Antioxidant Profile of Pepper (Capsicum annuum L.) Fruits Containing Diverse Levels of Capsaicinoids
Abstract
:1. Introduction
2. Materials and Methods
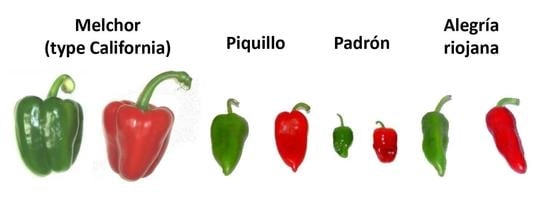
2.1. Plant Material
2.2. Determination of Capsaicin and Dihydrocapsaicin by High-Performance Liquid Chromatography-Electrospray Mass Spectrometry (HPLC-ES/MS)
2.3. Detection and Quantification of Ascorbate, GSH and GSSG by High-Performance Liquid Chromatography-Electrospray Mass Spectrometry (LC-ES/MS)
2.4. Preparation of Crude Extracts for Enzyme Activity
2.5. Enzyme Activity Assays
2.6. Immunoblot Analysis
2.7. Statistical Analysis
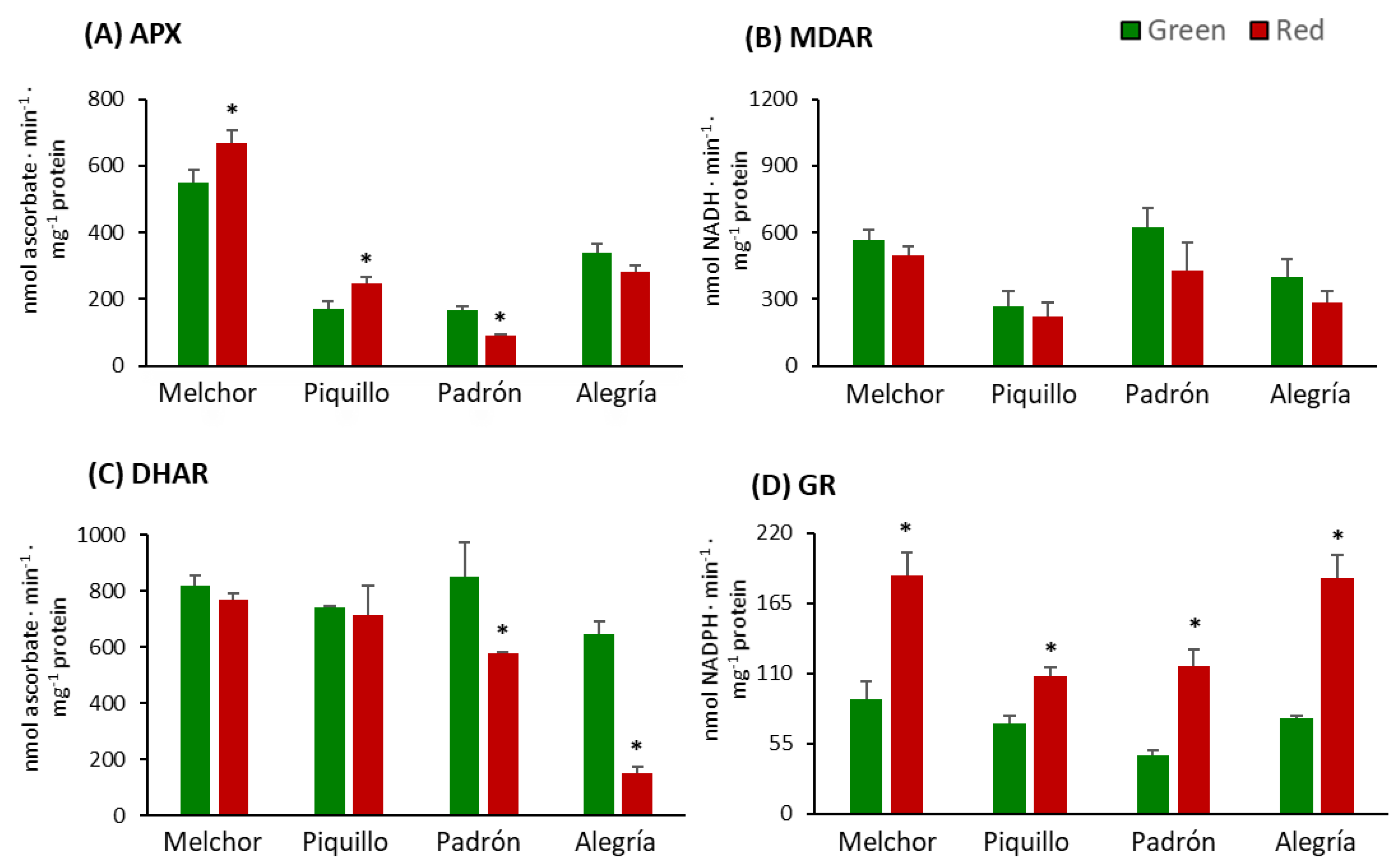
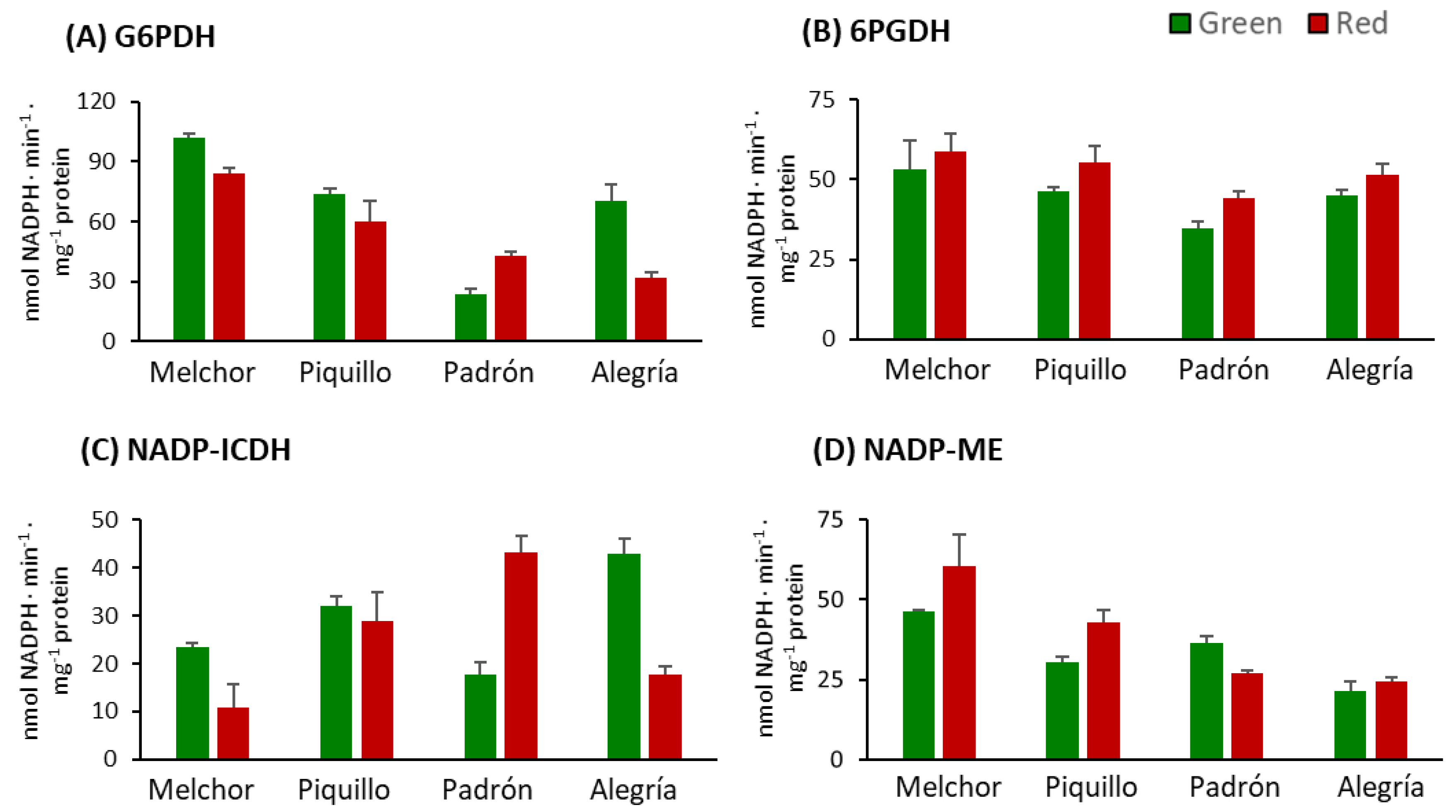
3. Results
4. Discussion
4.1. The Experimental Design Provided a Gradual Capsaicin Concentration Depending on the Pepper Variety and the Ripening Stage
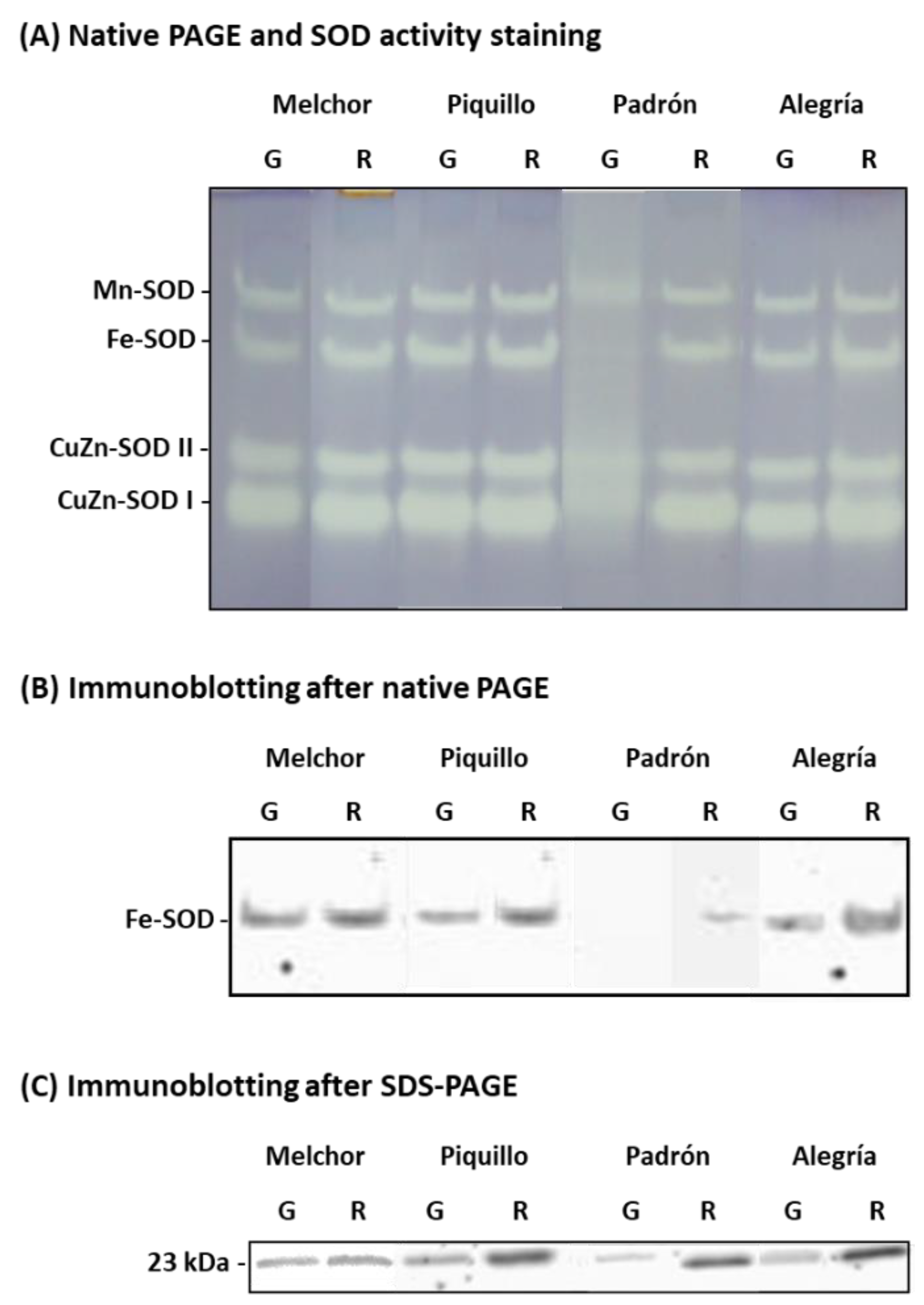
4.2. The Ripening Stage and the Capsaicinoids Content Alter the Metabolism of Enzymatic Antioxidants
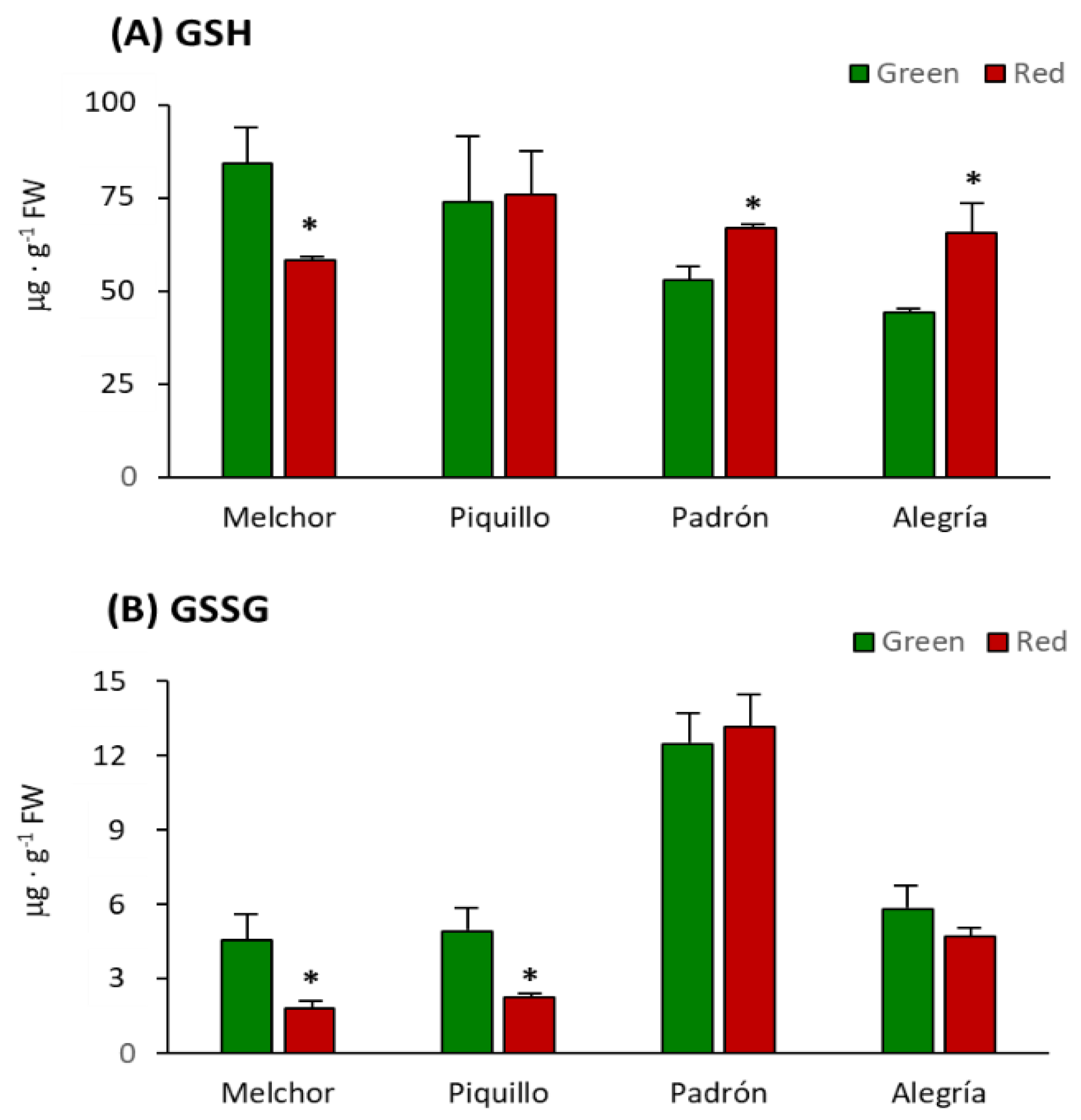
4.3. The Higher Capsaicinoids Level the Higher Ascorbate and Glutathione Content
5. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; Van Buren, L.; Wagner, E.; Wiseman, S.; Van De Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Rad. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Mariko, N.; Hassimoto, A.; Genovese, M.I.; Lajolo, F.M. Antioxidant capacity of Brazilian fruit, vegetables and commercially-frozen fruit pulps. J. Food Comp. Anal. 2009, 22, 394–396. [Google Scholar]
- Mateos, R.M.; Jiménez, A.; Román, P.; Romojaro, F.; Bacarizo, S.; Leterrier, M.; Gómez, M.; Sevilla, F.; Del Río, L.A.; Corpas, F.J.; et al. Antioxidant systems from pepper (Capsicum annuum L.): Involvement in the response to temperature changes in ripe fruits. Int. J. Mol. Sci. 2013, 14, 9556–9580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, J.M.; Sevilla, F.; Jiménez, A.; Del Río, L.A.; Corpas, F.J.; Álvarez de Morales, P.; Camejo, D.M. Physiology of pepper fruit and the metabolism of antioxidants: Chloroplasts, mitochondria and peroxisomes. Ann. Bot. 2015, 116, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Palma, J.M.; Corpas, F.J.; Ruiz, C.; Molina, T.; Campos-Ramos, M.J.; Juanena, A.; Torreira, J.R. Los pimientos de las variedades Padrón, Piquillo y Alegría riojana: Una buena fuente de macro y microelementos para nuestra dieta. Horticultura 2016, 323, 60–64. [Google Scholar]
- Rodríguez-Ruiz, M.; Mateos, R.M.; Codesido, V.; Corpas, F.J.; Palma, J.M. Characterization of the galactono-1,4-lactone dehydrogenase from pepper fruits and its modulation in the ascorbate biosynthesis. Role of nitric oxide. Redox Biol. 2017, 12, 171–181. [Google Scholar] [CrossRef]
- Corpas, F.J.; Freschi, L.; Rodríguez-Ruiz, M.; Mioto, P.T.; González-Gordo, S.; Palma, J.M. Nitro-oxidative metabolism during fruit ripening. J. Exp. Bot. 2018, 69, 3449–3463. [Google Scholar] [CrossRef]
- Song, W.; Derito, C.M.; Liu, M.K.; He, H.J.; Dong, M.; Hai Liu, R. Cellular antioxidant activity of common vegetables. J. Agric. Food Chem. 2010, 58, 6621–6629. [Google Scholar] [CrossRef]
- Palma, J.M.; Corpas, F.J.; Del Río, L.A. Proteome of plant peroxisomes: New perspectives on the role of these organelles in cell biology. Proteomics 2009, 9, 2301–2312. [Google Scholar] [CrossRef]
- Martí, M.C.; Camejo, D.; Vallejo, F.; Romojaro, F.; Bacarizo, S.; Palma, J.M.; Sevilla, F.; Jiménez, A. Influence of fruit ripening stage and harvest period on the antioxidant content of sweet pepper cultivars. Plant Foods Hum. Nut. 2011, 66, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; D’Acierno, A.; Cozzolino, A.; Spigno, P.; Riccardi, R.; Raimo, F.; Pane, C.; Zaccardelli, M.; Lombardo, V.T.; Tucci, M.; et al. Biochemical characterization of traditional varieties of sweet pepper (Capsicum annuum L.) of the Campania Region, Southern Italy. Antioxidants 2020, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.K.; De, A.K. Capsicum: Historical and Botanical Perspectives. In Capsicum. The Genus Capsicum; De, A.K., Ed.; Taylor & Francis: London, UK, 2003; pp. 1–15. ISBN 0-415-29991-8. [Google Scholar]
- Topuz, A.; Ozdemir, F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. J. Food Comp. Anal. 2007, 20, 596–602. [Google Scholar] [CrossRef]
- Roy, A. Bhut jolokia (Capsicum chinense jaqc): A review. Int. J. Pharm. Sci. Res. 2016, 7, 882–889. [Google Scholar]
- Aguiar, A.C.; Coutinho, J.P.; Fernández-Barbero, G.; Godoy, H.T.; Martinez, J. Comparative study of capsaicinoid composition in capsicum peppers grown in Brazil. Int. J. Food Prop. 2016, 19, 1292–1302. [Google Scholar] [CrossRef] [Green Version]
- Parvez, G.M.M. Current advances in pharmacological activity and toxic effects of various Capsicum species. Int. J. Pharm. Sci. Res. 2017, 8, 1900–1912. [Google Scholar]
- Kopta, T.; Sekara, A.; Pokluda, R.; Ferby, V.; Caruso, G. Screening of chilli pepper genotypes as a source of capsaicinoids and antioxidants under conditions of simulated drought stress. Plants 2020, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K. Biosynthesis of capsaicinoids in Capsicum. In Capsicum. The Genus Capsicum; De, A.K., Ed.; Taylor & Francis: London, UK, 2003; pp. 87–95. ISBN 0-415-29991-8. [Google Scholar]
- Reyes-Escogido, M.L.; González-Mondragón, E.G.; Vázquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [Green Version]
- Garcés-Claver, A.; Arnedo-Andrés, M.S.; Abadía, J.; Gil-Ortega, R.; Álvarez-Fernández, A. Determination of capsaicin and dihydrocapsaicin in Capsicum fruits by Liquid Chromatography−Electrospray/Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2007, 54, 9303–9311. [Google Scholar] [CrossRef] [Green Version]
- Al Othman, Z.A.; Ahmed, Y.B.; Habila, M.A.; Ghafar, A.A. Determination of capsaicin and dihydrocapsaicin in Capsicum fruit samples using high performance liquid chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lin, Y. The bioactivity of capsaicin on peach aphid and its combination with several insecticides. J. Pest Sci. 2003, 52, 94–96. [Google Scholar]
- Jin, M.S.; Liu, S.W.; Gu, Z.M.; Wei, S.H.; Wang, Y.Z. Repellent activity of capsaicin and its effects on glutathione-S-transferase and Na+, K+- ATPase activity in Plutella xylostella (Lepidoptera: Plutellidae). Acta Entomol. Sin. 2008, 51, 1039–1043. [Google Scholar]
- Aley, J.P.; Adams, N.J.; Ladyman, R.J.; Fraser, D.L. The efficacy of capsaicin as an equine repellent for chewing wood. J. Veter Behav. Clin. App. Res. 2015, 10, 243–247. [Google Scholar] [CrossRef]
- Dickens, J.C.; Bohbot, J.D. Neuromolecular basis of repellent action. In Insect Repellents Handbook, 2nd ed.; Debboun, M., Frances, S.P., Strickman, D.A., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 31–42. [Google Scholar]
- Li, B.; Yang, M.; Shi, R.; Ye, M. Insecticidal activity of natural capsaicinoids against several agricultural insects. Nat. Prod. Commun. 2019. [Google Scholar] [CrossRef] [Green Version]
- Sancho, R.; Lucena, C.; Macho, A.; Calzado, M.A.; Blanco-Molina, M.; Minassi, A.; Appendino, G.; Muñoz, E. Immunosuppressive activity of capsaicinoids: Capsiate derived from sweet peppers inhibits NF-kappa activation and is a potent antiinflammatory compound in vivo. Eur. J. Immunol. 2002, 32, 1753–1763. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, H.; Zhao, Z.; Wang, J.; Guo, D.; Liu, Y. Regulation of Actg1 and Gsta2 is possible mechanism by which capsaicin alleviates apoptosis in cell model of 6-OHDA-induced Parkinson’s disease. Biosci. Rep. 2020, 40, BSR20191796. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal chemistry, pharmacology, and clinical implications of TRPV1 receptor antagonists. Med. Res. Rev. 2017, 37, 936–983. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, J. Understand spiciness: Mechanism of TRPV1 channel activation by capsaicin. Protein Cell 2017, 8, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From plants to a cancer-suppressing agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.; Ho Lee, S. Anticancer properties of capsaicin against human cancer. Anticancer Res. 2016, 36, 837–844. [Google Scholar] [PubMed]
- Klie, S.; Osorio, S.; Tohge, T.; Drincovich, M.F.; Fait, A.; Giovannoni, J.J.; Fernie, A.R.; Nikoloski, Z. Conserved changes in the dynamics of metabolic processes during fruit development and ripening across species. Plant Physiol. 2014, 164, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palma, J.M.; Corpas, F.J.; Del Río, L.A. Proteomics as an approach to the understanding of the molecular physiology of fruit development and ripening. J. Proteom. 2011, 74, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
- Barsan, C.; Zouine, M.; Maza, E.; Bian, W.; Egea, I.; Rossignol, M.; Bouyssie, D.; Pichereaux, C.; Purgatto, E.; Bouzayen, M.; et al. Proteomic analysis of chloroplast to chromoplast transition in tomato reveals metabolic shifts coupled with disrupted thylakoid biogenesis machinery and elevated energy-production components. Plant Physiol. 2012, 160, 708–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpas, F.J.; Palma, J.M. Nitric oxide on/off in fruit ripening. Plant Biol. 2018, 20, 805–807. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.; Ren, L.; Li, A.; Chen, G.; Hu, Z. The SlFSR gene controls fruit shelf-life in tomato. J. Exp. Bot. 2018, 69, 2897–2909. [Google Scholar] [CrossRef] [Green Version]
- González-Gordo, S.; Bautista, R.; Claros, M.G.; Cañas, A.; Palma, J.M.; Corpas, F.J. Nitric oxide-dependent regulation of sweet pepper fruit ripening. J. Exp. Bot. 2019, 70, 4557–4570. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Ruiz, M.; González-Gordo, S.; Cañas, A.; Campos, M.J.; Paradela, A.; Corpas, F.J.; Palma, J.M. Sweet pepper (Capsicum annuum L.) fruits contain an atypical peroxisomal catalase that is modulated by reactive oxygen and nitrogen species. Antioxidants 2019, 8, 374. [Google Scholar] [CrossRef] [Green Version]
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, polyphenols and antioxidant activities of Capsicum annuum: Comparative study of the effect of ripening stage and cooking methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef] [Green Version]
- Cisternas-Jamet, J.; Salvatierra-Martínez, R.; Vega-Gálvez, A.; Stoll, A.; Uribe, E.; Goñi, M.G. Biochemical composition as a function of fruit maturity stage of bell pepper (Capsicum annum) inoculated with Bacillus amyloliquefaciens. Sci. Hortic. 2020, 263, 109107. [Google Scholar] [CrossRef]
- Ribes-Moya, A.M.; Adalid, A.M.; Raigón, M.D.; Hellín, P.; Fita, A.; Rodríguez-Burruezo, A. Variation in flavonoids in a collection of peppers (Capsicum sp.) under organic and conventional cultivation: Effect of the genotype, ripening stage, and growing system. J. Sci. Food Agric. 2020, 100, 2208–2223. [Google Scholar] [CrossRef]
- Martí, M.C.; Camejo, D.; Olmos, E.; Sandalio, L.M.; Fernández-García, N.; Jiménez, A.; Sevilla, F. Characterisation and changes in the antioxidant system of chloroplasts and chromoplasts isolated from green and mature pepper fruits. Plant Biol. 2009, 11, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.M.; Bonilla-Valverde, D.; Del Río, L.A.; Palma, J.M.; Corpas, F.J. NADP-dehydrogenases from pepper fruits: Effect of maturation. Physiol. Plant. 2009, 135, 130–139. [Google Scholar] [CrossRef]
- Ramírez-Serrano, R.; Larrinaga-Mayoral, J.A.; Murillo-Amador, B.; Hernández-Saavedra, N.Y.; Fujiyama, H. ntioxidant enzymatic response of hot pepper (Capsicum annuum L.) under saline stress conditions. Interciencia 2008, 33, 377–383. [Google Scholar]
- Boonsiri, K.; Ketsa, S.; Van Doorn, W.G. Seed browning of hot peppers during low temperature storage. Postharvest Biol. Technol. 2007, 45, 358–365. [Google Scholar] [CrossRef]
- Tan, C.K.; Ali, Z.M.; Zainal, Z. Changes in ethylene production, carbohydrase activity and antioxidant status in pepper fruits during ripening. Sci. Hortic. 2012, 142, 23–31. [Google Scholar] [CrossRef]
- Airaki, M.; Sánchez-Moreno, L.; Leterrier, M.; Barroso, J.B.; Palma, J.M.; Corpas, F.J. Detection and quantification of S-nitrosoglutathione (GSNO) in pepper (Capsicum annuum L.) plant organs by LC-ES/MS. Plant Cell Physiol. 2011, 52, 2006–2015. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Hossain, M.A.; Asada, K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: Its protection by ascorbate. Plant Cell Physiol. 1984, 25, 1285–1295. [Google Scholar]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplast and its participation in regeneration of ascorbate for scavenging of hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Dalton, D.A.; Baird, L.M.; Langeberg, L.; Taugher, C.Y.; Anyan, W.R.; Vance, C.P.; Sarath, G. Subcellular localization of oxygen defense enzymes in soybean (Glycine max [L.] Merr.) root nodules. Plant Physiol. 1993, 102, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Edwards, E.A.; Rawsthone, S.; Mullineaux, P.M. Subcellular distribution ofmultiple forms of glutathione reductase in leaves of pea (Pisum sativum L.). Planta 1990, 180, 278–284. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein. J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Houmani, H.; Rodríguez-Ruiz, M.; Palma, J.M.; Abdelly, C.; Corpas, F.J. Modulation of superoxide dismutase (SOD) isozymes by organ development and high long-term salinity in the halophyte Cakile maritima. Protoplasma 2016, 253, 885–894. [Google Scholar] [CrossRef]
- Pinilla, M.; Iglesias-Moya, J.; Campos, M.J.; Corpas, F.J.; Palma, J.M. Pomegranate (Punica granatum L.) Fruits: Characterization of the main enzymatic antioxidants (peroxisomal catalase and SOD isozymes) and the NADPH-regenerating system. Agronomy 2019, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Leterrier, M.; Airaki, M.; Palma, J.M.; Chaki, M.; Barroso, J.B.; Corpas, F.J. Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 2012, 166, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, M.; Barroso, J.B.; Palma, J.M.; Corpas, F.J. Cytosolic NADP-isocitrate dehydrogenase in Arabidopsis leaves and roots. Biol. Plant. 2012, 56, 705–710. [Google Scholar] [CrossRef]
- Barroso, J.B.; Peragon, J.; Contreras-Jurado, C.; Garcia-Salguero, L.; Corpas, F.J.; Esteban, F.J.; Peinado, M.A.; De la Higuera, M.; Lupiáñez, J.A. Impact of starvation-refeeding on kinetics and protein expression of trout liver NADPH-production systems. Am. J. Physiol. 1998, 274, R1578–R1587. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Sandalio, L.M.; Distefano, S.; Palma, J.M.; Lupiáñez, J.A.; Del Río, L.A. A dehydrogenase-mediated recycling system of NADPH peroxisomes. Biochem. J. 1998, 330, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Freschi, L.; Rodríguez-Ruiz, M.; González-Gordo, S.; Corpas, F.J. Nitric oxide in the physiology and quality of fleshy fruits. J. Exp. Bot. 2019, 70, 4405–4417. [Google Scholar] [CrossRef]
- Chu-Puga, Á.; González-Gordo, S.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J. NADPH oxidase (Rboh) activity is up regulated during sweet pepper (Capsicum annuum L.) fruit ripening. Antioxidants 2019, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- González-Gordo, S.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J. Superoxide radical metabolism in sweet pepper (Capsicum annuum L.) fruits is regulated by ripening and by a NO-enriched environment. Front. Plant Sci. 2020, 11, 485. [Google Scholar] [CrossRef]
- Goicoechea, N.; Aguirreolea, J.; Cenoz, S.; García-Mina, J.M. Verticillium dahliae modifies the concentrations of proline, soluble sugars, starch, soluble protein and abscisic acid in pepper plants. Eur. J. Plant Pathol. 2000, 106, 19–25. [Google Scholar] [CrossRef]
- Goicoechea, N.; Aguirreolea, J.; Cenoz, S.; García-Mina, J.M. Gas exchange and flowering in Verticillium-wilted pepper plants. J. Phytopathol. 2001, 149, 281–286. [Google Scholar] [CrossRef]
- Goicoechea, N.; Aguirreolea, J.; García-Mina, J.M. Alleviation of verticillium wilt in pepper (Capsicum annuum L.) by using the organic amendment COACH of natural origin. Sci. Hortic. 2004, 101, 23–37. [Google Scholar] [CrossRef]
- Pascual, I.; Azcona, I.; Morales, F.; Aguirreolea, J.; Sánchez-Díaz, M. Photosynthetic response of pepper plants to wilt induced by Verticillium dahliae and soil water déficit. J. Plant Physiol. 2010, 167, 701–708. [Google Scholar] [CrossRef]
- Garmendia, I.; Goicoechea, N.; Aguirreolea, J. Effectiveness of three Glomus species in protecting pepper (Capsicum annuum L.) against verticillium wilt. Biol. Control. 2004, 31, 296–305. [Google Scholar] [CrossRef]
- Goicoechea, N.; Garmendia, I.; Sánchez-Díaz, M.; Aguirreolea, J. Arbuscular mycorrhizal fungi (AMF) as bioprotector agents against wilt induced by Verticillium spp. in pepper. Span. J. Agric. Res. 2010, 8, S25–S42. [Google Scholar] [CrossRef] [Green Version]
- Pascual, I.; Avilés, M.; Aguirreolea, J.; Sánchez-Díaz, M. Effect of sanitized and non-sanitized sewage sludge on soil microbial community and the physiology of pepper plants. Plant Soil 2008, 310, 41. [Google Scholar] [CrossRef]
- Pascual, I.; Azcona, I.; Aguirreolea, J.; Morales, F.; Corpas, F.J.; Palma, J.M.; Rellán-Álvarez, R.; Sánchez-Díaz, M. Growth, yield, and fruit quality of pepper plants amended with two sanitized sewage sludges. J. Agric. Food Chem. 2010, 58, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- García, T.; Gutiérrez, J.; Veloso, J.; Gago-Fuentes, R.; Díaz, J. Wounding induces local resistance but systemic susceptibility to Botrytis cinerea in pepper plants. J. Plant Pathol. 2015, 176, 202–209. [Google Scholar] [CrossRef]
- Gayoso, C.; De la Ilarduya, O.M.; Pomar, F.; Merino, F. Assessment of real-time PCR as a method for determining the presence of Verticillium dahliae in different Solanaceae cultivars. Eur. J. Plant Pathol. 2007, 118, 199–209. [Google Scholar] [CrossRef]
- Silvar, C.; Merino, F.; Díaz, J. Diversity of Phytophthora capsici in northwest Spain: Analysis of virulence, metalaxyl response, and molecular characterization. Plant Dis. 2006, 90, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, A.; Romojaro, F.; Llanos, M.R.; Gómez, J.M.; León, A.; Sevilla, F. Antioxidant systems and their relationship with the response of pepper fruits to storage at 20 °C. J. Agric. Food Chem. 2003, 51, 6293–6299. [Google Scholar] [CrossRef]
- Chaki, M.; Álvarez de Morales, P.; Ruiz, C.; Begara-Morales, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M. Ripening of pepper (Capsicum annuum) fruit is characterized by an enhancement of protein tyrosine nitration. Ann. Bot. 2015, 116, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Tortosa, G.; González-Gordo, S.; Ruiz, C.; Bedmar, E.J.; Palma, J.M. “Alperujo” compost improves the ascorbate (Vitamin C) content in pepper (Capsicum annuum L.) fruits and influences their oxidative metabolism. Agronomy 2018, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.K.; Ali, Z.M.; Zainal, Z. MnSOD and 9-LOX gene expression along with antioxidant changes of Capsicum annuum cv. Kulai during postharvest treatment. Sci. Hortic. 2016, 213, 403–409. [Google Scholar] [CrossRef]
- Azuma, R.; Ito, N.; Nakayama, N.; Suwa, R.; Nguyen, T.N.; Larrinaga-Mayoral, J.A.; Esaka, M.; Fujiyama, H.; Saneoka, H. Fruits are more sensitive to salinity than leaves and stems in pepper plants (Capsicum annuum L.). Sci. Hortic. 2010, 125, 171–178. [Google Scholar] [CrossRef]
- Li, R.; Li, D.W.; Liu, H.P.; Hong, C.L.; Song, M.Y.; Dai, Z.X.; Liu, J.W.; Zhou, J.; Weng, H.X. Enhancing iodine content and fruit quality of pepper (Capsicum annuum L.) through biofortification. Sci. Hortic. 2017, 214, 165–173. [Google Scholar] [CrossRef]
- Padilha, H.K.M.; Madruga, N.D.; Aranha, B.C.; Hoffmann, J.F.; Lopes Crizel, R.L.; Barbieri, R.L.; Chaves, F.C. Defense responses of Capsicum spp. genotypes to post-harvest Colletotrichum sp. inoculation. Phytoparasitica 2019, 47, 557–573. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Cañas, A.; López-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide 2018, 81, 36–45. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Palma, J.M.; Corpas, F.J. Inhibition of NADP-malic enzyme activity by H2S and NO in sweet pepper (Capsicum annuum L.) fruits. Physiol. Plant. 2020, 168, 278–288. [Google Scholar]
- León, A.M.; Palma, J.M.; Corpas, F.J.; Gómez, M.; Romero-Puertas, M.C.; Chatterjee, D.; Mateos, R.M.; Del Río, L.A.; Sandalio, L.M. Antioxidative enzymes in cultivars of pepper plants with different sensitivity to cadmium. Plant Physiol. Biochem. 2002, 40, 813–820. [Google Scholar]
- Kopta, T.; Slosar, M.; Andrejiova, A.; Jurica, M.; Pokluda, R. The influence of genotype and season on the biological potential of chilli pepper cultivars. Folia Hortic. 2019, 31, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, D.C.; Hertwig, K.M. Peroxidase-catalyzed oxidation of capsaicinoids: Steady-state and transient-state kinetic studies. Arch. Biochem. Biophys. 2003, 417, 18–26. [Google Scholar] [CrossRef]
- Díaz-Vivancos, P.; De Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione—Linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef]
- Mateos, R.M.; León, A.M.; Sandalio, L.M.; Gómez, M.; Del Río, L.A.; Palma, J.M. Peroxisomes from pepper fruits (Capsicum annuum L): Purification, characterization and antioxidant activity. J. Plant Physiol. 2003, 160, 1507–1516. [Google Scholar] [CrossRef]
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-nitrosoglutathione. Biochim. Biophys. Acta 2013, 1830, 3173–3181. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Alché, J.D.; Barroso, J.B. Current overview of S-nitrosoglutathione (GSNO) in higher plants. Front. Plant Sci. 2013, 4, 126. [Google Scholar] [CrossRef] [Green Version]
- Belcastro, E.; Gaucher, C.; Corti, A.; Leroy, P.; Lartaud, I.; Pompella, A. Regulation of protein function by S-nitrosation and S-glutathionylation: Processes and targets in cardiovascular pathophysiology. Biol. Chem. 2017, 398, 1267–1293. [Google Scholar] [CrossRef] [PubMed]
- Mazourek, M.; Pujar, A.; Borovsky, Y.; Paran, I.; Mueller, L.; Jahn, M.M. A dynamic interface for capsaicinoid systems biology. Plant Physiol. 2009, 150, 1806–1821. [Google Scholar] [CrossRef] [Green Version]
- Aghdam, M.S.; Palma, J.M.; Corpas, F.J. NADPH as quality footprinting in horticultural crops marketability. Trends Food Sci. Technol. 2020, 168, 111244. [Google Scholar] [CrossRef]
- Palma, J.M.; Álvarez de Morales, P.; Del Río, L.A.; Corpas, F.J. The proteome of fruit peroxisomes: Sweet pepper (Capsicum annuum L.) as a model. Subcell Biochem. 2018, 89, 323–341. [Google Scholar]
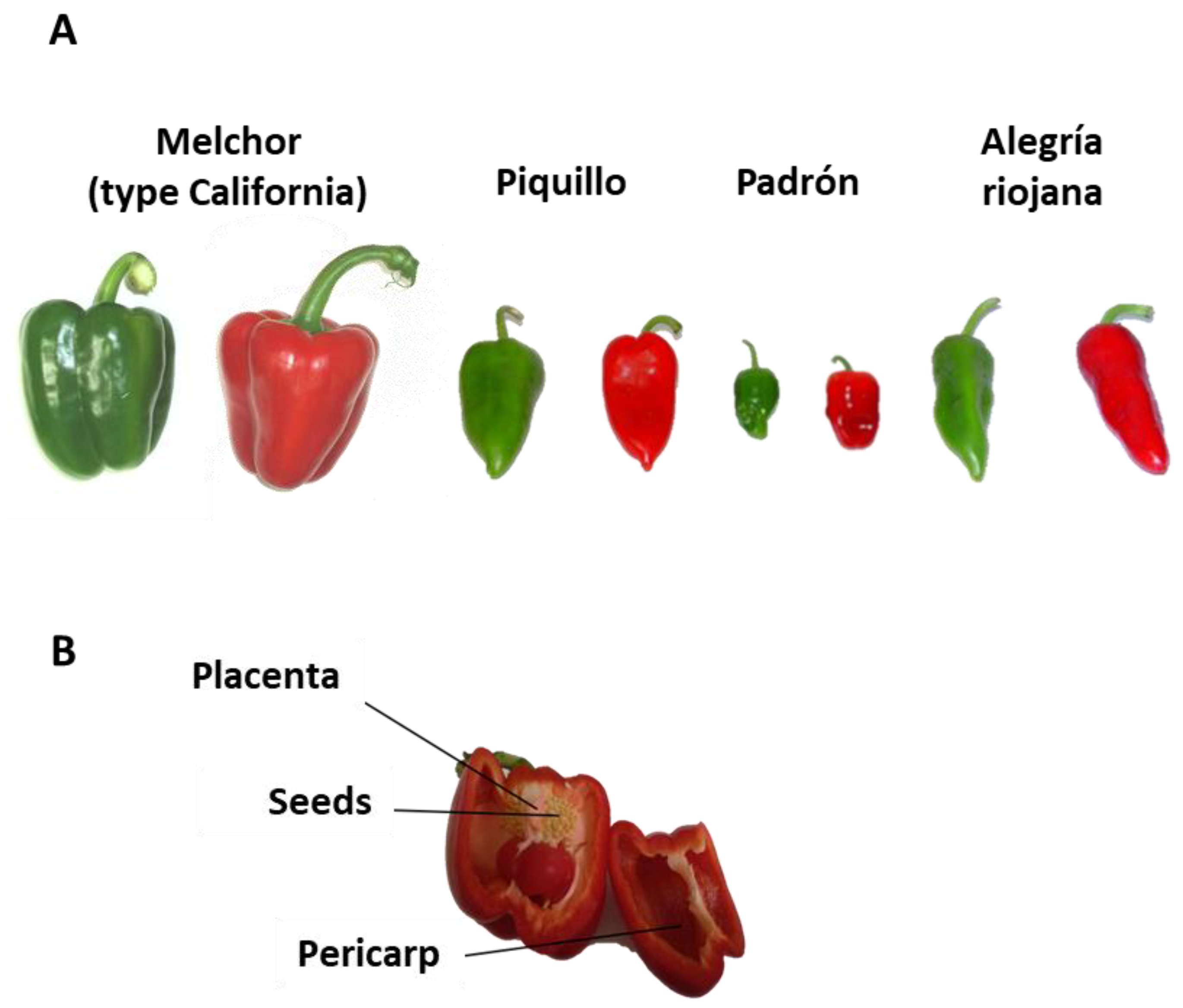


| Variety | FW Green (g) | FW Ripe Red (g) |
|---|---|---|
| Melchor | 245.22 ± 13.41 | 212.05 ± 12.45 |
| Piquillo | 43.50 ± 2.62 | 40.91 ± 6.69 |
| Padrón | 16.19 ± 1.91 | 24.37 ± 1.58 |
| Alegría | 43.78 ± 1.94 | 36.13 ± 2.38 |
| Variety | Ripening Stage | Tissue | Capsaicin (μg/g FW) | Dihydrocapsaicin (μg/g FW) | Capsaicin + Dihydrocapsaicin (μg/g FW) |
|---|---|---|---|---|---|
| Melchor | Green | Pericarp | 0 k | 0 k | 0 l |
| Placenta | 0 k | 0 k | 0 l | ||
| Red | Pericarp | 0 k | 0 k | 0 l | |
| Placenta | 0 k | 0 k | 0 l | ||
| Piquillo | Green | Pericarp | 0.40 ± 0.01 i | 0.56 ± 0.01 i | 0.96 ± 0.02 j |
| Placenta | 1.35 ± 0.63 gh | 0.24 ± 0.13 j | 1.59 ± 0.76 hijk | ||
| Red | Pericarp | 0.25 ± 0.02 j | 0.54 ± 0.01 i | 0.79 ± 0.03 k | |
| Placenta | 0.59 ± 0.03 h | 0.61 ± 0.01 h | 1.20 ±0.04 i | ||
| Padrón | Green | Pericarp | 2.11 ± 0.08 g | 0.03 ± 0.02 k | 2.14 ± 0.10 gh |
| Placenta | 244.09 ± 34.85 c | 33.10 ± 4.31 d | 277.19 ± 39.16 c | ||
| Red | Pericarp | 22.45 ± 2.26 e | 3.02 ± 0.19 f | 25.47 ± 2.45 e | |
| Placenta | 553.47 ± 29.59 b | 166.96 ± 5.00 b | 720.43 ± 34.59 b | ||
| Alegría | Green | Pericarp | 8.91 ± 1.69 f | 1.55 ± 0.21 g | 10.46 ± 1.90 f |
| Placenta | 205.23 ± 9.46 c | 72.96 ± 3.42 c | 278.19 ± 12.88 c | ||
| Red | Pericarp | 51.06 ± 0.55 d | 7.25 ± 0.35 e | 58.31 ± 0.90 d | |
| Placenta | 766.26 ± 37.00 a | 269.44 ± 27.77 a | 1035.70 ± 64.77 a |
| Variety | Ripening Stage | GSH + GSSG (μg·g−1 FW) | GSH/GSSG |
|---|---|---|---|
| Melchor | Green | 88.93 ± 12.84 | 18.55 |
| Melchor | Red | 60.12 ± 1.38 * | 32.02 * |
| Piquillo | Green | 78.83 ± 21.43 | 15.04 |
| Piquillo | Red | 78.84 ± 13.10 | 33.71 * |
| Padrón | Green | 65.24 ± 8.57 | 4.24 |
| Padrón | Red | 80.07 ± 5.75 * | 5.08 * |
| Alegría | Green | 50.08 ± 2.08 | 7.62 |
| Alegría | Red | 70.43 ± 4.16 * | 13.89 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, J.M.; Terán, F.; Contreras-Ruiz, A.; Rodríguez-Ruiz, M.; Corpas, F.J. Antioxidant Profile of Pepper (Capsicum annuum L.) Fruits Containing Diverse Levels of Capsaicinoids. Antioxidants 2020, 9, 878. https://doi.org/10.3390/antiox9090878
Palma JM, Terán F, Contreras-Ruiz A, Rodríguez-Ruiz M, Corpas FJ. Antioxidant Profile of Pepper (Capsicum annuum L.) Fruits Containing Diverse Levels of Capsaicinoids. Antioxidants. 2020; 9(9):878. https://doi.org/10.3390/antiox9090878
Chicago/Turabian StylePalma, José M., Fátima Terán, Alba Contreras-Ruiz, Marta Rodríguez-Ruiz, and Francisco J. Corpas. 2020. "Antioxidant Profile of Pepper (Capsicum annuum L.) Fruits Containing Diverse Levels of Capsaicinoids" Antioxidants 9, no. 9: 878. https://doi.org/10.3390/antiox9090878
APA StylePalma, J. M., Terán, F., Contreras-Ruiz, A., Rodríguez-Ruiz, M., & Corpas, F. J. (2020). Antioxidant Profile of Pepper (Capsicum annuum L.) Fruits Containing Diverse Levels of Capsaicinoids. Antioxidants, 9(9), 878. https://doi.org/10.3390/antiox9090878