Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Clinical Protocol
2.2. Vaccine
2.3. Randomization and Blinding
2.4. Outcomes
2.4.1. Assessment of Safety
2.4.2. Assessment of Immunogenicity
2.4.3. Statistical Analysis
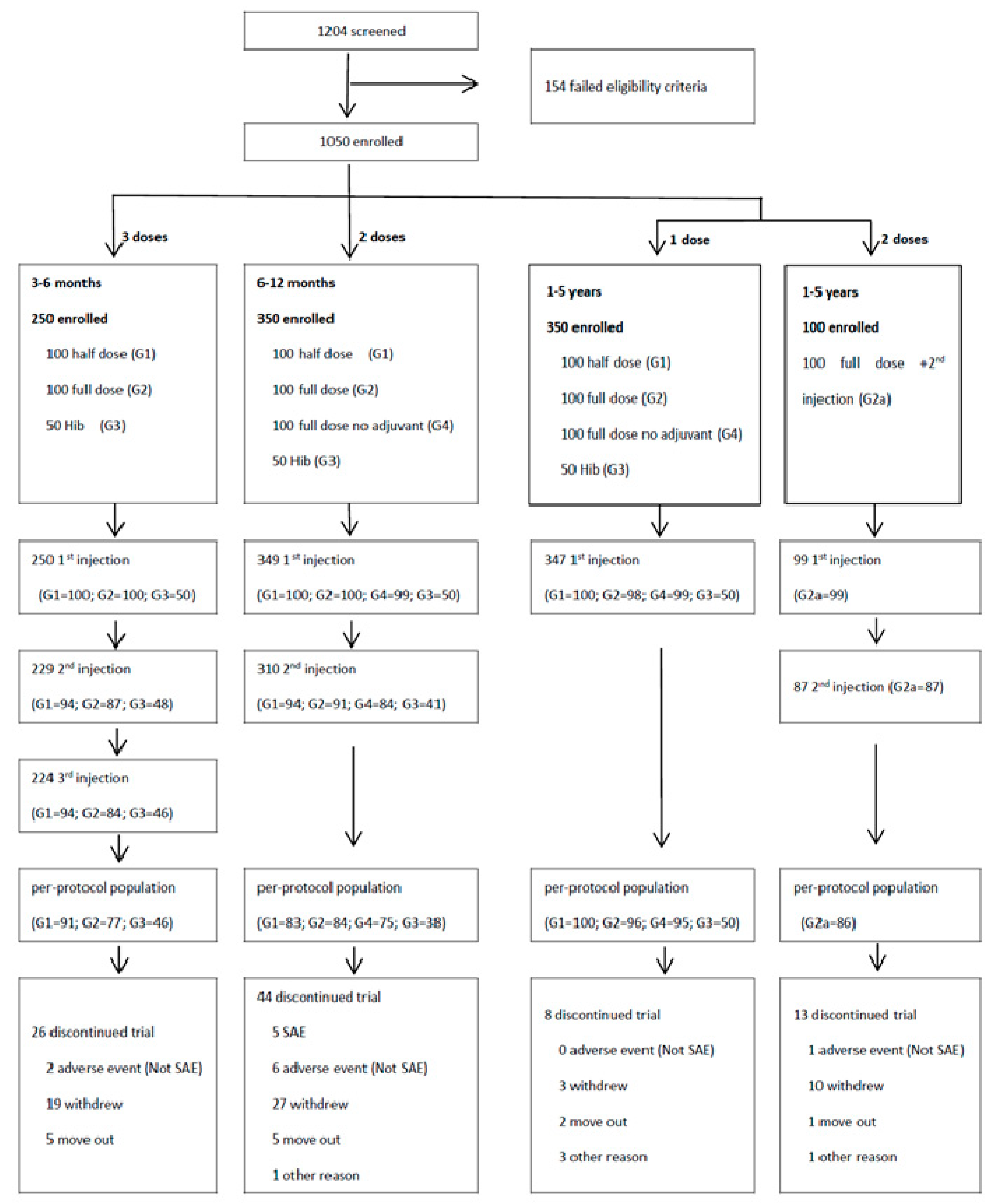
3. Results
3.1. Study Participants
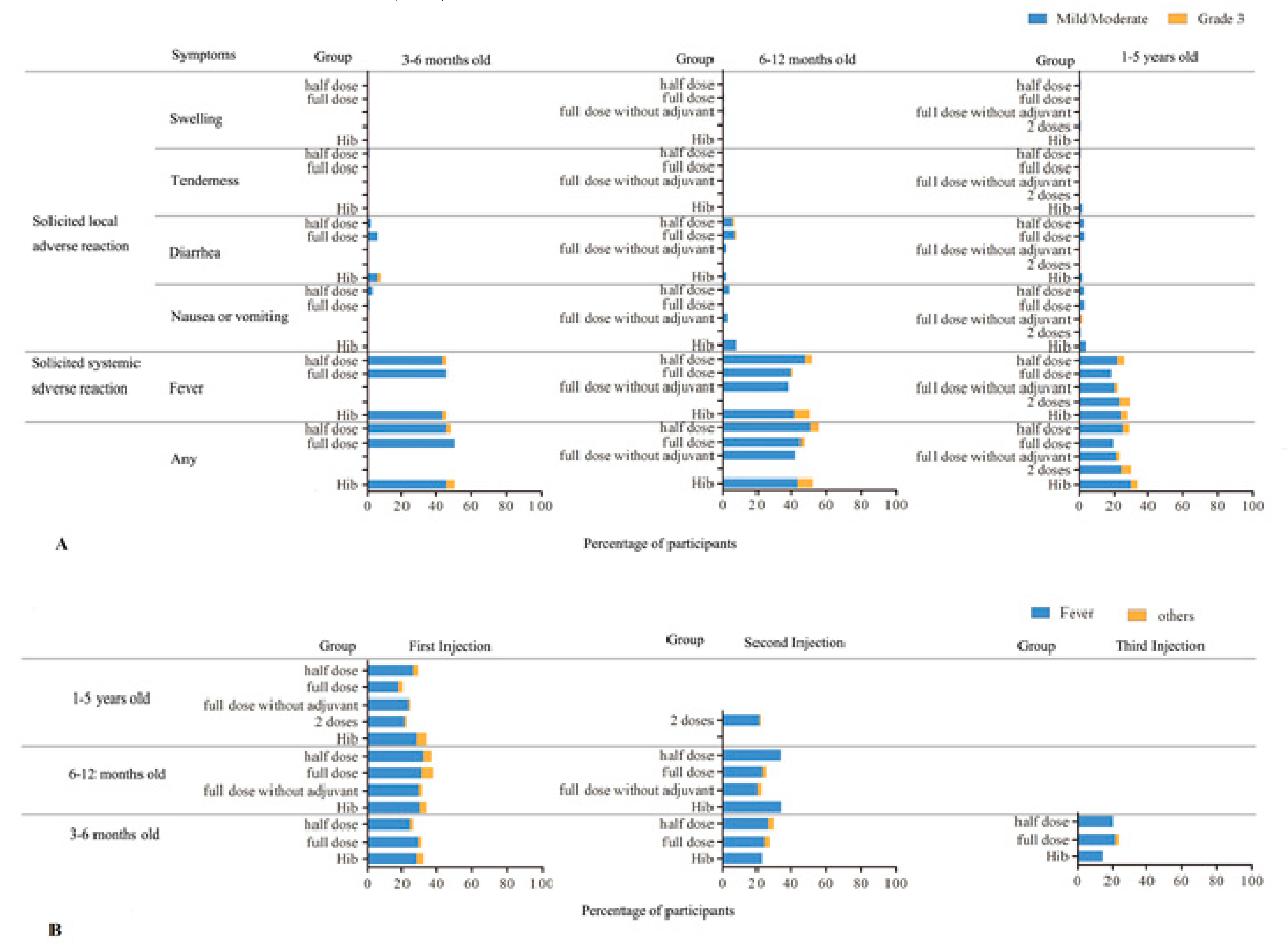
3.2. Adverse Reactions
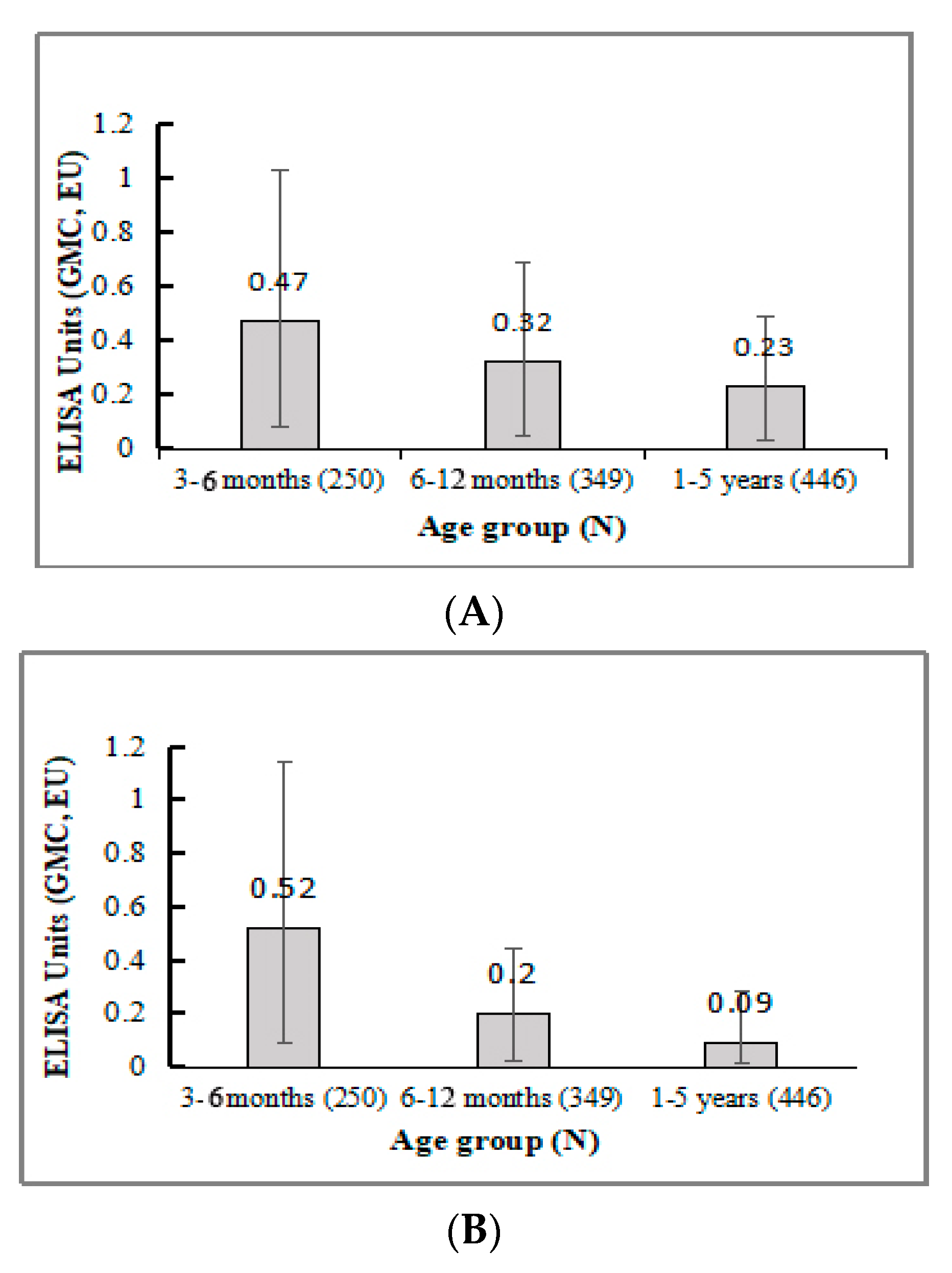
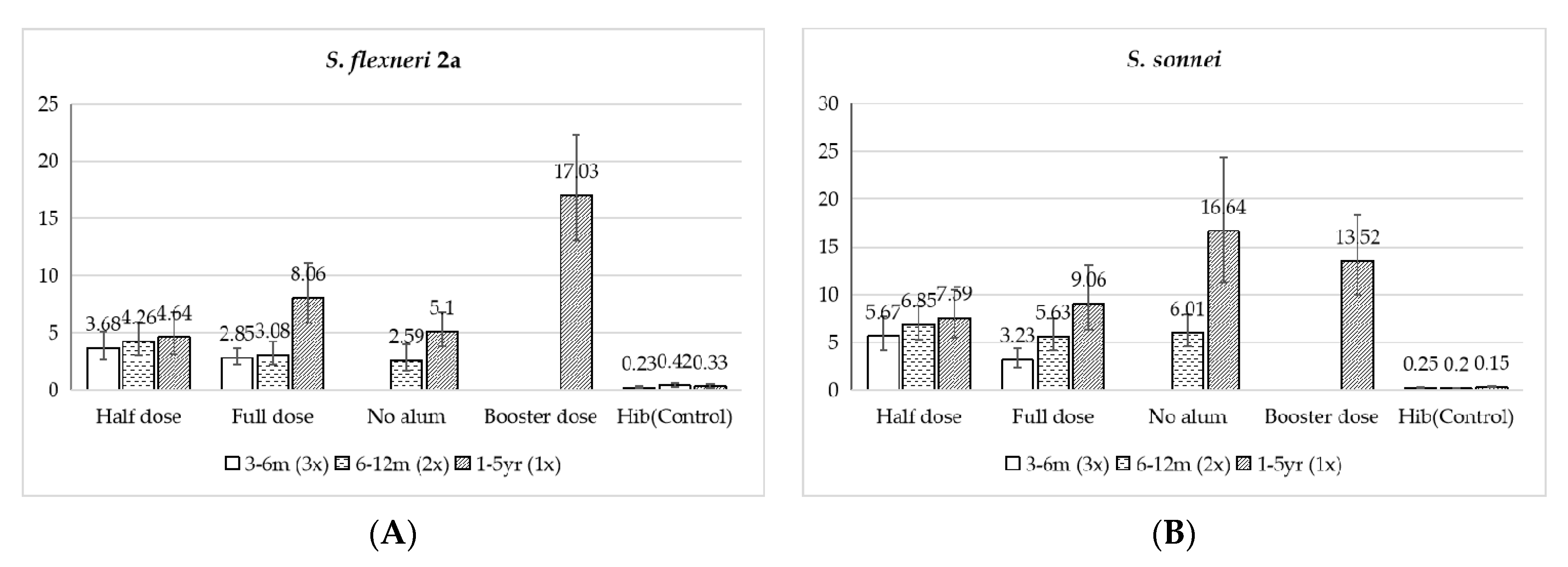
3.3. Immunogenicity
3.3.1. 3 to 6 Months Old
3.3.2. 6 to 12 Months Old
3.3.3. 1 to 5 Years Old
3.3.4. Antibody Response in All Three Age Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K.M. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- Baker, S.; The, H.C. Recent insights into Shigella. Curr. Opin. Infect. Dis. 2018, 31, 449–454. [Google Scholar] [CrossRef]
- Zachariah, O.H.; Lizzy, M.A.; Rose, K.; Angela, M.M. Multiple drug resistance of Campylobacter jejuni and Shigella isolated from diarrhoeic children at Kapsabet County referral hospital, Kenya. BMC Infect. Dis. 2021, 21, 109. [Google Scholar] [CrossRef]
- Rizi, K.S.; Farsiani, H.; Sasan, M.S. High rate of resistance to ceftriaxone and azithromycin among Shigella spp. isolates at three children’s referral hospitals in Northeast Iran. J. Infect. Chemother. 2020, 26, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to Shigella and Enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Liu, Z.; Tong, M.X.; Xiang, J.; Dear, K.; Wang, C.; Ma, W.; Lu, L.; Liu, Q.; Jiang, B.; Bi, P. Daily temperature and bacillary dysentery: Estimated effects, attributable risks, and future disease burden in 316 Chinese cities. Environ. Health Perspect. 2020, 128, 057008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Si, Y.; Wang, X.; Gong, P. Patterns of bacillary dysentery in China, 2005–2010. Int. J. Environ. Res. Public Health 2016, 13, 164. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, J.; Ran, L.; Sun, J.; Liu, F.; Luo, L.; Zeng, L.; Wang, L.; Li, Z.; Yu, H.; et al. The changing epidemiology of bacillary dysentery and characteristics of antimicrobial resistance of Shigella isolated in China from 2004–2014. BMC Infect. Dis. 2016, 16, 685. [Google Scholar] [CrossRef]
- China CDC. National data of class A, B and C communicable diseases in December 2020. Ji Bing Jian Ce 2021, 36, 1. [Google Scholar] [CrossRef]
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for Shigella. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef]
- Böhles, N.; Busch, K.; Hensel, M. Vaccines against human diarrheal pathogens: Current status and perspectives. Hum. Vaccines Immunother. 2014, 10, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Cassels, F.; Riddle, M.; Walker, R.; Wierzba, T. Vaccines against Shigella and Enterotoxigenic Escherichia coli: A summary of the 2018 VASE Conference. Vaccine 2019, 37, 4768–4774. [Google Scholar] [CrossRef] [PubMed]
- Barel, L.A.; Mulard, L.A. Classical and novel strategies to develop a Shigella glycoconjugate vaccine: From concept to efficacy in human. Hum. Vaccines Immunother. 2019, 15, 1338–1356. [Google Scholar] [CrossRef]
- Anderson, M.; Sansonetti, P.J.; Marteyn, B.S. Shigella Diversity and Changing Landscape: Insights for the Twenty-First Century. Front. Cell. Infect. Microbiol. 2016, 6, 45. [Google Scholar] [CrossRef]
- Nisa, I.; Qasim, M.; Yasin, N.; Ullah, R.; Ali, A. Shigella flexneri: An emerging pathogen. Folia Microbiol. 2020, 65, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Shad, A.A.; Shad, W.A. Shigella sonnei: Virulence and antibiotic resistance. Arch. Microbiol. 2021, 203, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef]
- Farzam, N.; Ramon-Saraf, R.; Banet-Levi, Y.; Lerner-Geva, L.; Ashkenazi, S.; Kubler-Kielb, J.; Vinogradov, E.; Robbins, J.B.; Schneerson, R. Vaccination with Shigella flexneri 2a conjugate induces type 2a and cross-reactive type 6 antibodies in humans but not in mice. Vaccine 2017, 35, 4990–4996. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Passwell, J.H.; Harlev, E.; Ashkenazi, S.; Chu, C.; Miron, D.; Ramon, R.; Farzan, N.; Shiloach, J.; Bryla, D.A.; Majadly, F.; et al. Safety and immunogenicity of improved Shigella O-specific polysaccharide-protein conjugate vaccines in adults in Israel. Infect. Immun. 2001, 69, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Passwell, J.H.; Ashkenazi, S.; Harlev, E.; Miron, D.; Ramon, R.; Farzam, N.; Lerner-Geva, L.; Levi, Y.; Chu, C.; Shiloach, J.; et al. Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2a-r EPAsucc conjugate vaccines in one- to four-year-old children. Pediatr. Infect. Dis. J. 2003, 22, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.-S.; Ogawa, Y.; Flippen-Anderson, J.L.; Kováč, P. Synthesis and crystal structure of methyl 4,6-dideoxy-4-(3-deoxy-l-glycero-tetronamido)-2-O-methyl-α-d-mannopyranoside, the methyl α-glycoside of the terminal unit, and presumed antigenic determinant, of the O-specific polysaccharide of Vibrio cholerae O:1, serotype Ogawa. Carbohydr. Res. 1995, 275, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, K.A.; Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.; Porter, C.K.; Chakraborty, S.; Brubaker, J.; Elwood, D.; et al. Immune response characterization in a human challenge study with a Shigella flexneri 2a bioconjugate vaccine. EBioMedicine 2021, 66, 103308. [Google Scholar] [CrossRef] [PubMed]
- Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.M.; Kaminski, R.W.; Porter, C.K.; Chakraborty, S.; Clarkson, K.A.; Brubaker, J.; et al. Human challenge study with a Shigella bioconjugate vaccine: Analyses of clinical efficacy and correlate of protection. EBioMedicine 2021, 66, 103310. [Google Scholar] [CrossRef]
- Cohen, D.; Atsmon, J.; Artaud, C.; Meron-Sudai, S.; Gougeon, M.-L.; Bialik, A.; Goren, S.; Asato, V.; Ariel-Cohen, O.; Reizis, A.; et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: A phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 2021, 21, 546–558. [Google Scholar] [CrossRef]
- Passwell, J.H.; Ashkenazi, S.; Banet-Levi, Y.; Ramon-Saraf, R.; Farzam, N.; Lerner-Geva, L.; Even-Nir, H.; Yerushalmi, B.; Chu, C.; Shiloach, J.; et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine 2010, 28, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, S.; Passwell, J.H.; Harlev, E.; Miron, D.; Dagan, R.; Farzan, N.; Ramon, R.; Majadly, F.; Bryla, D.A.; Karpas, A.B.; et al. Safety and immunogenicity of Shigella sonnei and Shigella flexneri 2a O-specific polysaccharide conjugates in children. J. Infect. Dis. 1999, 179, 1565–1568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cohen, D.; Ashkenazi, S.; Green, M.; Lerman, Y.; Slepon, R.; Robin, G.; Orr, N.; Taylor, D.N.; Sadoff, J.C.; Chu, C.; et al. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect. Immun. 1996, 64, 4074–4077. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG Placental Transfer in Healthy and Pathological Pregnancies. Clin. Dev. Immunol. 2011, 2012, 985646. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.M.; Crowcroft, N.S.; Friedman, L.; Deeks, S.L.; Halperin, S.A.; Severini, A.; Hatchette, T.F.; Bolotin, S. Waning of measles maternal antibody in infants in measles elimination settings–A systematic literature review. Vaccine 2018, 36, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.M.; Rench, M.A.; Swaim, L.S.; Timmins, A.; Vyas, A.; Sangi-Haghpeykar, H.; Ng, N.; Rajam, G.; Havers, F.; Schiffer, J.; et al. Kinetics of maternal pertussis-specific antibodies in infants of mothers vaccinated with tetanus, diphtheria and acellular pertussis (Tdap) during pregnancy. Vaccine 2020, 38, 5955–5961. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.B.; Chu, C.; Schneerson, R. Hypothesis for vaccine development: Protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin. Infect. Dis. 1992, 15, 346–361. [Google Scholar] [CrossRef]
- Li, J.B.; Li, Z.; Pan, B.; Gong, J.; Li, C.Y.; Shi, L.W.; Huang, Y.; Du, L. Epidemiological surveillance of bacillary dysentery in Zhongshan County, Guangxi. Ying Yong Yu Fang Yi Xue 2018, 24, 220–222. (In Chinese) [Google Scholar]
- Jones, C.; Pollock, L.; Barnett, S.M.; Battersby, A.; Kampmann, B. The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine 2014, 32, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Wu, C.G.; Xie, G.Z.; Gu, Q.W.; Wang, B.R.; Wang, L.Y.; Wang, H.F.; Ding, Z.S.; Yang, Y.; Tan, W.S.; et al. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China. Bull. World Health Organ. 2001, 79, 625–631. [Google Scholar]
- Klein, N.P.; Peyrani, P.; Yacisin, K.; Caldwell, N.; Xu, X.; Scully, I.L.; Scott, D.A.; Jansen, K.U.; Gruber, W.C.; Watson, W. A phase 3, randomized, double-blind study to evaluate the immunogenicity and safety of 3 lots of 20-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naive adults 18 through 49 years of age. Vaccine 2021, 39, 5428–5435. [Google Scholar] [CrossRef]
- Perez, J.L.; Absalon, J.; Beeslaar, J.; Balmer, P.; Jansen, K.U.; Jones, T.R.; Harris, S.; York, L.J.; Jiang, Q.; Radley, D.; et al. From research to licensure and beyond: Clinical development of MenB-FHbp, a broadly protective meningococcal B vaccine. Expert Rev. Vaccines 2018, 17, 461–477. [Google Scholar] [CrossRef]
- Wen, X.; Cao, D.; Jones, R.W.; Li, J.; Szu, S.; Hoshino, Y. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine 2012, 30, 6121–6126. [Google Scholar] [CrossRef] [PubMed]
| 3–6 Months Old | Half Dose (n = 100) | Full Dose (n = 100) | Hib (n = 50) | ||
|---|---|---|---|---|---|
| Age at first vaccination—mo | |||||
| Mean (SD) | 4.25 (0.92) | 4.16 (0.91) | 4.30 (0.86) | ||
| Median | 4.21 | 4.09 | 4.34 | ||
| Min, Max | 3.0, 5.8 | 3.0, 5.9 | 3.1, 5.8 | ||
| Sex—no. (%) | |||||
| male | 53 (53.00) | 59 (59.00) | 29 (58.00) | ||
| female | 47 (47.00) | 41 (41.00) | 21 (42.00) | ||
| 6–12 months old | half dose (n = 100) | full dose (n = 100) | full dose without adjuvant (n = 99) | Hib (n = 50) | |
| Age at first vaccination—mo | |||||
| Mean (SD) | 8.59 (1.83) | 7.71 (1.66) | 8.66 (1.92) | 8.61 (1.90) | |
| Median | 8.38 | 7.11 | 8.77 | 8.51 | |
| Min, Max | 6.0, 11.9 | 6.0, 11.8 | 6.0, 11.9 | 6.0, 11.9 | |
| Sex—no. (%) | |||||
| male | 44 (44.00) | 50 (50.00) | 55 (55.56) | 28 (56.00) | |
| female | 56 (56.00) | 50 (50.00) | 44 (44.44) | 22 (44.00) | |
| 1–6 years old | half dose (n = 100) | full dose (n = 98) | full dose without adjuvant (n = 99) | Hib (n = 50) | 2 doses (n = 99) |
| Age at first vaccination—yr | |||||
| Mean (SD) | 2.97 (1.3) | 3.01 (1.3) | 3.12 (1.2) | 2.83 (1.1) | 2.82 (1.5) |
| Median | 2.91 | 2.83 | 3.21 | 2.78 | 2.84 |
| Min, Max | 1.0, 5.6 | 1.0, 5.6 | 1.1, 5.9 | 1.1, 5.8 | 1.1, 5.6 |
| Sex—no. (%) | |||||
| male | 44 (44.00) | 37 (37.76) | 54 (54.55) | 23 (46.00) | 46 (46.46) |
| female | 56 (56.00) | 61 (62.24) | 45 (45.45) | 27 (54.00) | 53 (53.54) |
| 3–6 Months Old | Half Dose (n = 91) | Full Dose (n = 77) | Hib (n = 46) | |
|---|---|---|---|---|
| S. flexneri 2a | ||||
| Conversion rate (%) | 58.24 (47.43, 68.50) | 57.14 (45.35, 68.37) | 4.35 (0.53, 14.84) | |
| Con (EU/mL) | Pre- | 0.45 (0.34, 0.59) | 0.48 (0.35, 0.65) | 0.52 (0.36, 0.74) |
| Post- | 3.68 (2.65, 5.10) | 2.85 (2.19, 3.70) | 0.23 (0.16, 0.33) | |
| Fold rise | 8.26 (4.96, 13.73) | 5.99 (3.67, 9.76) | 0.45 (0.33, 0.62) | |
| S. Sonnei | ||||
| Conversion rate (%) | 67.03 (56.39, 76.53) | 53.25 (41.52, 64.71) | 4.35 (0.53, 14.84) | |
| Con (EU/mL) | Pre- | 0.43 (0.33, 0.57) | 0.60 (0.43, 0.83) | 0.58 (0.40, 0.84) |
| Post- | 5.67 (4.17, 7.73) | 3.23 (2.37, 4.41) | 0.25 (0.18, 0.34) | |
| Fold rise | 13.18 (7.78, 22.34) | 5.38 (3.02, 9.61) | 0.42 (0.31, 0.59) |
| 6–12 Months Old | Half Dose (n = 83) | Full Dose (n = 84) | Full Dose without Adjuvant (n = 75) | Hib (n = 38) | |
|---|---|---|---|---|---|
| S. flexneri 2a | |||||
| Conversion rate (%) | 66.27 (55.05, 76.28) | 64.29 (53.08, 74.45) | 64.00 (52.09, 74.77) | 15.79 (6.02, 31.25) | |
| Con (EU/mL) | Pre- | 0.36 (0.27, 0.48) | 0.30 (0.24, 0.39) | 0.30 (0.23, 0.40) | 0.29 (0.18, 0.46) |
| Post- | 4.26 (3.07, 5.91) | 3.08 (2.22, 4.28) | 2.59 (1.69, 3.97) | 0.42 (0.28, 0.65) | |
| Fold rise | 11.83 (7.99, 17.52) | 10.15 (6.97, 14.77) | 8.52 (5.61, 12.94) | 1.48 (1.09, 2.00) | |
| S. Sonnei | |||||
| Conversion rate (%) | 89.16 (80.41, 94.92) | 84.52 (74.99, 91.49) | 85.33 (75.27, 92.44) | 10.53 (2.94, 24.80) | |
| Con (EU/mL) | Pre- | 0.19 (0.14, 0.24) | 0.23 (0.17, 0.30) | 0.22 (0.17, 0.30) | 0.16 (0.11, 0.24) |
| Post- | 6.85 (5.28, 8.89) | 5.63 (4.22, 7.50) | 6.01 (4.57, 7.90) | 0.20 (0.15, 0.27) | |
| Fold rise | 36.47 (24.13, 55.11) | 24.64 (15.96, 38.04) | 27.02 (17.63, 41.42) | 1.25 (0.94, 1.66) |
| 1–5 Years Old | Half Dose (n = 100) | Full Dose (n = 96) | Full Dose without Adjuvant (n = 95) | Hib (n = 50) | 2 Doses (n = 86) | |
|---|---|---|---|---|---|---|
| S. flexneri 2a | ||||||
| Conversion rate (%) | 85.00 (76.47, 91.35) | 92.71 (85.55, 97.02) | 85.26 (76.51, 91.70) | 4.00 (0.49, 13.71) | 95.35 (88.52, 98.72) | |
| Con (EU/mL) | Pre- | 0.18 (0.13, 0.25) | 0.23 (0.17, 0.32) | 0.24 (0.17, 0.33) | 0.28 (0.18, 0.42) | 0.25 (0.18, 0.34) |
| Post- | 4.64 (3.17, 6.82) | 8.06 (5.84, 11.13) | 5.10 (3.81, 6.83) | 0.33 (0.20, 0.54) | 17.03 (13.04, 22.24) | |
| Fold rise | 25.75 (18.11, 36.63) | 35.04 (25.83, 47.53) | 21.50 (15.58, 29.67) | 1.20 (0.88, 1.64) | 68.15 (47.87, 97.02) | |
| S. Sonnei | ||||||
| Conversion rate (%) PPS | 95.00 (88.72, 98.36) | 94.79 (88.26, 98.29) | 95.79 (89.57, 98.84) | 6.00 (1.25, 16.55) | 96.51 (90.14, 99.27) | |
| Con (EU/mL) | Pre- | 0.09 (0.07, 0.11) | 0.08 (0.06, 0.10) | 0.11 (0.08, 0.14) | 0.09 (0.07, 0.13) | 0.09 (0.07, 0.12) |
| Post- | 7.59 (5.49, 10.50) | 9.06 (6.28, 13.09) | 16.64 (11.34, 24.42) | 0.15 (0.10, 0.22) | 13.52 (10.01, 18.26) | |
| Fold rise | 85.99 (59.79, 123.68) | 112.06 (76.16, 164.87) | 158.03 (102.69, 243.22) | 1.55 (1.22, 1.96) | 146.01 (103.11, 206.74) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, Y.; Fang, W.; Li, H.; Chen, J.; Hu, X.; Wang, B.; Feng, Z.; Shi, H.; He, Y.; Huang, D.; et al. Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China. Vaccines 2022, 10, 33. https://doi.org/10.3390/vaccines10010033
Mo Y, Fang W, Li H, Chen J, Hu X, Wang B, Feng Z, Shi H, He Y, Huang D, et al. Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China. Vaccines. 2022; 10(1):33. https://doi.org/10.3390/vaccines10010033
Chicago/Turabian StyleMo, Yi, Wenjian Fang, Hong Li, Junji Chen, Xiaohua Hu, Bin Wang, Zhengli Feng, Honghua Shi, Ying He, Dong Huang, and et al. 2022. "Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China" Vaccines 10, no. 1: 33. https://doi.org/10.3390/vaccines10010033
APA StyleMo, Y., Fang, W., Li, H., Chen, J., Hu, X., Wang, B., Feng, Z., Shi, H., He, Y., Huang, D., Mo, Z., Ye, Q., & Du, L. (2022). Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China. Vaccines, 10(1), 33. https://doi.org/10.3390/vaccines10010033





