Retinoprotective Effect of Wild Olive (Acebuche) Oil-Enriched Diet against Ocular Oxidative Stress Induced by Arterial Hypertension
Abstract
1. Introduction
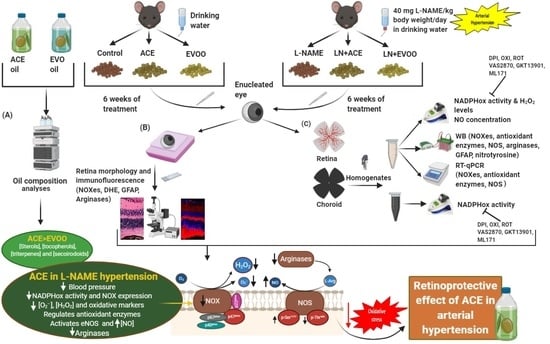
2. Materials and Methods
2.1. Study Design
2.2. Dietary Supplementation
2.3. Determination of ACE Oil and EVOO Chemical Composition
2.4. Animal Characteristics
2.5. Histomorphometric Studies
2.6. Tissue Isolation and Homogenization
2.7. NADPH Oxidase Activity Measurements
2.8. Retinal and Choroidal ROS Measurement
2.9. Immunohistofluorescence
2.10. Western Blotting Analyses
2.11. Real-Time PCR
2.12. Nitric Oxide (NO) Concentration
2.13. Statistical Analyses
3. Results
3.1. Oil Composition Analyses
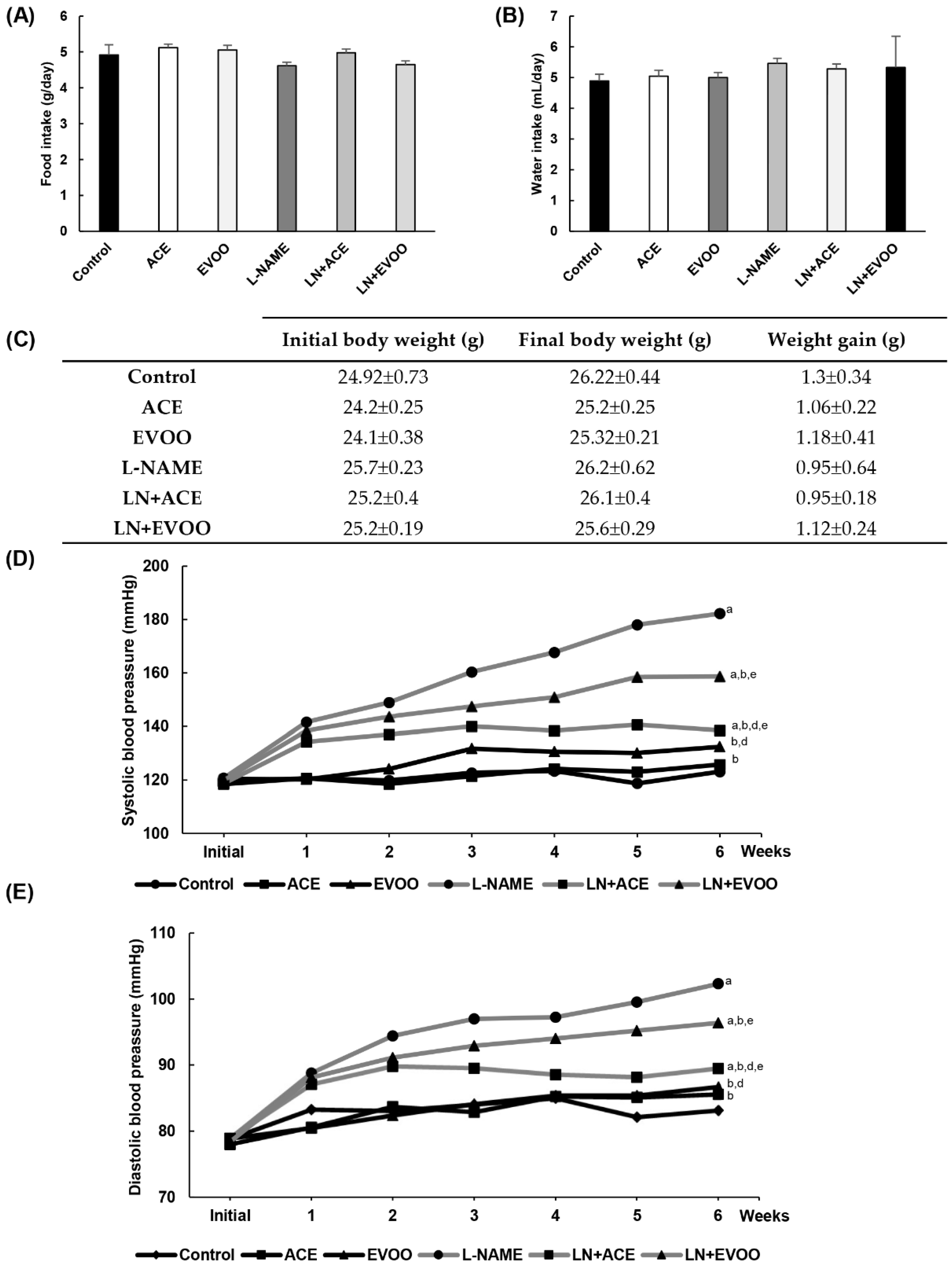
3.2. Characterization of the Experimental Model
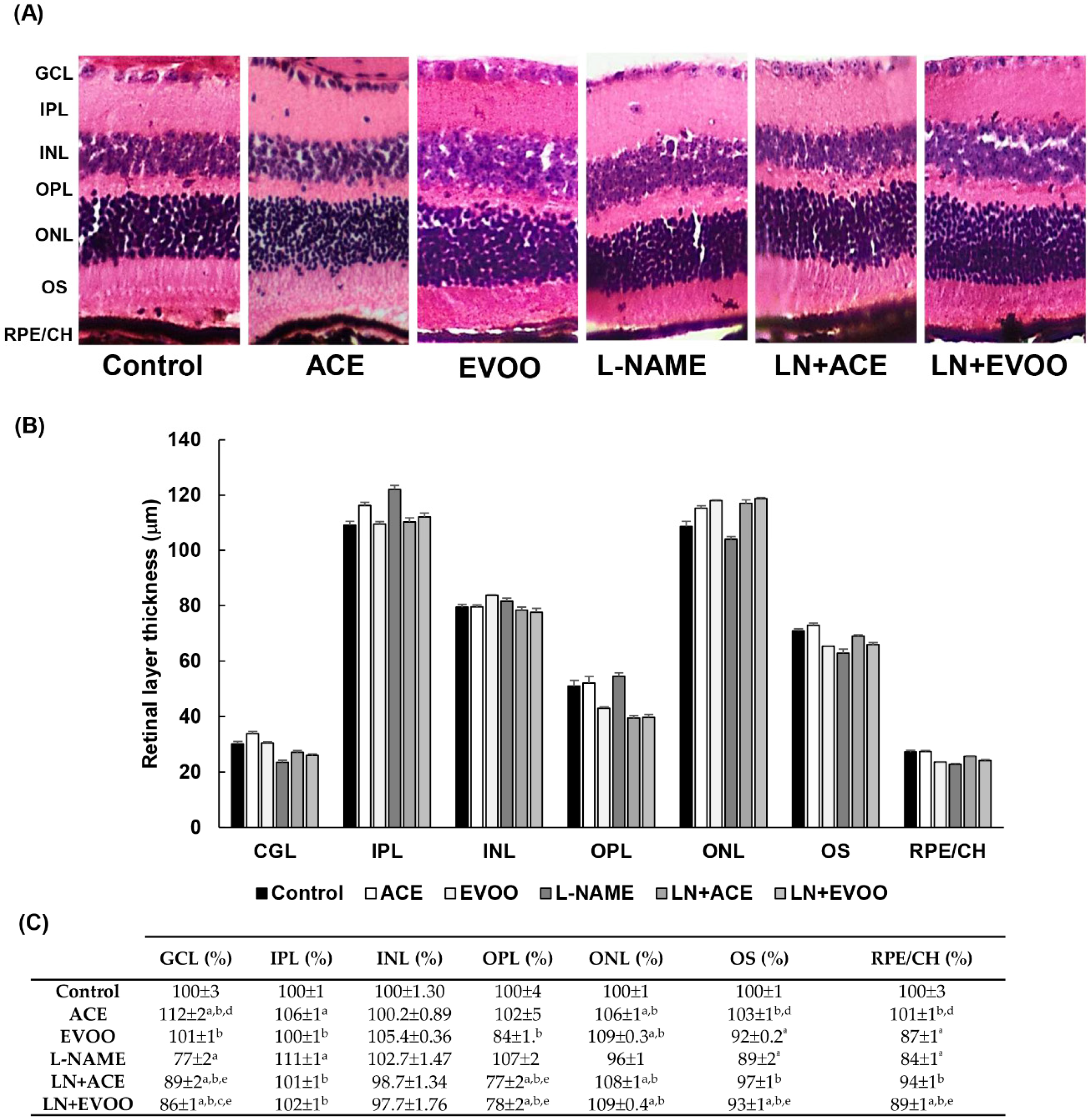
3.3. Histomorphometric Effects of Dietary Supplementation
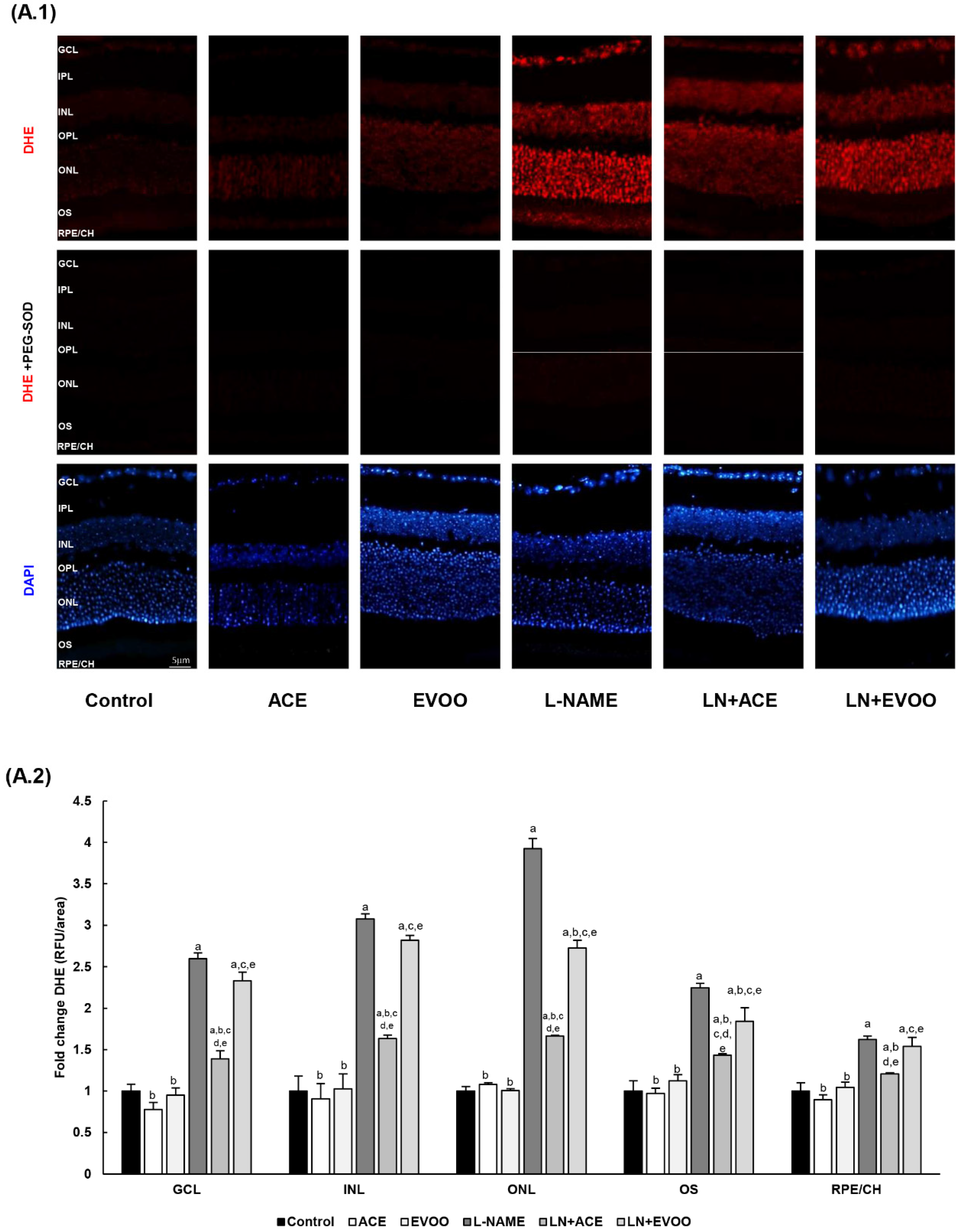
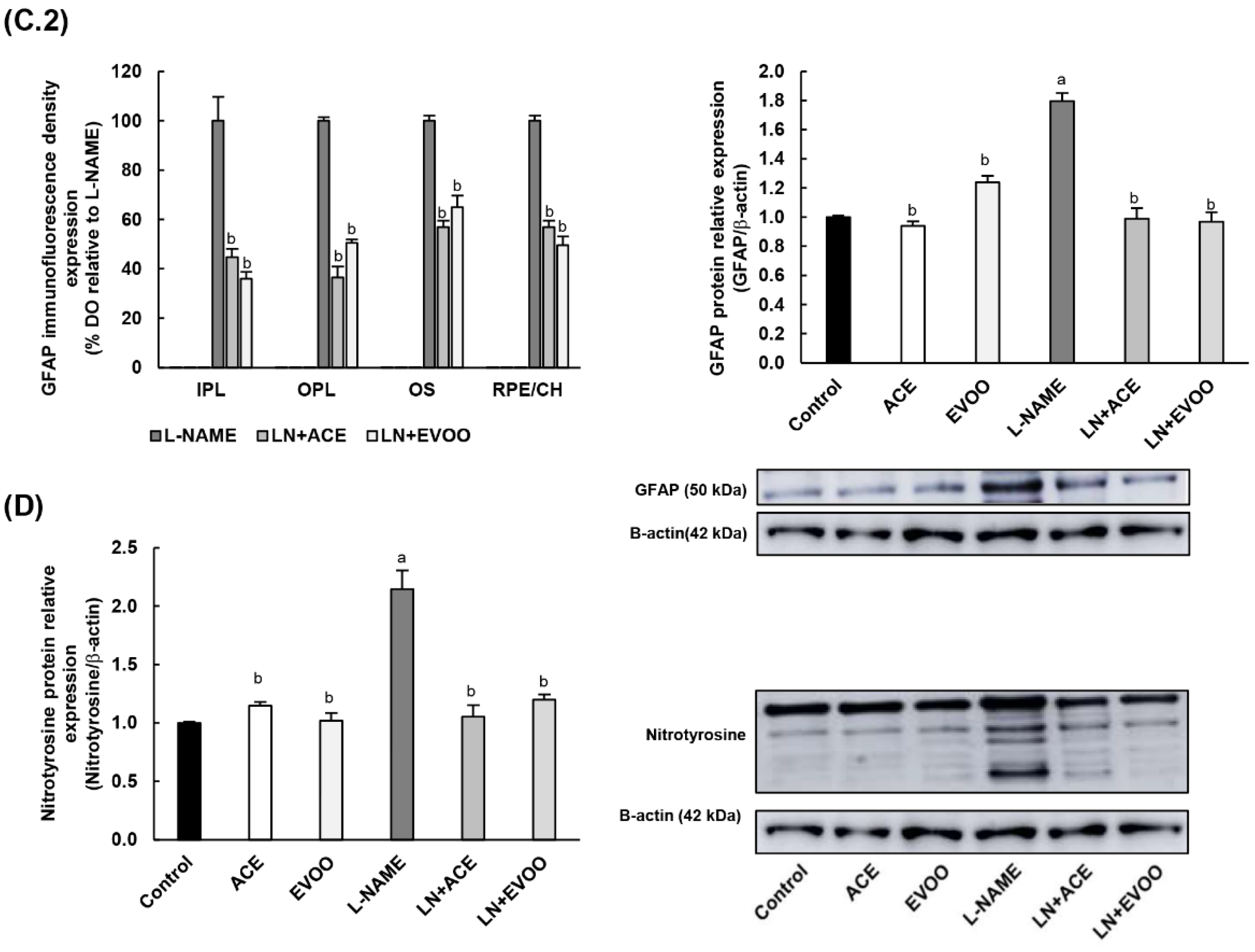
3.4. ROS Levels and Oxidative Stress Markers in Retina
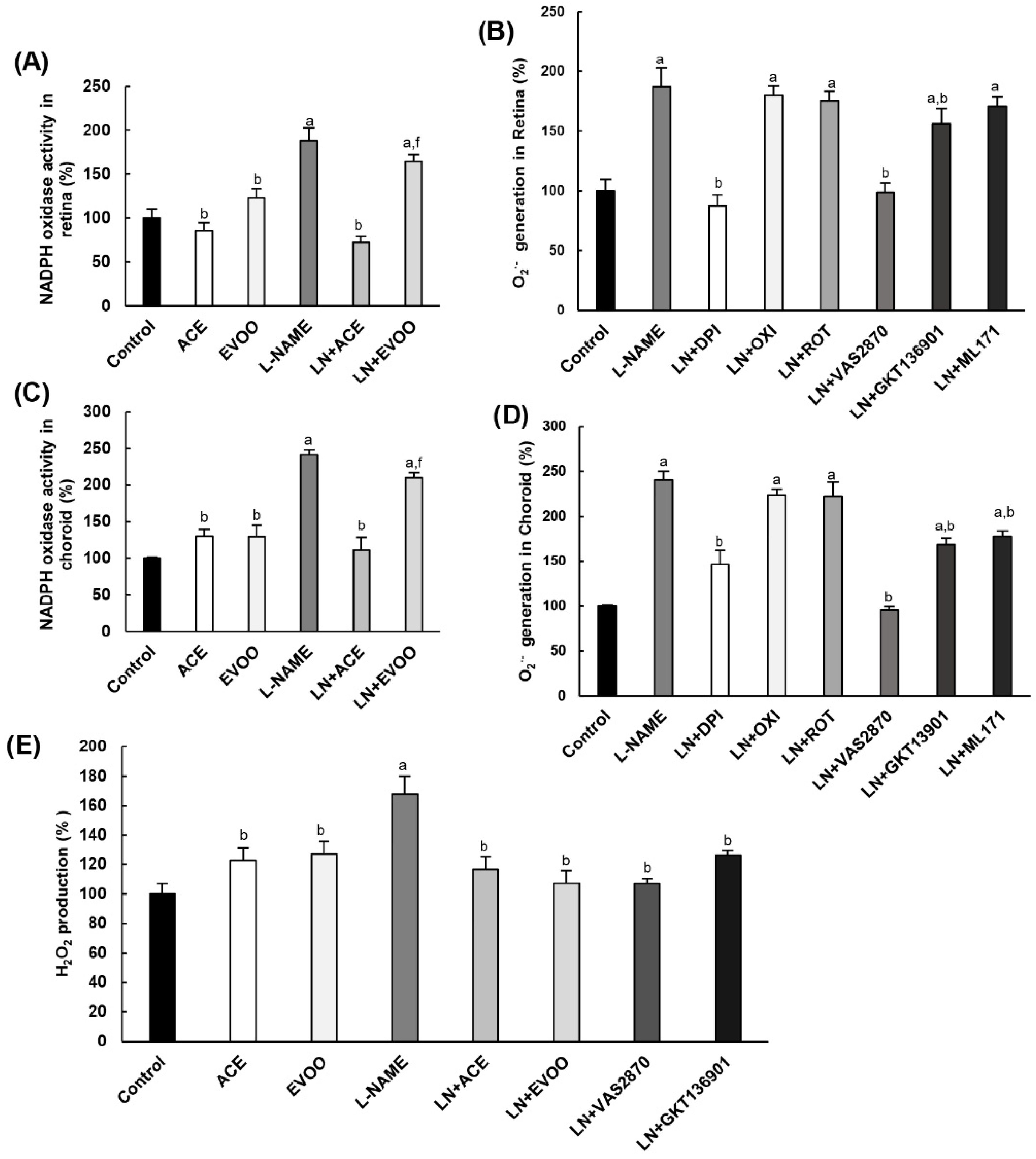
3.5. NADPH Oxidase Activity in Retina and Choroid
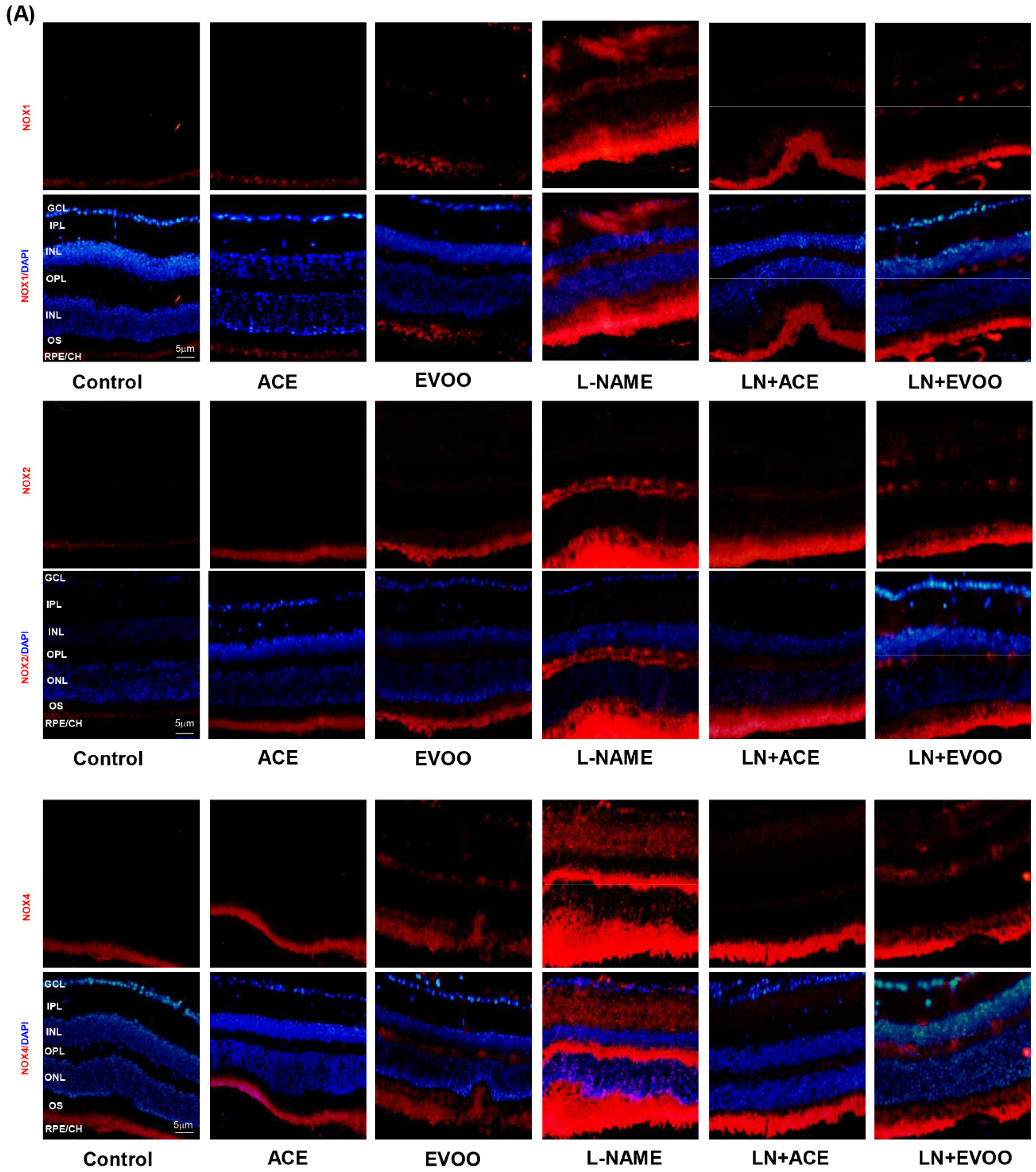
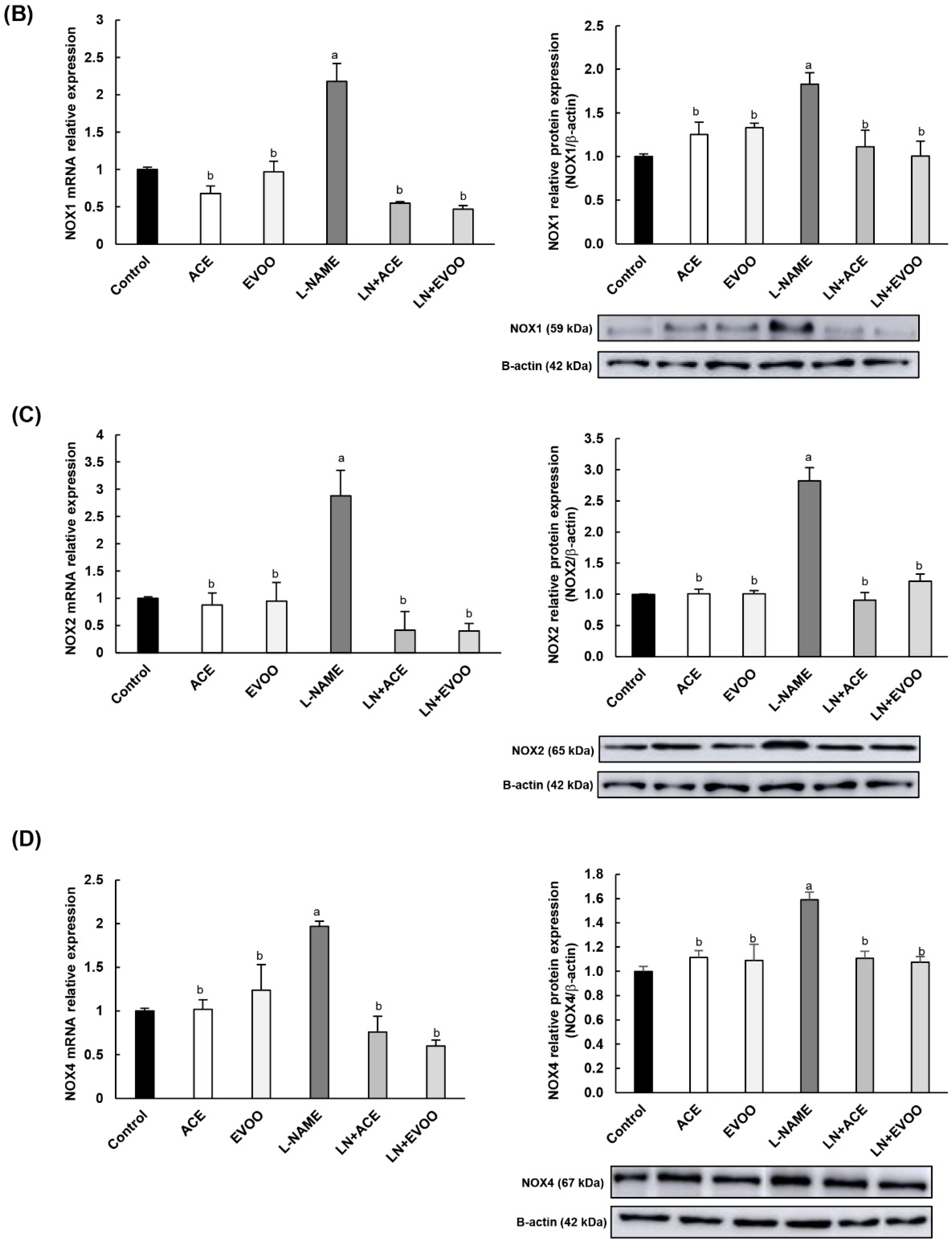
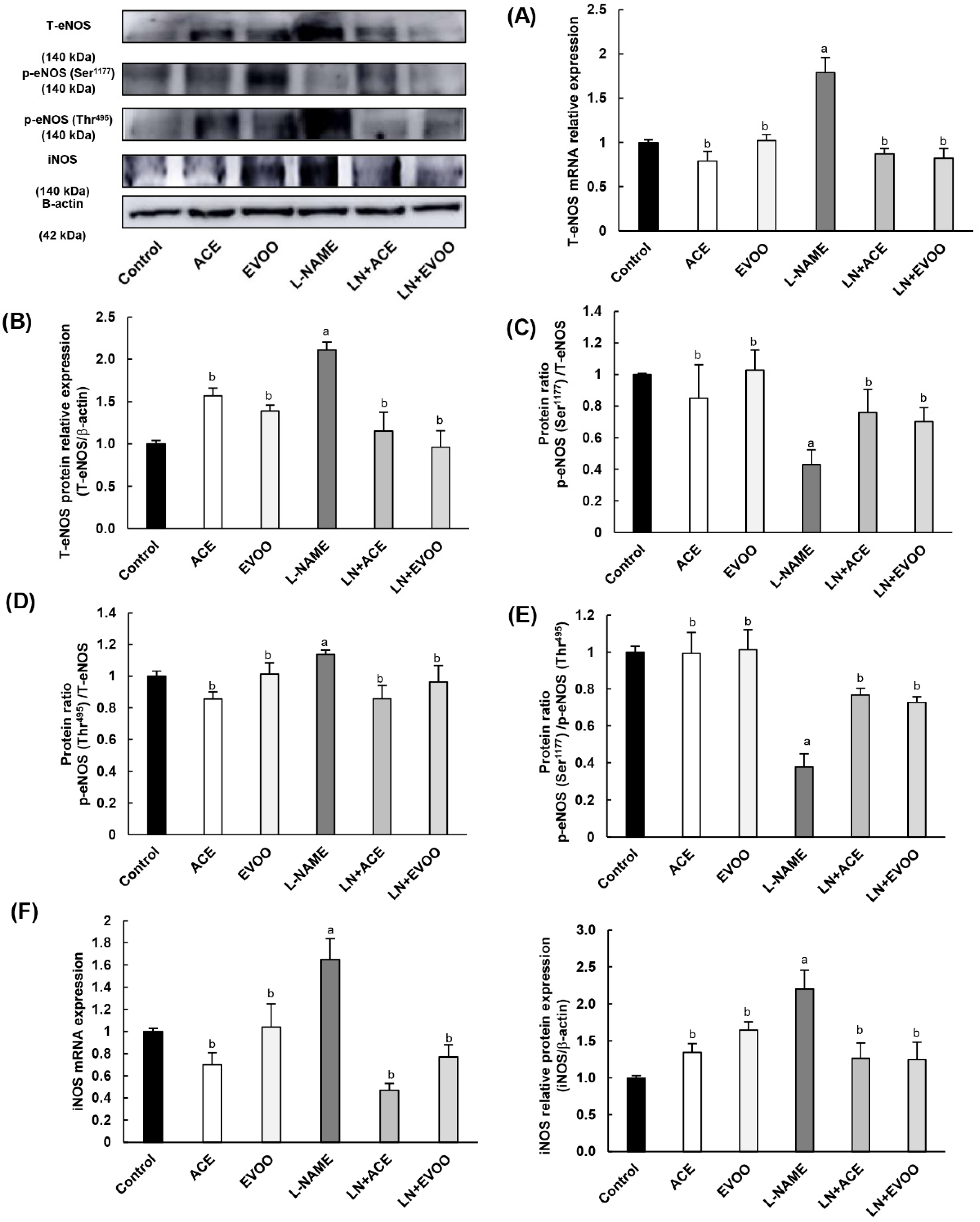
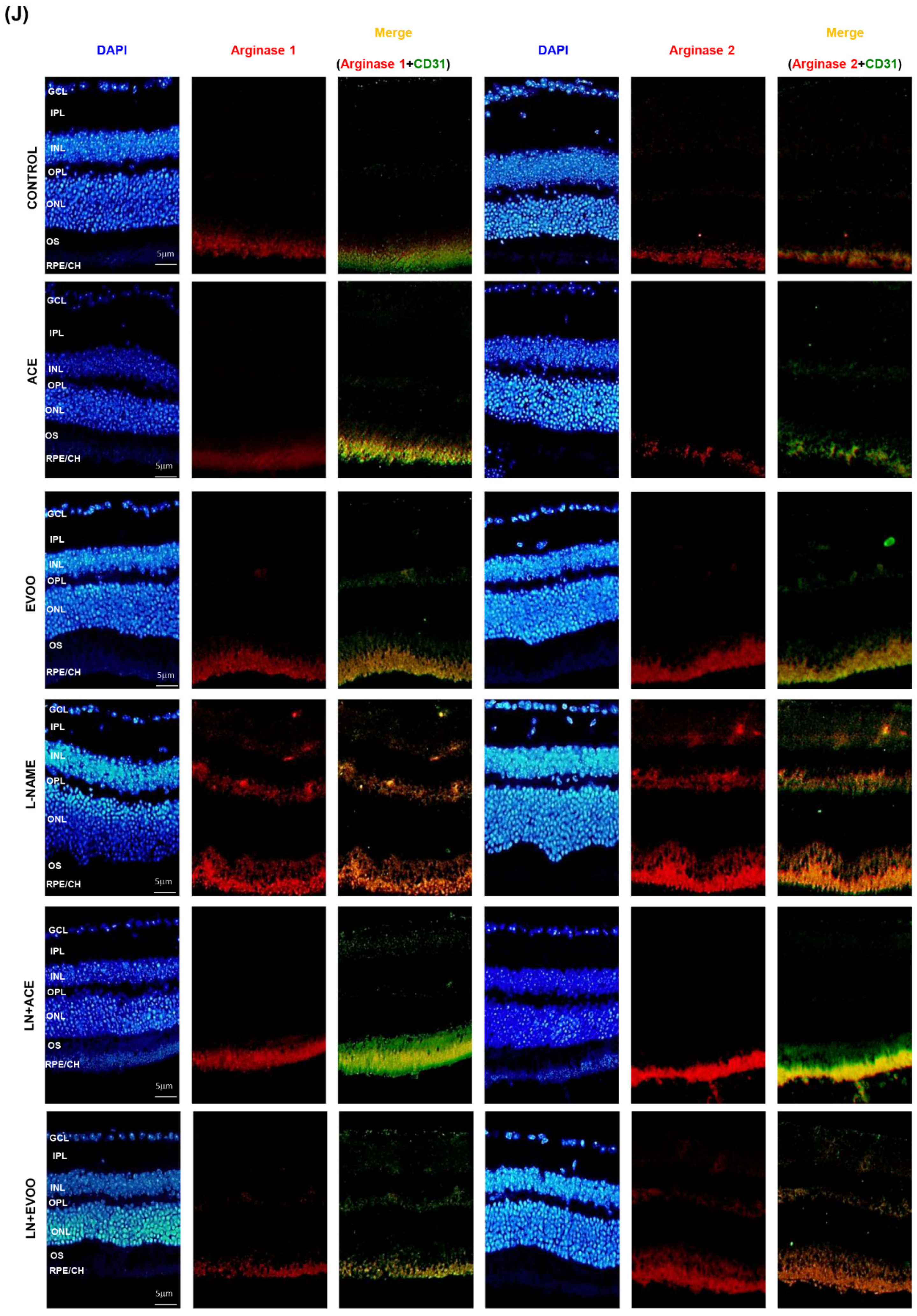
3.6. NOX Expression and Localization in Retinal Layers and Choroid
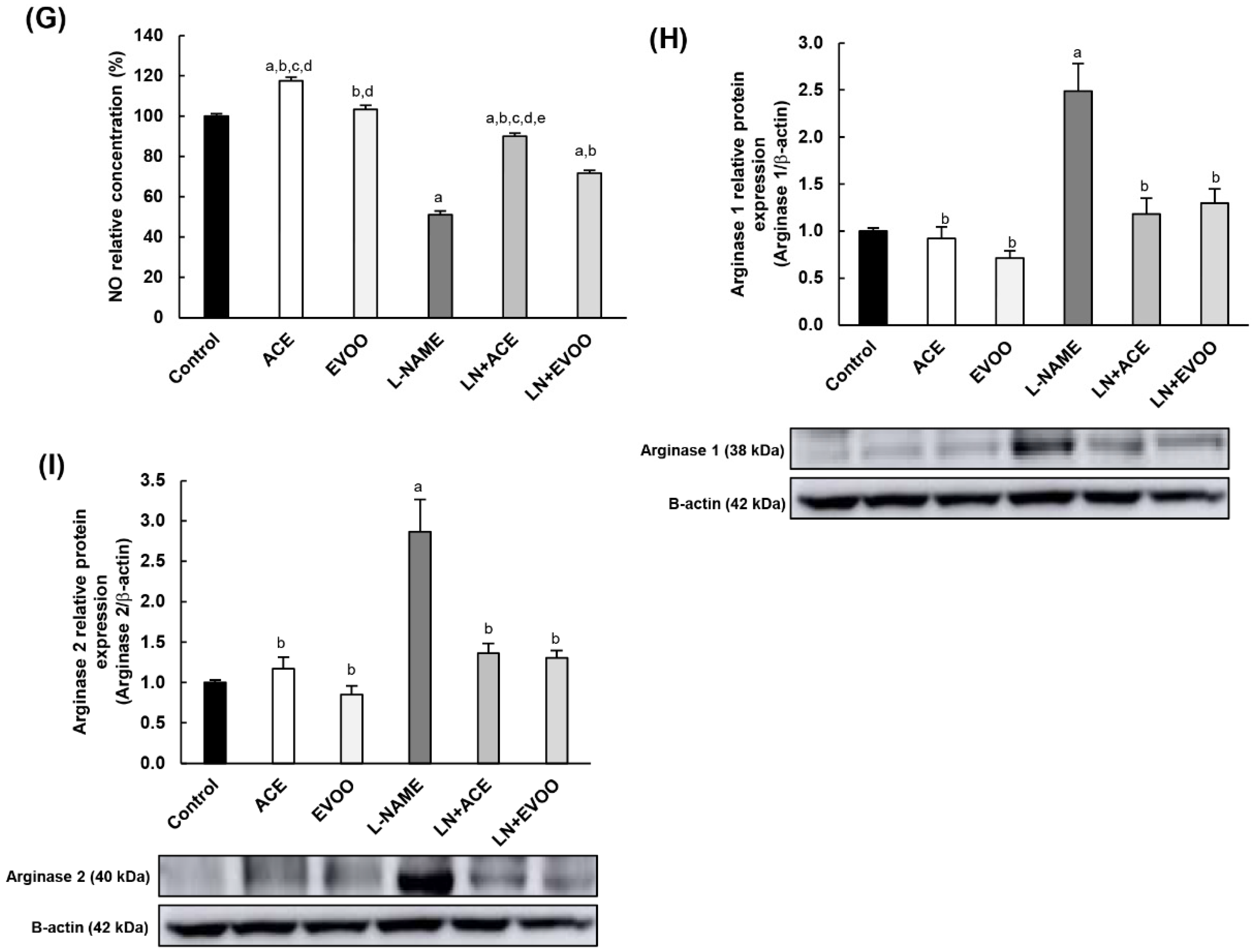
3.7. Nitric Oxide Synthase Expression, NO Concentration and Arginase Enzymes
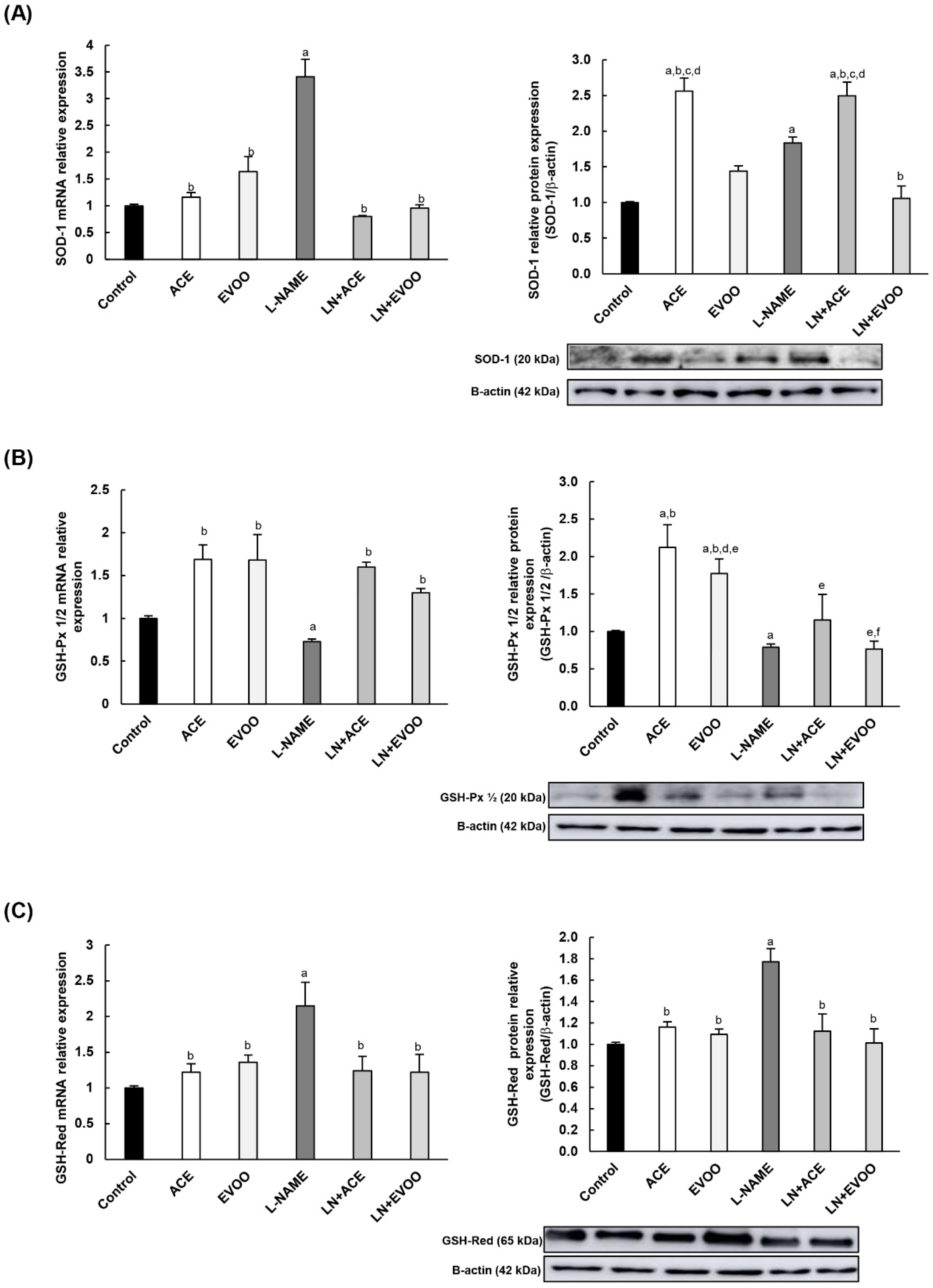
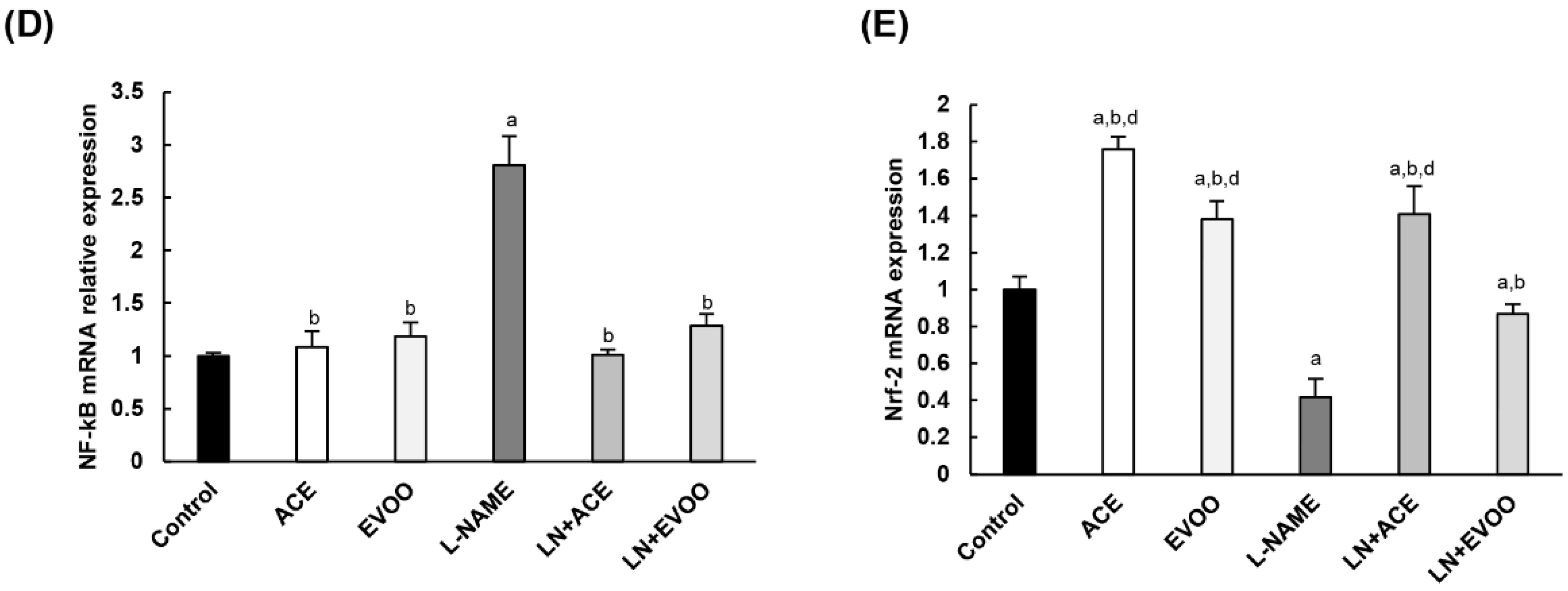
3.8. Antioxidant Enzymes and Transcription Factors in Retina Homogenates
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rachele, R. The EU olive and olive oil sector: Main features, challenges and prospects. Eur. Parliam. 2017. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE (accessed on 1 June 2020).
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of extra-virgin olive oil: A review. BioFactors 2017, 43, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; García-González, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- European Commission. COMMISSION REGULATION (EEC) No 2568/91; Official Journal of the European Communities; EU Publications: Luxembourg, 1991; Volume L269, pp. 1–15. [Google Scholar]
- European Parliament. Council of the European Union Regulation (EU) No 1308/2013 of 17 December 2013; Official Journal of the European Union; EU Publications: Luxembourg, 2013; Volume 2008, p. 184. [Google Scholar]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Liu, J.; Cheng, J.; Lu, Y.; Chen, Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1018/2013 of 23 October 2013 Amending Regulation (EU) No 432/2012 Establishing a List of Permitted Health Claims Made on Foods other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Heal. In Official Journal of the European Union; EU Publications: Luxembourg, 2013; Volume 56, pp. 43–45. [Google Scholar]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of olive oil phenols in neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Gammazza, A.M.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Declerck, K.; Szarc vel Szic, K.; Palagani, A.; Heyninck, K.; Haegeman, G.; Morand, C.; Milenkovic, D.; Vanden Berghe, W. Epigenetic control of cardiovascular health by nutritional polyphenols involves multiple chromatin-modifying writer-reader-eraser proteins. Curr. Top. Med. Chem. 2015, 16, 788–806. [Google Scholar] [CrossRef]
- Szymańska, R.; Nowicka, B.; Kruk, J. Vitamin E-Occurrence, Biosynthesis by Plants and Functions in Human Nutrition. Mini-Rev. Med. Chem. 2017, 17, 1039–1052. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; de Freitas, K.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; de Guimarães, R.C.A. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Fuster, V.P. Usefulness of plant sterols in the treatment of hypercholesterolemia. Nutr. Hosp. 2017, 34 (Suppl. 4), 62–67. [Google Scholar]
- Reboredo-Rodríguez, P.; Varela-López, A.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Bompadre, S.; Quiles, J.L.; et al. Phenolic compounds isolated from olive oil as nutraceutical tools for the prevention and management of cancer and cardiovascular diseases. Int. J. Mol. Sci. 2018, 19, 2305. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lourida, I.; Soni, M.; Thompson-Coon, J.; Purandare, N.; Lang, I.A.; Ukoumunne, O.C.; Llewellyn, D.J. Mediterranean diet, cognitive function, and dementia: A systematic review. Epidemiology 2013, 24, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.D.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A.D. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef]
- Merle, B.M.; Silver, R.E.; Rosner, B.; Seddon, J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015, 102, 1196–1206. [Google Scholar] [CrossRef]
- Mares, J.A.; Voland, R.P.; Sondel, S.A.; Millen, A.E.; LaRowe, T.; Moeller, S.M.; Klein, M.L.; Blodi, B.A.; Chappell, R.J.; Tinker, L.; et al. Healthy lifestyles related to subsequent prevalence of age-related macular degeneration. Arch. Ophthalmol. 2011, 129, 470–480. [Google Scholar] [CrossRef]
- Cougnard-Grégoire, A.; Merle, B.M.J.; Rougier, J.F.K.M.B.; Delyfer, M.N.; Le Goff, M.; Samieri, C.; Dartigues, J.F.; Delcourt, C. Olive oil consumption and age-related macular degeneration: The alienor study. PLoS ONE 2016, 11, e0160240. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef]
- Zou, X.; Feng, Z.; Li, Y.; Wang, Y.; Wertz, K.; Weber, P.; Fu, Y.; Liu, J. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: Activation of Nrf2 and JNK-p62/SQSTM1 pathways. J. Nutr. Biochem. 2012, 23, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- González-Correa, J.A.; Rodríguez-Pérez, M.D.; Márquez-Estrada, L.; López-Villodres, J.A.; Reyes, J.J.; Rodriguez-Gutierrez, G.; Fernández-Bolaños, J.; De La Cruz, J.P. Neuroprotective Effect of Hydroxytyrosol in Experimental Diabetic Retinopathy: Relationship with Cardiovascular Biomarkers. J. Agric. Food Chem. 2018, 66, 637–644. [Google Scholar] [CrossRef]
- Benlarbi, M.; Jemai, H.; Hajri, K.; Mbarek, S.; Amri, E.; Jebbari, M.; Hammoun, I.; Baccouche, B.; Boudhrioua Mihoubi, N.; Zemmal, A.; et al. Neuroprotective effects of oleuropein on retina photoreceptors cells primary culture and olive leaf extract and oleuropein inhibitory effects on aldose reductase in a diabetic model: Meriones shawi. Arch. Physiol. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Carnés Sánchez, J.; Iraola, V.M.; Sastre, J.; Florido, F.; Boluda, E.; Fernández-Caldas, L. Allergenicity and immunochemical characterization of six varieties of Olea europaea. Allergy Eur. J. Allergy Clin. Immunol. 2002, 57, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Maqueda, J.E. Estudio Analítico Comparado Entre el Aceite de Acebuchina y el Aceite de Oliva Virgen. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 2005. Available online: https://idus.us.es/handle/11441/16008?locale-attribute=en (accessed on 1 July 2020).
- Fanjul-Moles, M.L.; López-Riquelme, G.O. Relationship between oxidative stress, circadian rhythms, and AMD. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Moreno, M.L.; Mérida, S.; Bosch-Morell, F.; Miranda, M.; Villar, V.M. Autophagy dysfunction and oxidative stress, two related mechanisms implicated in retinitis pigmentosa. Front. Physiol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Hammes, H.P. Diabetic retinopathy: Hyperglycaemia, oxidative stress and beyond. Diabetologia 2018, 61, 29–38. [Google Scholar] [CrossRef]
- Karaca, M.; Coban, E.; Felek, R.; Unal, M. The association of oxidative stress with hypertensive retinopathy. Clin. Exp. Hypertens. 2013, 35, 16–19. [Google Scholar] [CrossRef]
- Small, H.Y.; Migliarino, S.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Hypertension: Focus on autoimmunity and oxidative stress. Free Radic. Biol. Med. 2018, 125, 104–115. [Google Scholar] [CrossRef]
- Camargo, L.L.; Harvey, A.P.; Rios, F.J.; Tsiropoulou, S.; Da Silva, R.D.N.O.; Cao, Z.; Graham, D.; McMaster, C.; Burchmore, R.J.; Hartley, R.C.; et al. Vascular Nox (NADPH Oxidase) compartmentalization, protein hyperoxidation, and endoplasmic reticulum stress response in hypertension. Hypertension 2018, 72, 235–246. [Google Scholar] [CrossRef]
- Augsburger, F.; Filippova, A.; Rasti, D.; Seredenina, T.; Lam, M.; Maghzal, G.; Mahiout, Z.; Jansen-Dürr, P.; Knaus, U.G.; Doroshow, J.; et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019, 26, 101272. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog. Retin. Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Opatrilova, R.; Kubatka, P.; Caprnda, M.; Büsselberg, D.; Krasnik, V.; Vesely, P.; Saxena, S.; Ruia, S.; Mozos, I.; Rodrigo, L.; et al. Nitric oxide in the pathophysiology of retinopathy: Evidences from preclinical and clinical researches. Acta Ophthalmol. 2018, 96, 222–231. [Google Scholar] [CrossRef]
- DellaCroce, J.T.; Vitale, A.T. Hypertension and the eye. Curr. Opin. Ophthalmol. 2008, 19, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- Pérez-Camino, M.C.; Cert, A. Quantitative determination of hydroxy pentacyclic triterpene acids in vegetable oils. J. Agric. Food Chem. 1999, 47, 1558–1562. [Google Scholar] [CrossRef]
- Lyzogubov, V.V.; Bora, N.S.; Tytarenko, R.G.; Bora, P.S. Polyethylene glycol induced mouse model of retinal degeneration. Exp. Eye Res. 2014, 127, 143–152. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Santana-Garrido, Á.; Reyes-Goya, C.; André, H.; Aramburu, Ó.; Mate, A.; Vázquez, C.M. Sunitinib-induced oxidative imbalance and retinotoxic effects in rats. Life Sci. 2020, 257, 118072. [Google Scholar] [CrossRef]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef]
- Zambrano, S.; Blanca, A.J.; Ruiz-Armenta, M.V.; Miguel-Carrasco, J.L.; Revilla, E.; Santa-María, C.; Mate, A.; Vázquez, C.M. The renoprotective effect of L-carnitine in hypertensive rats is mediated by modulation of oxidative stress-related gene expression. Eur. J. Nutr. 2013, 52, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Marzinzig, M.; Nussler, A.K.; Stadler, J.; Marzinzig, E.; Barthlen, W.; Nussler, N.C.; Beger, H.G.; Morris, S.M.; Brückner, U.B. Improved methods to measure end products of nitric oxide in biological fluids: Nitrite, nitrate, and S-nitrosothiols. Nitric Oxide Biol. Chem. 1997, 1, 177–189. [Google Scholar] [CrossRef] [PubMed]
- He, W.S.; Zhu, H.; Chen, Z.Y. Plant Sterols: Chemical and Enzymatic Structural Modifications and Effects on Their Cholesterol-Lowering Activity. J. Agric. Food Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef]
- Hernáez, A.; Farràs, M.; Fitó, M. Olive oil phenolic compounds and high-density lipoprotein function. Curr. Opin. Lipidol. 2016, 27, 47–53. [Google Scholar] [CrossRef][Green Version]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef]
- Qian, Y.; Tang, X.; Guan, T.; Li, Y.; Sun, H. Neuroprotection by combined administration with maslinic acid, a natural product from Olea europaea, and MK-801 in the cerebral ischemia model. Molecules 2016, 21, 1093. [Google Scholar] [CrossRef]
- Wang, Z.H.; Mong, M.C.; Yang, Y.C.; Yin, M.C. Asiatic acid and maslinic acid attenuated kainic acid-induced seizure through decreasing hippocampal inflammatory and oxidative stress. Epilepsy Res. 2018, 139, 28–34. [Google Scholar] [CrossRef]
- Parkinson, L.; Cicerale, S. The health benefiting mechanisms of virgin olive oil phenolic compounds. Molecules 2016, 21, 1734. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tuomilehto, J. Olive oil nutraceuticals in the prevention and management of diabetes: From molecules to lifestyle. Int. J. Mol. Sci. 2018, 19, 2024. [Google Scholar] [CrossRef] [PubMed]
- Meza-Miranda, E.R.; Rangel-Zúñiga, O.A.; Marín, C.; Pérez-Martínez, P.; Delgado-Lista, J.; Haro, C.; Peña-Orihuela, P.; Jiménez-Morales, A.I.; Malagón, M.M.; Tinahones, F.J.; et al. Virgin olive oil rich in phenolic compounds modulates the expression of atherosclerosis-related genes in vascular endothelium. Eur. J. Nutr. 2016, 55, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Castejón, M.L.; Montoya, T.; Alarcón-de-la-lastra, C.; Sánchez-hidalgo, M. Potential protective role exerted by secoiridoids from olea europaea l. In cancer, cardiovascular, neurodegenerative, aging-related, and immunoinflammatory diseases. Antioxidants 2020, 9, 149. [Google Scholar] [CrossRef]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive Polyphenols and the Metabolic Syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef]
- Akay, F.; Gundogan, F.C.; Yolcu, U.; Toyran, S.; Uzun, S. Choroidal thickness in systemic arterial hypertension. Eur. J. Ophthalmol. 2015, 26, 152–157. [Google Scholar] [CrossRef]
- Lim, H.B.; Lee, M.W.; Park, J.H.; Kim, K.; Jo, Y.J.; Kim, J.Y. Changes in Ganglion Cell–Inner Plexiform Layer Thickness and Retinal Microvasculature in Hypertension: An Optical Coherence Tomography Angiography Study. Am. J. Ophthalmol. 2019, 199, 167–176. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Montoya, T.; Castejón, M.L.; Sánchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón de-la-Lastra, C. Oleocanthal Modulates LPS-Induced Murine Peritoneal Macrophages Activation via Regulation of Inflammasome, Nrf-2/HO-1, and MAPKs Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5552–5559. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Monticolo, R.; D’Amico, A.; Stefanini, L.; Pagano, F.; Pastori, D.; Cangemi, R.; et al. Antioxidant activity from extra virgin olive oil via inhibition of hydrogen peroxide-mediated NADPH-oxidase 2 activation. Nutrition 2018, 55–56, 36–40. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; D’Amore, S.; Gnoni, A.; Pellegrino, M.; Storelli, C.; De Caterina, R.; Palasciano, G.; Carluccio, M.A. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J. Nutr. Biochem. 2016, 28, 19–29. [Google Scholar] [CrossRef]
- Wang, W.; Shang, C.; Zhang, W.; Jin, Z.; Yao, F.; He, Y.; Wang, B.; Li, Y.; Zhang, J.; Lin, R. Hydroxytyrosol NO regulates oxidative stress and NO production through SIRT1 in diabetic mice and vascular endothelial cells. Phytomedicine 2019, 52, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.C.; Silva, K.C.; Biswas, S.K.; Martins, N.; Lopes De Faria, J.B.; Lopes De Faria, J.M. Arterial hypertension exacerbates oxidative stress in early diabetic retinopathy. Free Radic. Res. 2007, 41, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Alonso-Bellido, I.M.; Rodríguez-Gómez, J.A.; Troncoso, A.M.; García-Parrilla, M.C.; Venero, J.L.; Espinosa-Oliva, A.M.; de Pablos, R.M. Hydroxytyrosol Decreases LPS- and α-Synuclein-Induced Microglial Activation In Vitro. Antioxidants 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, J.W.; Kim, D.M.; Oh, S.; Lee, J.S.; Park, S.S.; Kim, J.Y. The relation between endothelial nitric oxide synthase polymorphisms and normal tension glaucoma. J. Glaucoma 2017, 26, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.A.; Baba, T.; Merges, C.; McLeod, D.S.; Lutty, G.A. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD). Exp. Eye Res. 2010, 90, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Q.; Shi, X.; Guo, Y. Maslinic acid inhibits impairment of endothelial functions induced by high glucose in HAEC cells through improving insulin signaling and oxidative stress. Biomed. Pharmacother. 2017, 95, 904–913. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Stankevicius, E.; Herrera, M.D.; Østergaard, L.; Andersen, M.R.; Ruiz-Gutierrez, V.; Simonsen, U. Oleanolic acid induces relaxation and calcium-independent release of endothelium-derived nitric oxide. Br. J. Pharmacol. 2008, 155, 535–546. [Google Scholar] [CrossRef]
- Garhöfer, G.; Schmetterer, L. Nitric oxide: A drug target for glaucoma revisited. Drug Discov. Today 2019, 24, 1614–1620. [Google Scholar] [CrossRef]
- Forrester, J.V. Bowman lecture on the role of inflammation in degenerative disease of the eye. Eye 2013, 27, 340–352. [Google Scholar] [CrossRef]
- Bahr, H.I.; Abdelghany, A.A.; Galhom, R.A.; Barakat, B.M.; Arafa, E.S.A.; Fawzy, M.S. Duloxetine protects against experimental diabetic retinopathy in mice through retinal GFAP downregulation and modulation of neurotrophic factors. Exp. Eye Res. 2019, 186, 107742. [Google Scholar] [CrossRef]
- Michell, D.L.; Andrews, K.L.; Chin-Dusting, J.P. Endothelial dysfunction in hypertension: The role of arginase. Front. Biosci. 2011, S3, 946. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Rojas, M.; Narayanan, S.P.; Zhang, W.; Xu, Z.; Lemtalsi, T.; Jittiporn, K.; Caldwell, R.W.; Caldwell, R.B. Arginase as a mediator of diabetic retinopathy. Front. Immunol. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Shosha, E.; Xu, Z.; Yokota, H.; Saul, A.; Rojas, M.; William Caldwell, R.; Caldwell, R.B.; Priya Narayanan, S. Arginase 2 promotes neurovascular degeneration during ischemia/reperfusion injury. Cell Death Dis. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Xu, Z.; Fouda, A.Y.; Lemtalsi, T.; Shosha, E.; Rojas, M.; Liu, F.; Patel, C.; Caldwell, R.W.; Narayanan, S.P.; Caldwell, R.B. Retinal Neuroprotection From Optic Nerve Trauma by Deletion of Arginase 2. Front. Neurosci. 2018, 12, 1–13. [Google Scholar] [CrossRef]
- Suwanpradid, J.; Rojas, M.; Behzadian, M.A.; Caldwell, R.W.; Caldwell, R.B. Arginase 2 deficiency prevents oxidative stress and limits hyperoxia-induced retinal vascular degeneration. PLoS ONE 2014, 9, 3–12. [Google Scholar] [CrossRef]
- Rojas, M.; Lemtalsi, T.; Toque, H.; Xu, Z.; Fulton, D.; Caldwell, R.; Caldwell, R. NOX2-Induced Activation of Arginase and Diabetes-Induced Retinal Endothelial Cell Senescence. Antioxidants 2017, 6, 43. [Google Scholar] [CrossRef]
- Tavafi, M.; Ahmadvand, H.; Toolabi, P. Inhibitory effect of olive leaf extract on gentamicin-induced nephrotoxicity in rats. Iran. J. Kidney Dis. 2012, 6, 25–32. [Google Scholar] [PubMed]
- Beiranvand, A.; Rasoulian, B.; Alizerai, M.; Hashemi, P.; Pilevarian, A.A.; Ezatpor, B.; Tavafi, M.; Chash, S. Pretreatment with olive leaf extract partially attenuates cisplatin-induced nephrotoxicity in rats. Yafteh 2020. Available online: http://yafte.lums.ac.ir/browse.php?a_id=190&sid=1& (accessed on 9 June 2020).
- Kaeidi, A.; Sahamsizadeh, A.; Allahtavakoli, M.; Fatemi, I.; Rahmani, M.; Hakimizadeh, E.; Hassanshahi, J. The effect of oleuropein on unilateral ureteral obstruction induced-kidney injury in rats: The role of oxidative stress, inflammation and apoptosis. Mol. Biol. Rep. 2020, 47, 1371–1379. [Google Scholar] [CrossRef]
- Lei, X.G.; Zhu, J.-H.; Cheng, W.-H.; Bao, Y.; Ho, Y.-S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S.J. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Physiol. Rev. 2016, 96, 307–364. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Ávila, V.F.; Foresto-Neto, O.; Arias, S.C.A.; Faustino, V.D.; Malheiros, D.M.A.C.; Camara, N.O.S.; Zatz, R.; Fujihara, C.K. Pathogenic role of angiotensin II and the NF-κB system in a model of malignant hypertensive nephrosclerosis. Hypertens. Res. 2019, 42, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Bai, J.; Li, J.M.; Che, Y.L.; Lin, Q.Y.; Zhang, Y.L.; Li, H.H. The immunoproteasome subunit LMP10 mediates angiotensin II-induced retinopathy in mice. Redox Biol. 2018, 16, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, J.P.; Sangiovanni, P.M.; Sapieha, P.; De Guire, V. MiRNAs, single nucleotide polymorphisms (SNPs) and age-related macular degeneration (AMD). Clin. Chem. Lab. Med. 2017, 55, 763–775. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mishra, M.; Kumar, B. Diabetic retinopathy and transcriptional regulation of a small molecular weight G-Protein, Rac1. Exp. Eye Res. 2016, 147, 72–77. [Google Scholar] [CrossRef]
- Wongwarawipat, T.; Papageorgiou, N.; Bertsias, D.; Siasos, G.; Tousoulis, D. Olive Oil-related Anti-inflammatory Effects on Atherosclerosis: Potential Clinical Implications. Endocr. Metab. Immune Disord.-Drug Targets 2017, 18, 51–62. [Google Scholar] [CrossRef]
- Ampofo, E.; Berg, J.J.; Menger, M.D.; Laschke, M.W. Maslinic acid alleviates ischemia/reperfusion-induced inflammation by downregulation of NFκB-mediated adhesion molecule expression. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Lee, W.; Kim, J.; Park, E.K.; Bae, J.S. Maslinic acid ameliorates inflammation via the downregulation of NF-κB and STAT-1. Antioxidants 2020, 9, 106. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, S.H.; Punia, S.; Sharma, K.A. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016, 10, 21–33. [Google Scholar] [CrossRef]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.M.; Li, J.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Lambros, M.L.; Plafker, S.M. Oxidative Stress and the Nrf2 Anti-Oxidant Transcription Factor in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2016, 854, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, H.; Chen, X. Protective effects of sulforaphane on diabetic retinopathy: Activation of the nrf2 pathway and inhibition of nlrp3 inflammasome formation. Exp. Anim. 2019, 68, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Deliyanti, D.; Lee, J.Y.; Petratos, S.; Meyer, C.J.; Ward, K.W.; Wilkinson-Berka, J.L.; de Haan, J.B. A potent Nrf2 activator, dh404, bolsters antioxidant capacity in glial cells and attenuates ischaemic retinopathy. Clin. Sci. 2016, 130, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huélamo, M.; Rodríguez-Morató, J.; Boronat, A.; de la Torre, R. Modulation of Nrf2 by olive oil and wine polyphenols and neuroprotection. Antioxidants 2017, 6, 73. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, R.; Wu, R.; Ramirez, C.N.; Sargsyan, D.; Li, S.; Wang, L.; Cheng, D.; Wang, C.; Hudlikar, R.; et al. DNA methylome and transcriptome alterations and cancer prevention by triterpenoid ursolic acid in UVB-induced skin tumor in mice. Mol. Carcinog. 2019, 58, 1738–1753. [Google Scholar] [CrossRef]

| Class | Compound | Extra Virgin Olive Oil (EVOO) | Acebuchina Oil (ACE Oil) |
|---|---|---|---|
| Fatty acids (%) | Myristic acid. C14:0 | <LOD 1 | <LOD |
| Palmitic acid. C16:0 | 10.56 | 12.90 | |
| Palmitoleic acid. C16:1 | 0.79 | 1.25 | |
| Margaric acid. C17:0 | 0.11 | 0.09 | |
| Margaroleic acid. C17:1 | 0.17 | 0.16 | |
| Stearic acid. C18:0 | 2.97 | 2.36 | |
| Oleic acid. C18:1 | 76.61 | 74.42 | |
| Linoleic acid. C18:2 | 7.18 | 7.34 | |
| Arachidonic acid. C20:0 | 0.44 | 0.39 | |
| Linolenic acid. C18:3 | 0.77 | 0.70 | |
| Gondoic acid. C20:1 | 0.31 | 0.29 | |
| Behenic acid. C22:0 | 0.11 | 0.12 | |
| Lignoceric acid. C24:0 | <LOD | <LOD | |
| Total (%) | 100.0 | 100.0 | |
| Acidity (%C18:1) | ≤ 0.8 | 0.14 | |
| Sterols (%) | Cholesterol | 0.36 | 0.37 |
| Bassicasterol | <LOD | <LOD | |
| 24-Methylenecholesterol | <LOD | <LOD | |
| Campesterol | 3.06 | 3.59 | |
| Campestanol | <LOD | <LOD | |
| Stigmasterol | 0.59 | 0.96 | |
| Δ7-Campesterol | <LOD | <LOD | |
| Δ5,23-Stigmastadienol | <LOD | <LOD | |
| Clerosterol | 1.07 | 1.02 | |
| Δ-Sitosterol | 84.60 | 85.89 | |
| Sitostanol | 1.46 | 1.1 | |
| Δ5-Avenasterol | 7.44 | 7.89 | |
| Δ5,24-Stigmastadienol | 0.37 | 0.64 | |
| Δ7-Stigmastenol | 0.31 | 0.25 | |
| Δ7-Avenasterol | 0.49 | 0.46 | |
| Total (mg/kg) | 1531.4 | 1735 | |
| Tocopherols (%) | α-Tocopherol | 94.60 | 97.2 |
| β-Tocopherol | 2.4 | 0.8 | |
| γ-Tocopherol | 3.0 | 2.1 | |
| δ-Tocopherol | <LOD | <LOD | |
| Total (mg/kg) | 221.76 | 343.8 | |
| Pentacyclic triterpenes | |||
| Triterpene acids (%) | Oleanolic acid | 27.03 | 34.5 |
| Ursolic acid | 37.93 | 24.9 | |
| Maslinic acid | 34.46 | 40.6 | |
| Total (mg/kg) | 153.12 | 340.3 | |
| Triterpene alcohols | Erythrodiol+Uvaol | 37 | 45.6 |
| (mg/kg) | |||
| Polyphenols | Ortodiphenols | 125 | 86.7 |
| (mg/kg) | Secoiridoids | 83 | 147.8 |
| Total (mg/kg) | 250 | 261.8 | |
| Total (mg/kg in Tyrosol) | 162 | 170 |
| Primary Antibody | Origin | Dilution | Reference |
|---|---|---|---|
| Anti-NOX1 | Mouse monoclonal | 1:200 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| Anti-NOX2 | Rabbit monoclonal | 1:100 | Epitomics-Abcam, Burlingame, CA, USA |
| Anti-NOX4 | Rabbit monoclonal | 1:500 | Epitomics-Abcam |
| Anti-GFAP | Mouse monoclonal | 1:500 | Santa Cruz Biotechnology |
| Anti-Arginase 1 | Mouse monoclonal | 1:100 | Santa Cruz Biotechnology |
| Anti-Arginase 2 | Mouse monoclonal | 1:100 | Santa Cruz Biotechnology |
| Anti-CD31 | Rabbit monoclonal | 1:200 | Rockland Immunochemicals, Limerick, PA, USA |
| Primary Antibody | Origin | Dilution | Secondary Antibody | Dilution | Reference |
|---|---|---|---|---|---|
| Anti-NOX1 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | Santa Cruz Biotechnology, CA, USA |
| Anti-NOX2 | Rabbit monoclonal | 1:8000 | Goat Anti-Rabbit | 1:9000 | Epitomics-Abcam, Burlingame, CA, USA |
| Anti-NOX4 | Rabbit monoclonal | 1:7000 | Goat Anti-Rabbit | 1:8000 | Epitomics-Abcam |
| Anti-T-eNOS | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | Santa Cruz Biotechnology |
| Anti-p-eNOS Ser1177 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | Santa Cruz Biotechnology |
| Anti-p-eNOS Thr495 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | Santa Cruz Biotechnology |
| Anti-iNOS | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | Santa Cruz Biotechnology |
| Anti-Nitrotyrosine | Mouse Monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | Santa Cruz Biotechnology |
| Anti-GFAP | Mouse monoclonal | 1:2000 | Goat Anti-Mouse | 1:4000 | Santa Cruz Biotechnology |
| Anti-GSH-Px1/2 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:4000 | Santa Cruz Biotechnology |
| Anti-GSH-Red | Rabbit polyclonal | 1:5000 | Goat Anti-Rabbit | 1:8000 | Santa Cruz Biotechnology |
| Anti-SOD-1 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | Santa Cruz Biotechnology |
| Anti-Arginase 1 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | Santa Cruz Biotechnology |
| Anti-Arginase 2 | Mouse monoclonal | 1:1000 | Goat Anti-Mouse | 1:2000 | Santa Cruz Biotechnology |
| Anti-β-Actin | Mouse monoclonal | 1:20,000 | Goat Anti-Mouse | 1:30,000 | Santa Cruz Biotechnology |
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Accesion Number |
|---|---|---|---|
| NOX1 | TTCACCAATTCCCAGGATTGAAGTGGATGGTC | GACCTGTCACGATGTCAGTGGCCTTGTCAA | AY174116.1 |
| NOX2 | CCCTTTGGTACAGCCAGTGAAGAT | CAATCCCACGTCCCACTAACATCA | FJ168469.1 |
| NOX4 | ATCACAGAAGGTCCCTAGCA | TAACCATGAGGAACAATACCAC | AF276957.1 |
| eNOS | AACTCCTGTCTTCCATCAAGAG | TTCACTGCATTGGCTACTTCC | U53142.1 |
| iNOS | TTTGTGCGAAGTGTCAGTGG | CCTCCTTTGAGCCCTTTGTG | BC062378.1 |
| GSH-Px1/2 | GGAGAATGGCAAGAATGAAGA | CCGCAGGAAGGTAAAGAG | NM001329528.1 |
| GSH-Red | CACCTCTTCCTTCGACTACC | GCTTGATGACATGCCAACTG | BC056358.1 |
| SOD-1 | CGTCATTCACTTCGAGCAGAAGG | GTCTGAGACTCAGACCACATA | AF223251.1 |
| NF-κB | CCCTAAAGATTGTGCCAAGAG | GAAAGAGGTTATCCTGAAATCCC | BC138535.1 |
| Nrf-2 | ACATTCCCAAACAAGATGCC | GGTATTAAGACACTGTAATTCGGG | BC026943.1 |
| GAPDH | GCCAAAAGGGTCATCATCTCCGC | GGATGACCTTGCCCACAGCCTTG | XM017321385.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Garrido, Á.; Reyes-Goya, C.; Pérez-Camino, M.C.; André, H.; Mate, A.; Vázquez, C.M. Retinoprotective Effect of Wild Olive (Acebuche) Oil-Enriched Diet against Ocular Oxidative Stress Induced by Arterial Hypertension. Antioxidants 2020, 9, 885. https://doi.org/10.3390/antiox9090885
Santana-Garrido Á, Reyes-Goya C, Pérez-Camino MC, André H, Mate A, Vázquez CM. Retinoprotective Effect of Wild Olive (Acebuche) Oil-Enriched Diet against Ocular Oxidative Stress Induced by Arterial Hypertension. Antioxidants. 2020; 9(9):885. https://doi.org/10.3390/antiox9090885
Chicago/Turabian StyleSantana-Garrido, Álvaro, Claudia Reyes-Goya, M. Carmen Pérez-Camino, Helder André, Alfonso Mate, and Carmen M. Vázquez. 2020. "Retinoprotective Effect of Wild Olive (Acebuche) Oil-Enriched Diet against Ocular Oxidative Stress Induced by Arterial Hypertension" Antioxidants 9, no. 9: 885. https://doi.org/10.3390/antiox9090885
APA StyleSantana-Garrido, Á., Reyes-Goya, C., Pérez-Camino, M. C., André, H., Mate, A., & Vázquez, C. M. (2020). Retinoprotective Effect of Wild Olive (Acebuche) Oil-Enriched Diet against Ocular Oxidative Stress Induced by Arterial Hypertension. Antioxidants, 9(9), 885. https://doi.org/10.3390/antiox9090885