Tawfeeq, H.M.; Rostam, H.M. SARS-CoV-2 Reinfection in Patients Negative for Immunoglobulin G Following Recovery from COVID-19. New Microbes New Infect. 2021, 43, 100926. [Google Scholar] [CrossRef]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
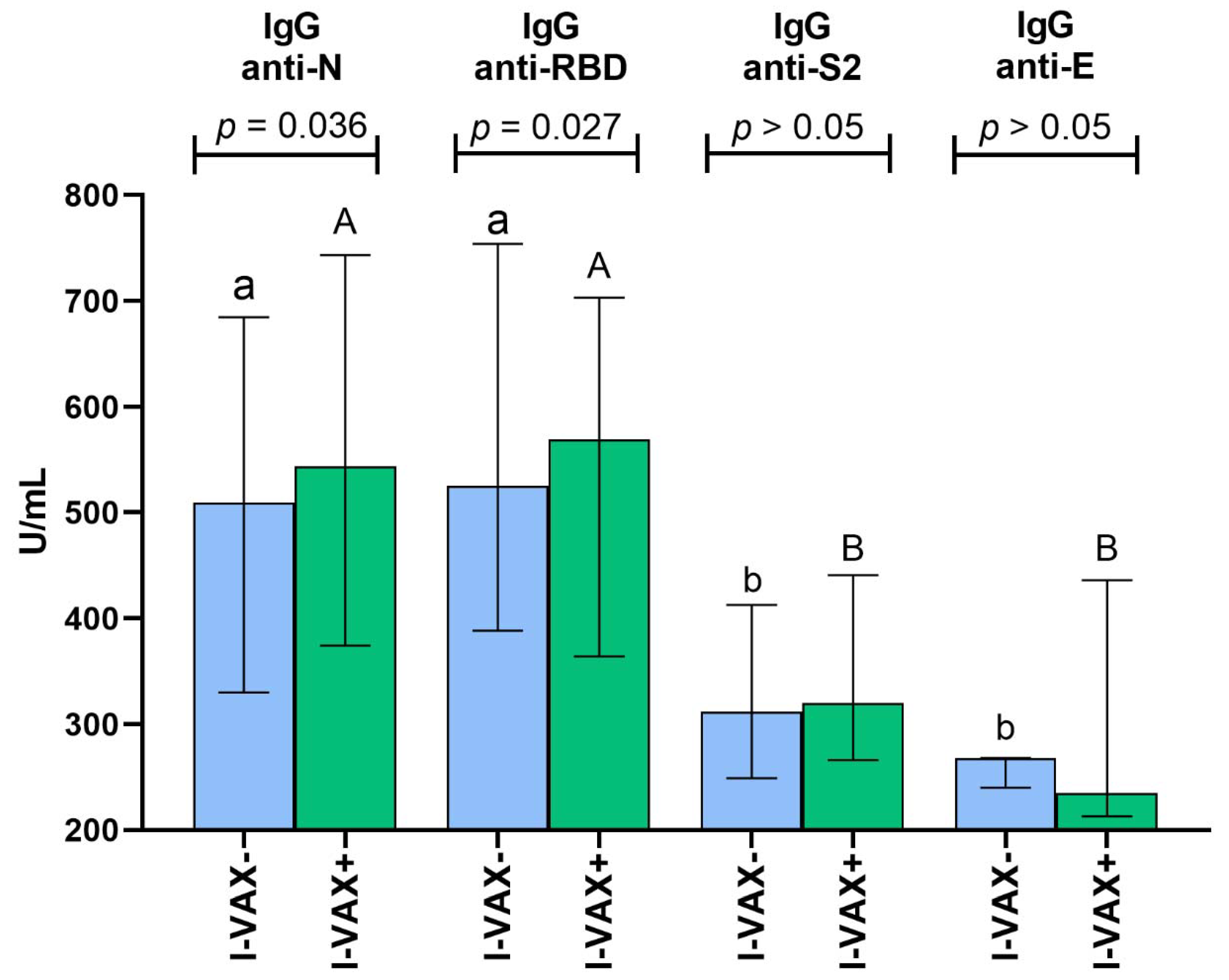
| Parameter | Unvaccinated against Influenza (n = 292) | Vaccinated against Influenza (n = 489) | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 35.8 ± 8.5 | 37.0 ± 10.3 | >0.05 |
| ≥50 years, % (n) | 5.1 (15) | 11.9 (58) | 0.002 |
| Women/men, % (n) | 17.1 (50)/82.9 (242) | 23.3 (114)/76.7 (375) | >0.05 |
| Comorbidities, % (n) | 1.7 (5) | 5.1 (25) | 0.02 |
| IgG Antibodies | Unvaccinated against Influenza (n = 292) | Vaccinated against Influenza (n = 489) | p-Value | Total (n = 781) |
|---|---|---|---|---|
| anti-N | 65.8 | 74.6 | 0.008 | 71.3 |
| anti-RBD | 76.7 | 85.1 | 0.001 | 81.6 |
| anti-S2 | 39.7 | 42.9 | >0.05 | 41.7 |
| anti-E | 1.7 | 1.4 | >0.05 | 1.5 |
| IgG Antibodies | Unvaccinated against Influenza (n = 292) | Vaccinated against Influenza (n = 489) | ||||||
|---|---|---|---|---|---|---|---|---|
| anti-N | anti-RBD | anti-S2 | anti-E | anti-N | anti-RBD | anti-S2 | anti-E | |
| anti-N | - | 0.56 | 0.24 | 0.15 | - | 0.38 | 0.21 | 0.14 |
| p < 0.05 | p < 0.05 | p > 0.05 | p < 0.05 | p < 0.05 | p > 0.05 | |||
| anti-RBD | - | - | 0.19 | 0.67 | - | - | 0.38 | 0.32 |
| p > 0.05 | p > 0.05 | p < 0.05 | p > 0.05 | |||||
| anti-S2 | - | - | - | 0.32 | - | - | - | 0.04 |
| p > 0.05 | p > 0.05 | |||||||
| anti-E | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poniedziałek, B.; Hallmann, E.; Sikora, D.; Szymański, K.; Kondratiuk, K.; Żurawski, J.; Rzymski, P.; Brydak, L. Relationship between Humoral Response in COVID-19 and Seasonal Influenza Vaccination. Vaccines 2022, 10, 1621. https://doi.org/10.3390/vaccines10101621
Poniedziałek B, Hallmann E, Sikora D, Szymański K, Kondratiuk K, Żurawski J, Rzymski P, Brydak L. Relationship between Humoral Response in COVID-19 and Seasonal Influenza Vaccination. Vaccines. 2022; 10(10):1621. https://doi.org/10.3390/vaccines10101621
Chicago/Turabian StylePoniedziałek, Barbara, Ewelina Hallmann, Dominika Sikora, Karol Szymański, Katarzyna Kondratiuk, Jakub Żurawski, Piotr Rzymski, and Lidia Brydak. 2022. "Relationship between Humoral Response in COVID-19 and Seasonal Influenza Vaccination" Vaccines 10, no. 10: 1621. https://doi.org/10.3390/vaccines10101621
APA StylePoniedziałek, B., Hallmann, E., Sikora, D., Szymański, K., Kondratiuk, K., Żurawski, J., Rzymski, P., & Brydak, L. (2022). Relationship between Humoral Response in COVID-19 and Seasonal Influenza Vaccination. Vaccines, 10(10), 1621. https://doi.org/10.3390/vaccines10101621











