How Does Severe Acute Respiratory Syndrome-Coronavirus-2 Affect the Brain and Its Implications for the Vaccines Currently in Use
Abstract
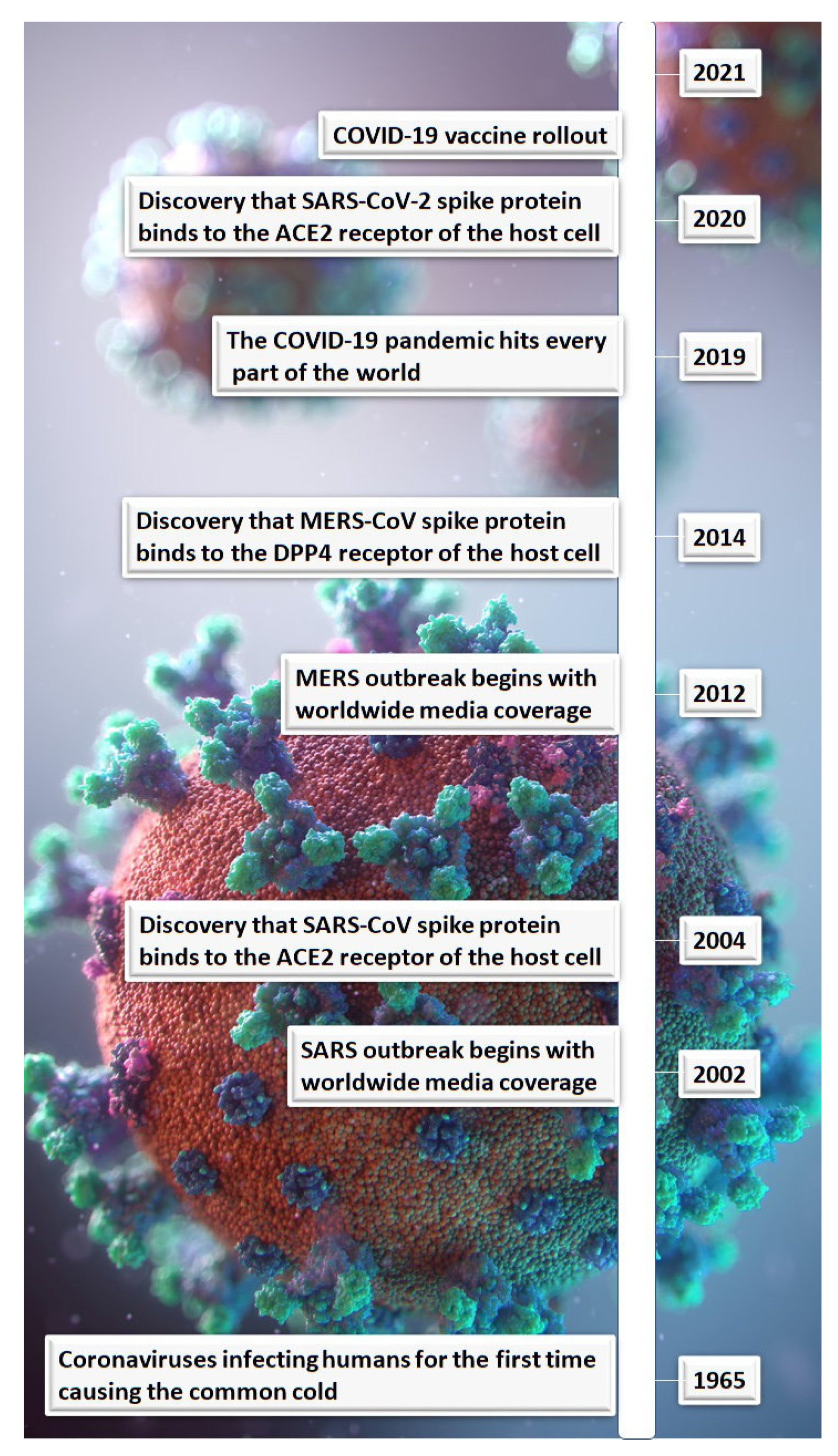
:1. Introduction
2. Lessons Learnt from Similar Coronaviruses
3. SARS-CoV-2 and Neurological Symptoms
4. COVID-19 and a Possible Connection with Parkinson’s Disease
5. Pathology Attributed to the SARS-CoV-2 Spike Protein
6. What Are the Implications for the COVID-19 Vaccines Currently Being Used?
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nath, A. Neurologic complications of coronavirus infections. Neurology 2020, 94, 809–810. [Google Scholar] [CrossRef]
- Alquisiras-Burgos, I.; Peralta-Arrieta, I.; Alonso-Palomares, L.A.; Zacapala-Gómez, A.E.; Salmerón-Bárcenas, E.; Aguilera, P. Neurological Complications Associated with the Blood-Brain Barrier Damage Induced by the Inflammatory Response during SARS-CoV-2 Infection. Mol. Neurobiol. 2020, 58, 520–535. [Google Scholar] [CrossRef]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Inf. 2020, 26, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.C.; Zumla, A. Severe Acute Respiratory Syndrome, Historical, Epidemiologic, and Clinical Features. Infect. Dis Clin. N. Am. 2019, 33, 869–889. [Google Scholar] [CrossRef]
- Saad, M.; Omrani, A.S.; Baig, K.; Bahloul, A.; Elzein, F.; Matin, M.A.; Selim, M.A.A.; Al-Mutairi, M.; Al-Nakhli, D.; Al-Aidaroos, A.Y.; et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: A single-center experience in Saudi Arabia. Int. J. Infect. Dis. 2014, 29, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Government of Canada. COVID-19 Sgns, Symptoms and Severity of Disease: A Clinician Guide. Available online: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/guidance-documents/signs-symptoms-severity.html (accessed on 17 June 2021).
- Mian, M.S.; Khan, L.R.S.; Hussain, N.; Razaq, M. Pathological Findings and Management of COVID-19 Patients: A Brief Overview of Modern-day Pandemic. Cureus 2020, 12, e8136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chu, H.; Li, C.; Wong, B.H.-Y.; Cheng, Z.-S.; Poon, V.W.-M.; Sun, T.; Lau, C.C.-Y.; Wong, K.K.-Y.; Chan, J.Y.-W.; et al. Active Replication of Middle East Respiratory Syndrome Coronavirus and Aberrant Induction of Inflammatory Cytokines and Chemokines in Human Macrophages: Implications for Pathogenesis. J. Infect. Dis. 2014, 209, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, M.-L.; Chien, C.-S.; Yarmishyn, A.A.; Yang, Y.-P.; Lai, W.-Y.; Luo, Y.-H.; Lin, Y.-T.; Chen, Y.-J.; Chang, P.-C.; et al. Highlight of Immune Pathogenic Response and Hematopathologic Effect in SARS-CoV, MERS-CoV, and SARS-CoV-2 Infection. Front. Immu. 2020, 11, 1022. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Pinsky, M.R. Heart-lung interactions during mechanical ventilation: The basics. Ann. Transl. Med. 2018, 6, 349. [Google Scholar] [CrossRef]
- Ahmed, H.; Patel, K.; Greenwood, D.C.; Halpin, S.; Lewthwaite, P.; Salawu, A.; Eyre, L.; Breen, A.; O’Connor, R.; Jones, A.; et al. Long-term Clinical Outcomes in Survivors of Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) Coronavirus Outbreaks After Hospitalisation or ICU Admission: A Systematic Review and Meta-Analysis. J. Rehabil. Med. 2020, 52, 1–11. [Google Scholar] [CrossRef]
- Ngai, J.C.; Ko, F.W.; Ng, S.S.; To, K.-W.; Tong, M.; Hui, D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010, 15, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldrini, M.; Canoll, P.D.; Klein, R.S. How COVID-19 Affects the Brain. JAMA Psych. Clin. Rev. Educ. 2021, 78, 682–683. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.-J.; Fletcher, R.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19 associated anosmia. bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Fodoulian, L.; Tuberosa, J.; Rossier, D.; Boillat, M.; Kan, C.; Pauli, V.; Egervari, K.; Lobrinus, J.A.; Landis, B.N.; Carleton, A.; et al. SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. bioRxiv 2020. preprint. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 1–22. [Google Scholar] [CrossRef]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.-E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, 1–18. [Google Scholar] [CrossRef]
- Lippi, A.; Domingues, R.; Setz, C.; Outeiro, T.F.; Krisko, A. SARS-CoV-2: At the Crossroad between Aging and Neurodegeneration. Mov. Disord. 2020, 35, 716–720. [Google Scholar] [CrossRef] [Green Version]
- Philippens, I.H.C.H.M.; Böszörményi, K.P.; Wubben, J.A.; Fagrouch, Z.C.; van Driel, N.; Mayenburg, A.Q.; Lozovagia, D.; Roos, E.; Schurink, B.; Bugiani, M.; et al. SARS-CoV-2 causes brain inflammation and induces Lewy body formation in macaques. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Brundin, P.; Nath, A.; Beckham, D.J. Is COVID-19 a perfect storm for Parkinson’s disease. Trends Neurosci. 2020, 43, 931–933. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; DeOre, B.J.; Bawdwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef]
- Ogata, A.F.; Cheng, C.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating SARS-CoV-2 Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clin. Infect. Dis. 2021, ciab465. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Kizaki, T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon 2021, 7, 2–10. [Google Scholar] [CrossRef]
- FDA. FDA Briefing Document Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.fda.gov/media/144245/download (accessed on 27 April 2021).
- European Medicines Agency. Assessment Report-COVID-19 Vaccine Moderna (EMA/15689/2021). Available online: https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-moderna-epar-public-assessment-report_en.pdf (accessed on 6 January 2021).
- European Medicines Agency. Assessment Report-COVID-19 Vaccine Janssen (EMA/158424/2021). Available online: https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-janssen-epar-public-assessment-report_en.pdf (accessed on 11 March 2021).
- FDA. BLA (STN: BL 125742/0) Approval Letter. Available online: https://www.fda.gov/media/151710/download (accessed on 23 August 2021).
- Lu, L.; Xiong, W.; Mu, J.; Zhang, Q.; Zhang, H.; Zou, L.; Li, W.; He, L.; Sander, J.W.; Zhou, D. The potential neurological effect of the COVID-19 vaccines: A review. Acta Neurol. Scand. 2021, 144, 3–12. [Google Scholar] [CrossRef]
- Goss, A.L.; Samudralwar, R.D.; Das, R.R.; Nath, A. ANA Investigates: Neurological Complications of COVID-19 Vaccines. Ann. Neurol. 2021, 89, 856–857. [Google Scholar] [CrossRef]
- Jing-Han, N.; Chaudhuri, K.R.; Tan, E.-K. Functional Neurological Disorders and COVID-19 Vaccination. Ann. Neurol. 2020, 90, 328. [Google Scholar]
- Lu, L.; Xiong, W.; Mu, J.; Zhang, Q.; Zhang, H.; Zou, L.; Li, W.; He, L.; Sander, J.W.; Zhou, D. Neurological side effects of COVID-19 vaccines are rare. Acta Neurol. Scand. 2021, 144, 111–112. [Google Scholar] [CrossRef] [PubMed]
- The Label. COMIRNATY® (COVID-19 Vaccine, mRNA) Suspension for Injection, for Intramuscular Use. Initial U.S. Approval: 2021. Available online: https://www.fda.gov/media/151707/download (accessed on 25 August 2021).
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 1–6. Available online: www.jimmunol.org/cgi/doi/10.4049/jimmunol.2100637 (accessed on 15 October 2021). [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines among Adolescents and Young Adults. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html (accessed on 10 December 2021).
- FDA-Summary Basis for Regulatory Action Date: 23 August 2021. Submitted a Biologics License Application (BLA) STN BL 125742 for Licensure of COVID-19 Vaccine, mRNA. Available online: https://www.fda.gov/media/151733/download (accessed on 23 August 2021).
- FDA-Guidance for Industry Preclinical Assessment of Investigational Cellular and Gene Therapy Products—(Issued November 2013). Available online: https://www.fda.gov/media/87564/download (accessed on 7 November 2021).
- FDA-Development and Licensure of Vaccines to Prevent COVID-19 Guidance for Industry—(Issued June 2020). Available online: https://www.fda.gov/media/139638/download (accessed on 7 November 2021).
| Coronavirus | Host Cell Target Receptor [3] | Clinical Symptoms | Death Rate (%) [3] |
|---|---|---|---|
| SARS-CoV | Angiotensin-converting enzyme 2 (ACE2) | Fever, Tiredness, Chills, Muscle aches, Dry cough, Difficulty breathing, Headaches, Sore throat, Diarrhea [4] | 9.5 |
| MERS-CoV | Dipeptidyl peptidase 4 | Fever, Cough, Shortness of breath [5] | 34.4 |
| SARS-CoV-2 | ACE2 | Fever, Dry cough, Chills, Difficulty breathing, Tiredness, Body aches, Headaches, Loss of taste or smell, Sore throat, Diarrhea [6,7] | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oldfield, P.R.; Hibberd, J.; Bridle, B.W. How Does Severe Acute Respiratory Syndrome-Coronavirus-2 Affect the Brain and Its Implications for the Vaccines Currently in Use. Vaccines 2022, 10, 1. https://doi.org/10.3390/vaccines10010001
Oldfield PR, Hibberd J, Bridle BW. How Does Severe Acute Respiratory Syndrome-Coronavirus-2 Affect the Brain and Its Implications for the Vaccines Currently in Use. Vaccines. 2022; 10(1):1. https://doi.org/10.3390/vaccines10010001
Chicago/Turabian StyleOldfield, Philip R., Jennifer Hibberd, and Byram W. Bridle. 2022. "How Does Severe Acute Respiratory Syndrome-Coronavirus-2 Affect the Brain and Its Implications for the Vaccines Currently in Use" Vaccines 10, no. 1: 1. https://doi.org/10.3390/vaccines10010001
APA StyleOldfield, P. R., Hibberd, J., & Bridle, B. W. (2022). How Does Severe Acute Respiratory Syndrome-Coronavirus-2 Affect the Brain and Its Implications for the Vaccines Currently in Use. Vaccines, 10(1), 1. https://doi.org/10.3390/vaccines10010001








