Vitamin C and E Treatment Blunts Sprint Interval Training–Induced Changes in Inflammatory Mediator-, Calcium-, and Mitochondria-Related Signaling in Recreationally Active Elderly Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Outline
2.2. Muscle Function
2.3. Muscle Biopsies
2.4. Protein Analysis
2.5. Gene Expression Analysis
2.6. Blood Lactate Measurement
2.7. Statistical Analysis
3. Results
3.1. Performance during SIT Sessions
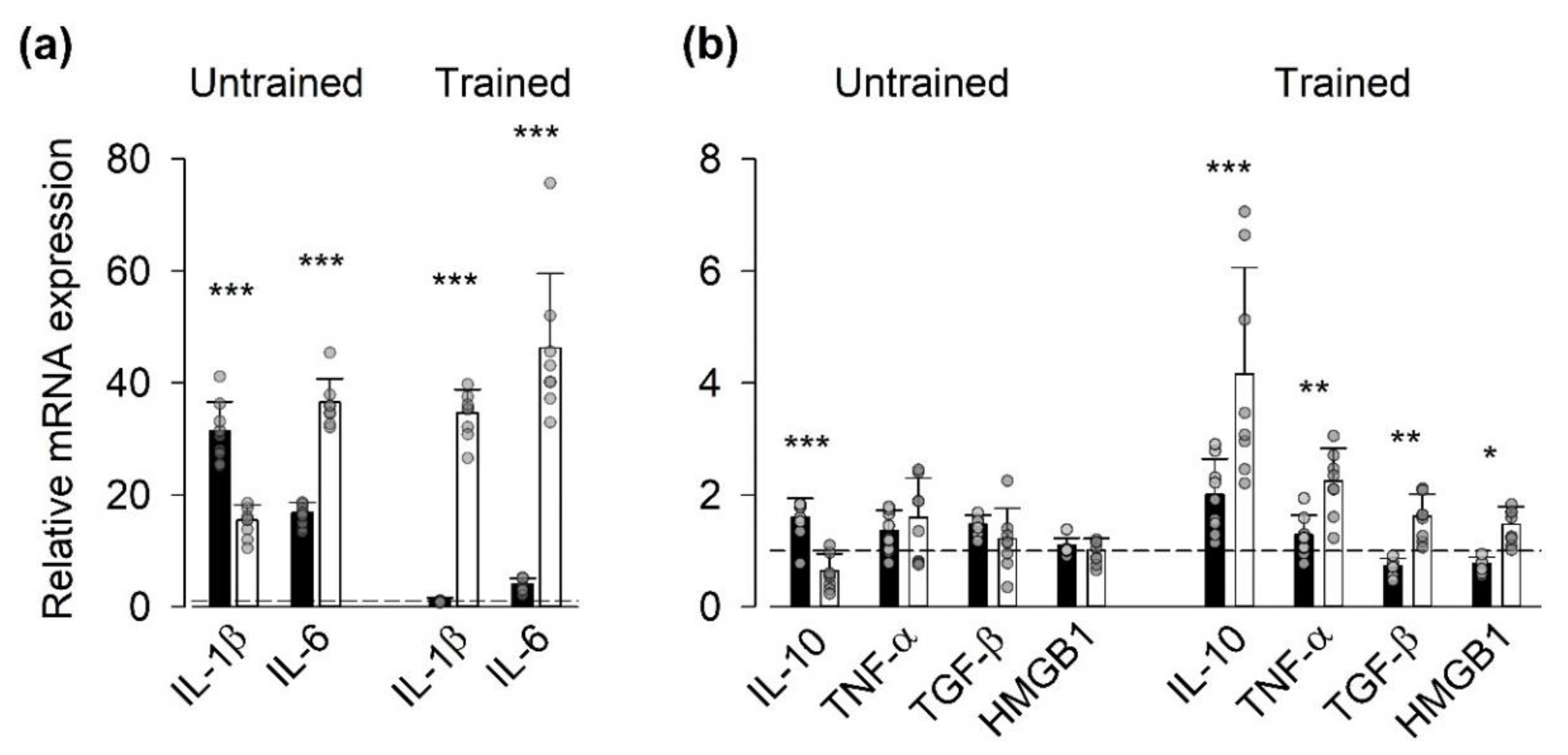
3.2. Gene Expression of Inflammatory Mediators
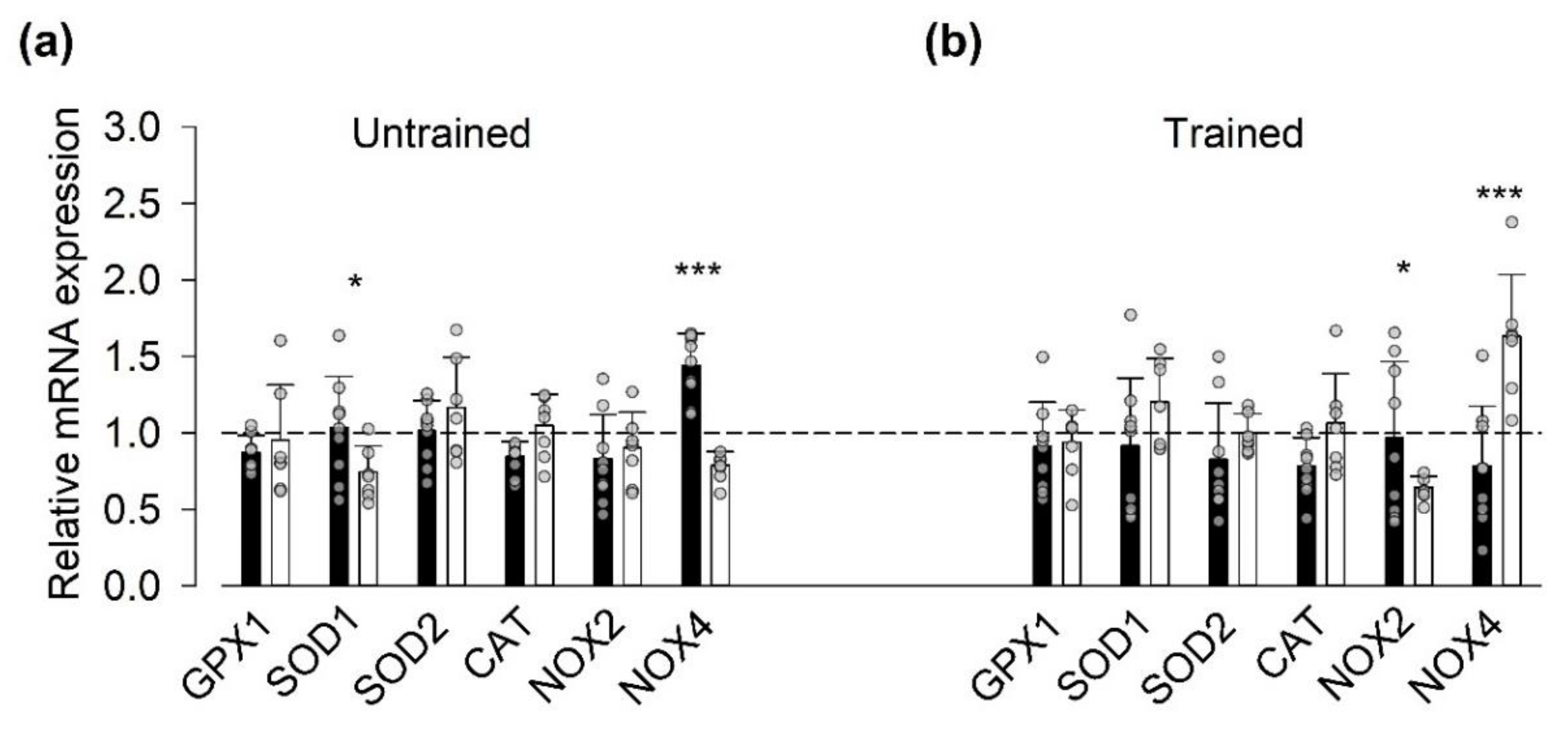
3.3. Gene Expression of ROS-Related Proteins
3.4. Protein and Gene Expression of SR Ca2+-Handling Proteins
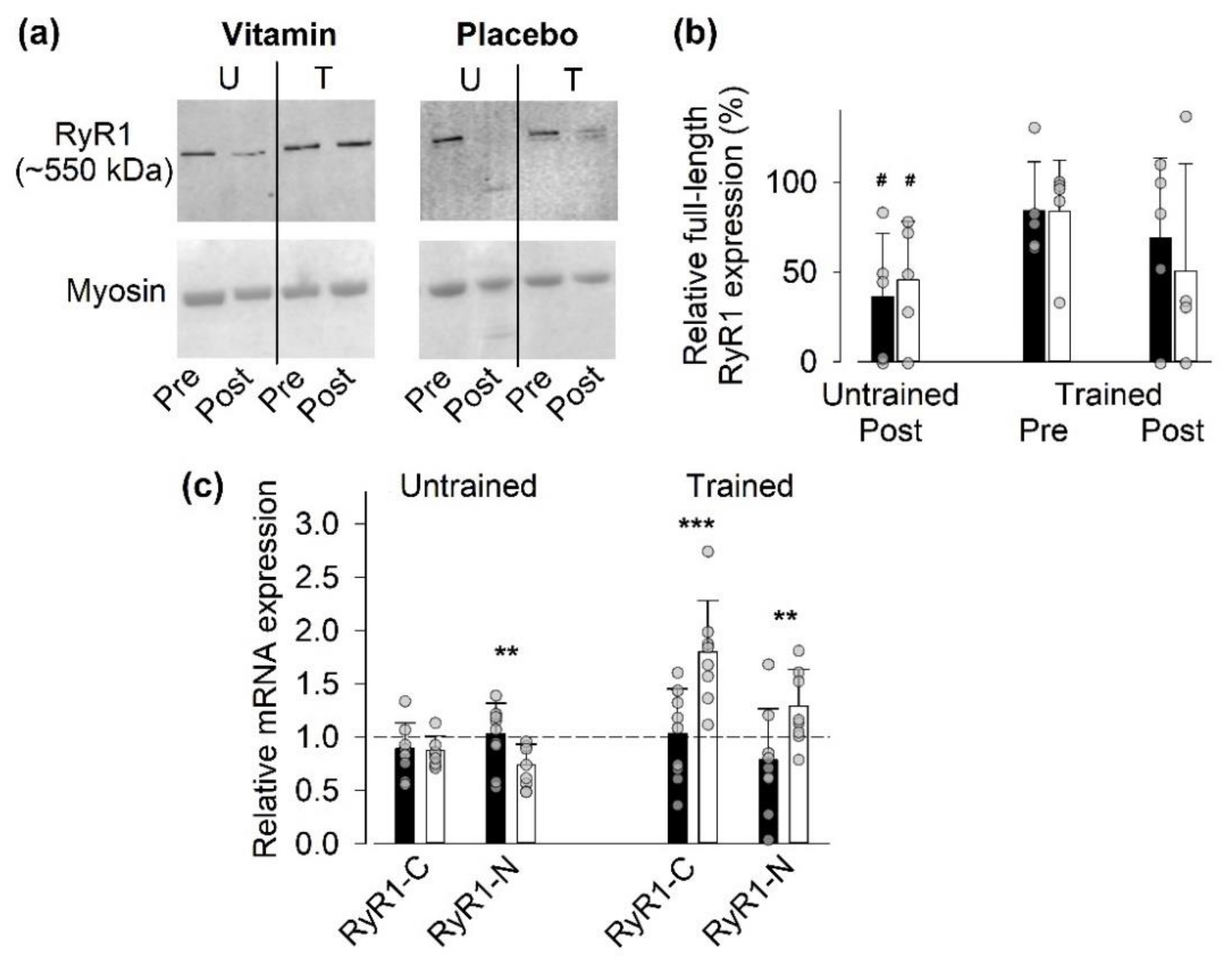
3.4.1. Full-Length RyR1 Protein Expression
3.4.2. Gene Expression of RyR1
3.4.3. Gene and Protein Expression of SR Ca2+-Handling Proteins Other Than RyR1
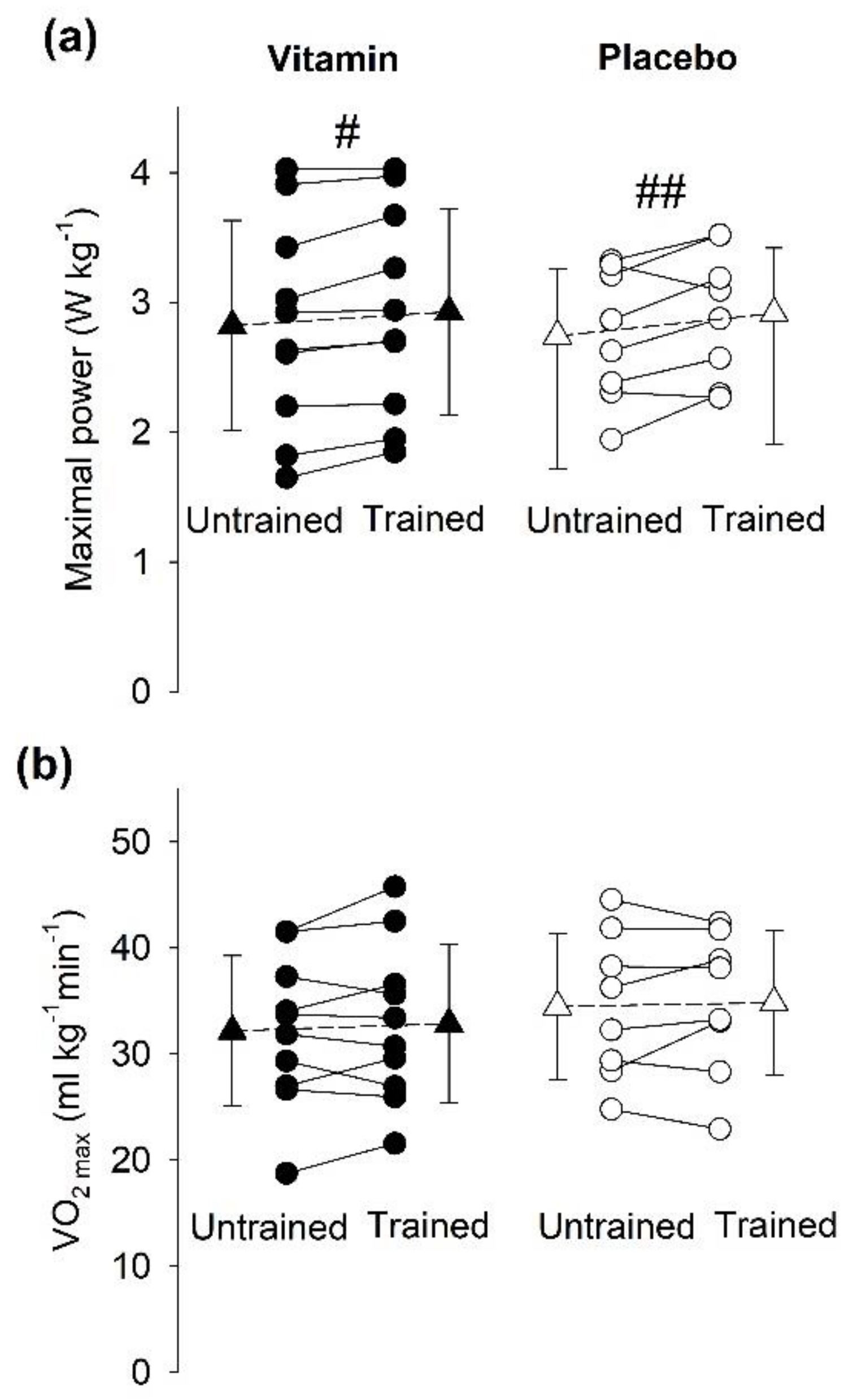
3.5. Aerobic Capacity
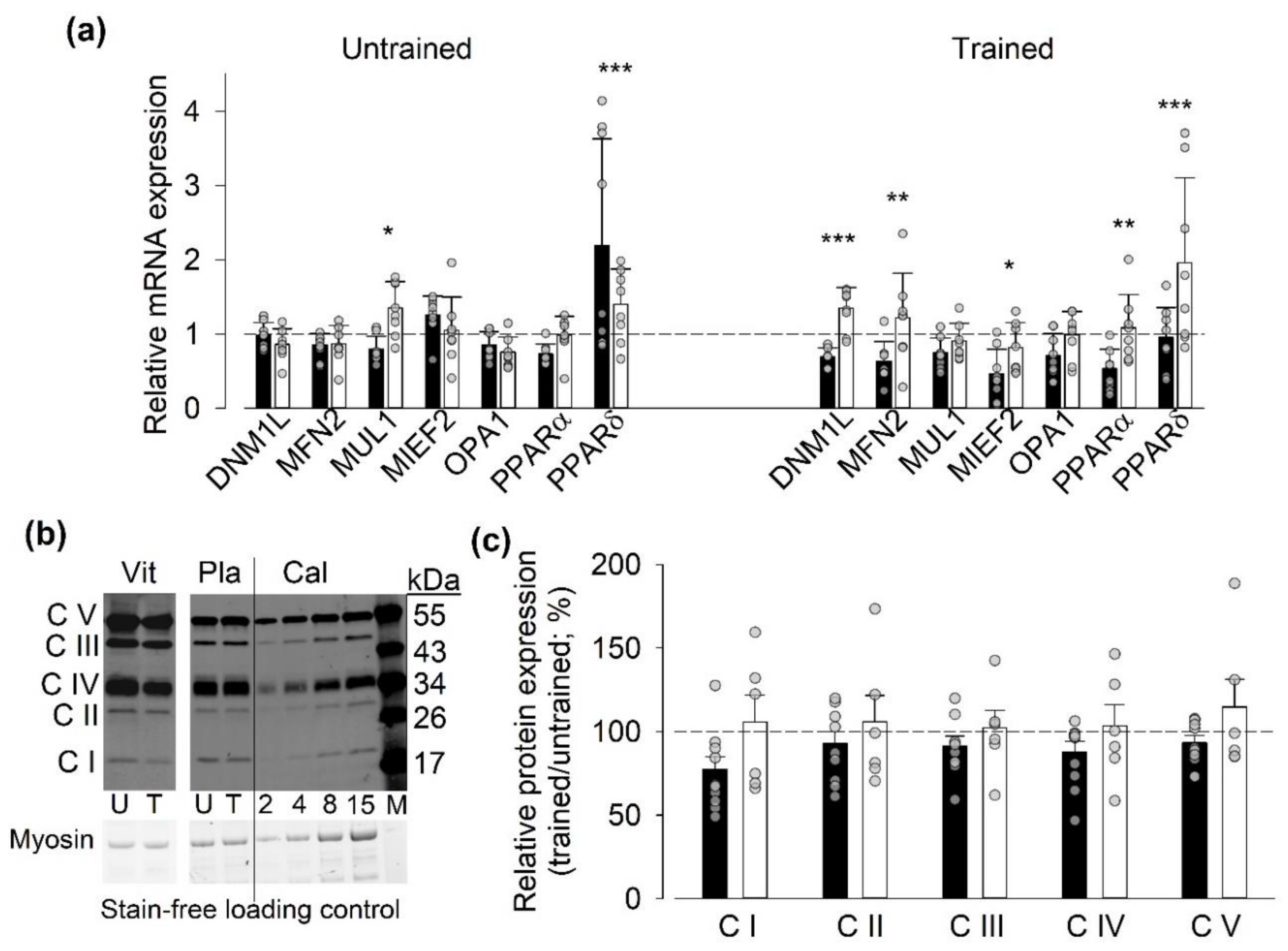
3.5.1. Gene and Protein Expression of Mitochondria-Related Proteins
3.5.2. Incremental Cycling Test to Assess VO2max
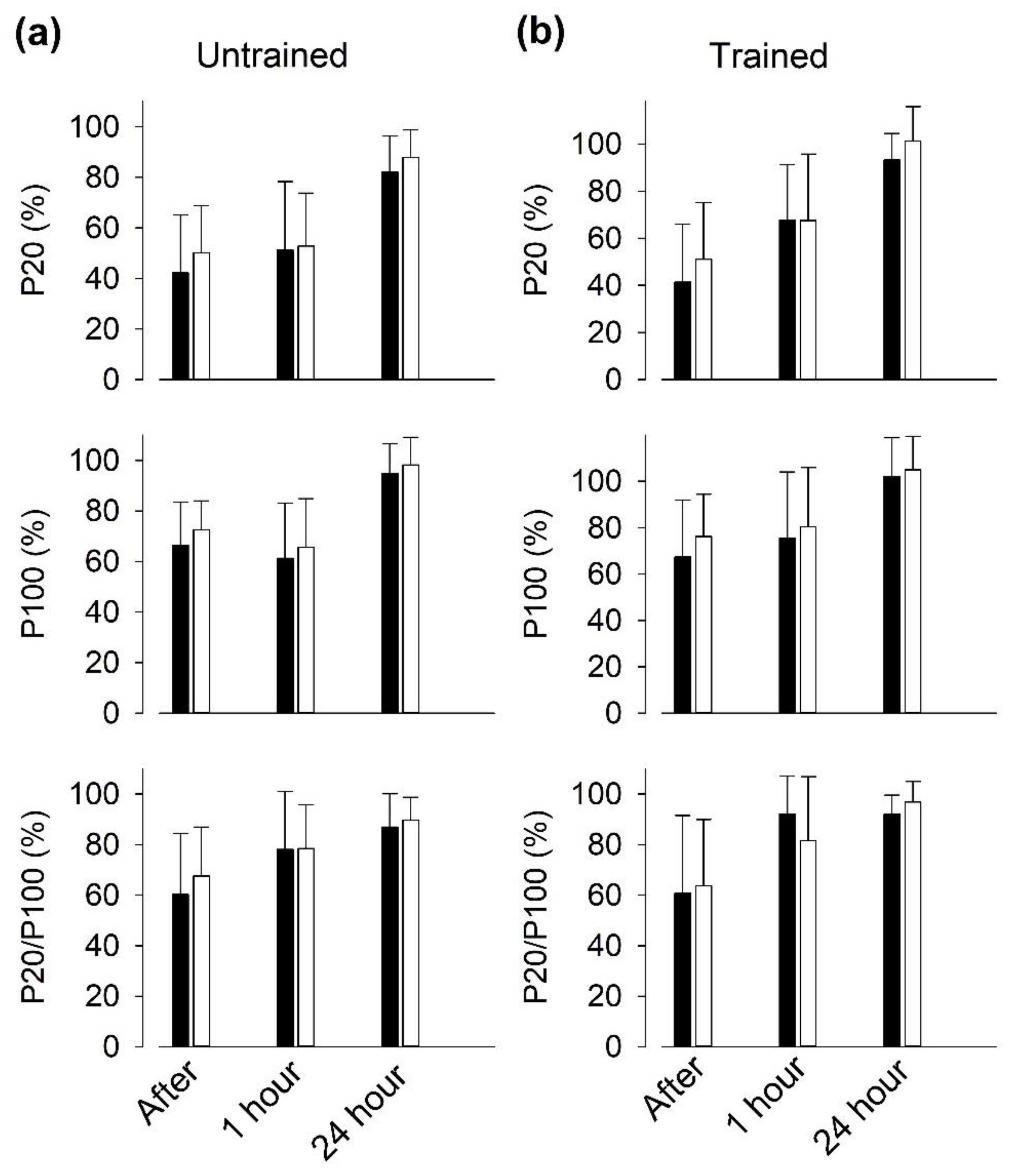
3.6. Recovery of Isometric Force after SIT Sessions
4. Discussion
4.1. Effectiveness of SIT in Young vs. Elderly Individuals
4.2. Expression of Genes Encoding for Inflammatory Mediators
4.3. SR Ca2+-Handling
- (i)
- Vitamin treatment gave some protection against the exercise-induced decrease in full-length RyR1 expression, as judged from a reduction to <50% of the pre-exercise value after the last SIT session in only one of five vitamin-treated individual as compared to three of four placebo-treated individuals.
- (ii)
- The relative RyR1 mRNA expression after the last SIT session was markedly smaller in the vitamin than in the placebo group, which indicates a reduced demand for synthesis of new RyR1 protein with vitamin treatment.
- (iii)
- The RyR1 mRNA expression before SIT sessions was lower in the trained than in the untrained state in the vitamin group. This, combined with an almost unaffected full-size RyR1 protein expression before the last SIT session, again indicates a reduced RyR1 protein turnover in trained vitamin-treated participants.
- (iv)
- After the last SIT session, the relative mRNA expression was higher for NOX2 and smaller for NOX4 in the vitamin than in the placebo group. A recent study on genetically and pharmacologically manipulated adult mouse skeletal muscle revealed a reciprocal interaction between NOX2 and NOX4 expression, where a decrease in NOX2 was accompanied by increases in NOX4 expression and RyR1-mediated SR Ca2+ leak [50]. Thus, it can be speculated that the vitamin treatment counteracted this tentative SIT-induced SR Ca2+ leak-promoting shift in the relation between NOX2 and NOX4 protein concentration.
4.4. PLFFD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Marini, F.; Cesari, M.; Buford, T.W.; Manini, T.M.; Pahor, M.; Leeuwenburgh, C.; Bernabei, R.; Landi, F.; Marzetti, E. Systemic inflammation, body composition, and physical performance in old community-dwellers. J. Cachexia Sarcopenia Muscle 2017, 8, 69–77. [Google Scholar] [CrossRef]
- Morrisette-Thomas, V.; Cohen, A.A.; Fulop, T.; Riesco, E.; Legault, V.; Li, Q.; Milot, E.; Dusseault-Belanger, F.; Ferrucci, L. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech. Ageing Dev. 2014, 139, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Booth, F.W.; Roberts, C.K.; Thyfault, J.P.; Ruegsegger, G.N.; Toedebusch, R.G. Role of inactivity in chronic diseases: Evolutionary insight and pathophysiological mechanisms. Physiol. Rev. 2017, 97, 1351–1402. [Google Scholar] [CrossRef]
- Peake, J.M.; Della Gatta, P.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar]
- Munoz-Canoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Crescioli, C. Targeting age-dependent functional and metabolic decline of human skeletal muscle: The geroprotective role of exercise, myokine IL-6, and vitamin D. Int. J. Mol. Sci. 2020, 21, 1010. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallen, J.; Ronnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Ostgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Strobel, N.A.; Peake, J.M.; Matsumoto, A.; Marsh, S.A.; Coombes, J.S.; Wadley, G.D. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2011, 43, 1017–1024. [Google Scholar] [CrossRef]
- Wadley, G.D.; McConell, G.K. High-dose antioxidant vitamin C supplementation does not prevent acute exercise-induced increases in markers of skeletal muscle mitochondrial biogenesis in rats. J. Appl. Physiol. 2010, 108, 1719–1726. [Google Scholar] [CrossRef] [Green Version]
- Higashida, K.; Kim, S.H.; Higuchi, M.; Holloszy, J.O.; Han, D.H. Normal adaptations to exercise despite protection against oxidative stress. Am. J. Physiol. Endocrin. Metab. 2011, 301, E779–E784. [Google Scholar] [CrossRef] [Green Version]
- Yfanti, C.; Åkerström, T.; Nielsen, S.; Nielsen, A.R.; Mounier, R.; Mortensen, O.H.; Lykkesfeldt, J.; Rose, A.J.; Fischer, C.P.; Pedersen, B.K. Antioxidant supplementation does not alter endurance training adaptation. Med. Sci. Sports Exerc. 2010, 42, 1388–1395. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Place, N.; Ivarsson, N.; Venckunas, T.; Neyroud, D.; Brazaitis, M.; Cheng, A.J.; Ochala, J.; Kamandulis, S.; Girard, S.; Volungevicius, G.; et al. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc. Natl. Acad. Sci. USA 2015, 112, 15492–15497. [Google Scholar] [CrossRef] [Green Version]
- Bruton, J.D.; Aydin, J.; Yamada, T.; Shabalina, I.G.; Ivarsson, N.; Zhang, S.J.; Wada, M.; Tavi, P.; Nedergaard, J.; Katz, A.; et al. Increased fatigue resistance linked to Ca2+-stimulated mitochondrial biogenesis in muscle fibres of cold-acclimated mice. J. Physiol. 2010, 588, 4275–4288. [Google Scholar] [CrossRef]
- Wright, D.C.; Geiger, P.C.; Han, D.H.; Jones, T.E.; Holloszy, J.O. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J. Biol. Chem. 2007, 282, 18793–18799. [Google Scholar] [CrossRef] [Green Version]
- Tavi, P.; Westerblad, H. The role of in vivo Ca2+ signals acting on Ca2+–calmodulin-dependent proteins for skeletal muscle plasticity. J. Physiol. 2011, 589, 5021–5031. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.J.; Bruton, J.D.; Lanner, J.T.; Westerblad, H. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J. Physiol. 2015, 593, 457–472. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.J.; Yamada, T.; Rassier, D.; Andersson, D.C.; Westerblad, H.; Lanner, J.T. Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J. Physiol. 2016, 594, 5149–5160. [Google Scholar] [CrossRef] [Green Version]
- Fodor, J.; Al-Gaadi, D.; Czirják, T.; Oláh, T.; Dienes, B.; Csernoch, L.; Szentesi, P. Improved calcium homeostasis and force by selenium treatment and training in aged mouse skeletal muscle. Sci. Rep. 2020, 10, 1707. [Google Scholar] [CrossRef] [PubMed]
- Schlittler, M.; Neyroud, D.; Tanga, C.; Zanou, N.; Kamandulis, S.; Skurvydas, A.; Kayser, B.; Westerblad, H.; Place, N.; Andersson, D.C. Three weeks of sprint interval training improved high-intensity cycling performance and limited ryanodine receptor modifications in recreationally active human subjects. Eur. J. Appl. Physiol. 2019, 119, 1951–1958. [Google Scholar] [CrossRef]
- Jones, D.A. High-and low-frequency fatigue revisited. Acta Physiol. Scand. 1996, 156, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Skurvydas, A.; Mamkus, G.; Kamandulis, S.; Dudoniene, V.; Valanciene, D.; Westerblad, H. Mechanisms of force depression caused by different types of physical exercise studied by direct electrical stimulation of human quadriceps muscle. Eur. J. Appl. Physiol. 2016, 116, 2215–2224. [Google Scholar] [CrossRef] [Green Version]
- Magistris, M.R.; Kohler, A.; Pizzolato, G.; Morris, M.A.; Baroffio, A.; Bernheim, L.; Bader, C.R. Needle muscle biopsy in the investigation of neuromuscular disorders. Muscle Nerve 1998, 21, 194–200. [Google Scholar] [CrossRef]
- Perry, C.G.R.; Lally, J.; Holloway, G.P.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010, 588, 4795–4810. [Google Scholar] [CrossRef]
- Weston, M.; Taylor, K.L.; Batterham, A.M.; Hopkins, W.G. Effects of low-volume high-intensity interval training (HIT) on fitness in adults: A meta-analysis of controlled and non-controlled trials. Sports Med. 2014, 44, 1005–1017. [Google Scholar] [CrossRef] [Green Version]
- Grundtman, C.; Bruton, J.; Yamada, T.; Östberg, T.; Pisetsky, D.S.; Harris, H.E.; Andersson, U.; Lundberg, I.E.; Westerblad, H. Effects of HMGB1 on in vitro responses of isolated muscle fibers and functional aspects in skeletal muscles of idiopathic inflammatory myopathies. FASEB J. 2010, 24, 570–578. [Google Scholar] [CrossRef]
- Perry, C.G.R.; Hawley, J.A. Molecular basis of exercise-induced skeletal muscle mitochondrial biogenesis: Historical advances, current knowledge, and future challenges. Cold Spring Harb. Perspect. Med. 2018, 8, a029686. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Creer, A.; Jemiolo, B.; Trappe, S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J. Appl. Physiol. 2005, 98, 1745–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, C.L.; Wu, Y.T.; Chou, C.H. Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: A meta-analysis. J. Cardiopulm. Rehabil. Prev. 2011, 31, 378–385. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjønna, A.E.; Stølen, T.O.; Bye, A.; Volden, M.; Slørdahl, S.A.; Odegard, R.; Skogvoll, E.; Wisløff, U. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin. Sci. 2009, 116, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, C.; Kayser, B. Preoperative high-intensity interval training is effective and safe in deconditioned patients with lung cancer: A randomized clinical trial. J. Rehabil. Med. 2019, 51, 712–718. [Google Scholar] [CrossRef] [Green Version]
- McKenna, M.J.; Heigenhauser, G.J.; McKelvie, R.S.; Obminski, G.; MacDougall, J.D.; Jones, N.L. Enhanced pulmonary and active skeletal muscle gas exchange during intense exercise after sprint training in men. J. Physiol. 1997, 501, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Hazell, T.J.; Macpherson, R.E.; Gravelle, B.M.; Lemon, P.W. 10 or 30-s sprint interval training bouts enhance both aerobic and anaerobic performance. Eur. J. Appl. Physiol. 2010, 110, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Hughes, S.C.; Heigenhauser, G.J.; Bradwell, S.N.; Gibala, M.J. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J. Appl. Physiol. 2005, 98, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2011, 2, 1143–1211. [Google Scholar] [CrossRef] [Green Version]
- Sloth, M.; Sloth, D.; Overgaard, K.; Dalgas, U. Effects of sprint interval training on VO2max and aerobic exercise performance: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2013, 23, e341–e352. [Google Scholar] [CrossRef] [PubMed]
- Gist, N.H.; Fedewa, M.V.; Dishman, R.K.; Cureton, K.J. Sprint interval training effects on aerobic capacity: A systematic review and meta-analysis. Sports Med. 2014, 44, 269–279. [Google Scholar] [CrossRef]
- Knowles, A.-M.; Herbert, P.; Easton, C.; Sculthorpe, N.; Grace, F.M. Impact of low-volume, high-intensity interval training on maximal aerobic capacity, health-related quality of life and motivation to exercise in ageing men. Age 2015, 37, 25. [Google Scholar] [CrossRef] [Green Version]
- Herbert, P.; Grace, F.M.; Sculthorpe, N.F. Exercising caution: Prolonged recovery from a single session of high-intensity interval training in older men. J. Am. Geriatr. Soc. 2015, 63, 817–818. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Da Rocha, A.L.; Pinto, A.P.; Kohama, E.B.; Pauli, J.R.; de Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; da Silva, A.S.R. The proinflammatory effects of chronic excessive exercise. Cytokine 2019, 119, 57–61. [Google Scholar] [CrossRef]
- Ivarsson, N.; Mattsson, C.M.; Cheng, A.J.; Bruton, J.D.; Ekblom, B.; Lanner, J.T.; Westerblad, H. SR Ca2+ leak in skeletal muscle fibers acts as an intracellular signal to increase fatigue resistance. J. Gen. Physiol. 2019, 151, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Cully, T.R.; Rodney, G.G. Nox4–RyR1–Nox2: Regulators of micro-domain signaling in skeletal muscle. Redox Biol. 2020, 36, 101557. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Vitamin C + E (n = 10) | Placebo (n = 8) | |
|---|---|---|
| Age (years) | 67.0 ± 7.8 | 64.3 ± 6.0 |
| Height (cm) | 174.3 ± 4.0 | 179.6 ± 6.7 |
| Body mass (kg) | 81.9 ± 11.2 | 84.4 ± 12.1 |
| BMI (kg m−2) | 26.4 ± 2.7 | 26.0 ± 2.4 |
| Incremental cycling test | ||
| VO2max (mL kg−1 min−1) | 32.2 ± 7.1 | 34.5 ± 6.9 |
| Maximal power (W kg−1) | 2.82 ± 0.81 | 2.74 ± 0.51 |
| Maximal heart rate (min−1) | 154 ± 15 | 160 ± 19 |
| RERmax | 1.15 ± 0.08 | 1,18 ± 0.05 |
| Protein | Abbreviation | Primer: FWD 5’- 3/ REV 5´- 3 | NCBI Reference Sequence |
|---|---|---|---|
| Inflammatory Mediators | |||
| Interleukin-1β | IL-1β | GGCATCCAGCTACGAATCTC/ GAACCAGCATCTTCCTCAGC | NM_000576.3 |
| Interleukin-6 | IL-6 | GAAAGCAGCAAAGAGGCACT/ TTTCACCAGGCAAGTCTCCT | NM_14584.1 |
| Interleukin-10 | IL-10 | CCAAGCTGAGAACCAAGACC/ GGGAAGAAATCGATGACAGC | NM_000572.3 |
| Tumor necrosis factor-α | TNF-α | AACCTCCTCTCTGCCATCAA/ GGAAGACCCCTCCCAGATAG | NM_000594.4 |
| Transforming growth factor-β1 | TGF-β1 | ACATTGACTTCCGCAAGGAC/ GTCCAGGCTCCAAATGTAGG | NM_000660.7 |
| High mobility group box 1 | HMGB1 | CACCCAGATGCTTCAGTCAA/ TCCGCTTTTGCCATATCTTC | NM_002128.7 |
| ROS-Related Proteins | |||
| Glutathione peroxidase 1 | GPX1 | ACGATGTTGCCTGGAACTTT/ TCGATGTCAATGGTCTGGAA | NM_000581.4 |
| Superoxide dismutase 1 | SOD1 | TGGCCGATGTGTCTATTGAA/ ACCTTTGCCCAAGTCATCTG | NM_000454.4 |
| Superoxide dismutase 2 | SOD2 | GTTGGCCAAGGGAGATGTTA/ TAGGGCTGAGGTTTGTCCAG | NM_000636.4 |
| Catalase 1 | CAT1 | CGTGCTGAATGAGGAACAGA/ TTGACCGCTTTCTTCTGGAT | NM_001752.4 |
| NADPH oxidase 2 | NOX2 | AAATGGTGGCATGGATGATT/ TATTGACTCGGGCATTCACA | NM_000397.3 |
| NADPH oxidase 4 | NOX4 | TGTTGGATGACTGGAAACCA/ AATCTGCAAACCAACGGAAG | NM_016931.5 |
| SR Ca2+-Handling Proteins | |||
| SR Ca2+-ATPase 1 | SERCA1 | TAAGAAGCTTGCCCTCCGTA/ CAGACATCTGGTTGGTGGTG | NM_004320.4 |
| SR Ca2+-ATPase 2a | SERCA2a | CTGAAGAAAGCCGAGATTGG/ GCCACAATGGTGGAGAAGTT | NM_170665.4 |
| Calsequestrin 1 | CSQ1 | TCCCATACTGGGAGAAGACG/ TCCTCCTCATCGTCCATTTC | NM_001231.5 |
| Calsequestrin 2 | CSQ2 | GACAAAGGGGTTGCAAAGAA/ CTCCACCAGCTCCTCTTCTG | NM_001232.3 |
| Dihydropyridine receptor | DHRP | ACTGTATTGCCTGGGTGGAG/ GCTTGATCAGCCTCATGACA | U09784.1 |
| Ryanodine receptor 1, C-terminal | RyR1-C | ACAGGGTGGTCTTCGACATC/ GTCTCGGAGCTCACCAAAAG | NM_000540.2 |
| Ryanodine receptor 1, N-terminal | RyR1-N | TGCTGCAGACAAACCTCATC/ ATTTGCTGTACTGCGTGGTG | NM_000540.2 |
| Mitochondria-Related Proteins | |||
| Dynamin 1-like protein | DNM1L | AAATCGTCGTAGTGGGAACG/ CGGGTGACAATTCCAGTACC | NM_012062.4 |
| Mitofusin 2 | MFN2 | GGCCAAACATCTTCATCCTG/ CTGGTACAACGCTCCATGTG | NM_014874.3 |
| Mitochondrial E3 ubiquitin protein ligase 1 | MUL1 | GAGAAGTTCCACCCCTCGAT/ TCAGCATCTCCTCGGTCTCT | NM_024544.3 |
| Mitochondrial elongation factor 2 | MIEF2 | GACTTCCTCCTGGCCAATG/ TGGCCCTGTCAATGAACC | NM_139162.4 |
| Mitochondrial dynamin-like GTPase | OPA1 | GGGTTGTTGTGGTTGGAGAT/ GTCATCATCTCCCCAGATCC | NM_015560.2 |
| Peroxisome proliferator-activated receptor α | PPARα | CCTTGCAGCACAAGAAAACA/ CTGCTTCGTCGTCAAAAACA | NM_013261.5 |
| Peroxisome proliferator-activated receptor δ | PPARδ | ACTGAGTTCGCCAAGAGCATC/ ACGCCATACTTGAGAAGGGTAA | NM_138712.4 |
| House-Keeping Gene | |||
| Hypoxanthine-guanine Phosphoribosyltransferase | HPRT | GAAAAGGACCCCACGAAGTGT/ AGTCAAGGGCATATCCTACAA | NM_000194 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyckelsma, V.L.; Venckunas, T.; Brazaitis, M.; Gastaldello, S.; Snieckus, A.; Eimantas, N.; Baranauskiene, N.; Subocius, A.; Skurvydas, A.; Pääsuke, M.; et al. Vitamin C and E Treatment Blunts Sprint Interval Training–Induced Changes in Inflammatory Mediator-, Calcium-, and Mitochondria-Related Signaling in Recreationally Active Elderly Humans. Antioxidants 2020, 9, 879. https://doi.org/10.3390/antiox9090879
Wyckelsma VL, Venckunas T, Brazaitis M, Gastaldello S, Snieckus A, Eimantas N, Baranauskiene N, Subocius A, Skurvydas A, Pääsuke M, et al. Vitamin C and E Treatment Blunts Sprint Interval Training–Induced Changes in Inflammatory Mediator-, Calcium-, and Mitochondria-Related Signaling in Recreationally Active Elderly Humans. Antioxidants. 2020; 9(9):879. https://doi.org/10.3390/antiox9090879
Chicago/Turabian StyleWyckelsma, Victoria L., Tomas Venckunas, Marius Brazaitis, Stefano Gastaldello, Audrius Snieckus, Nerijus Eimantas, Neringa Baranauskiene, Andrejus Subocius, Albertas Skurvydas, Mati Pääsuke, and et al. 2020. "Vitamin C and E Treatment Blunts Sprint Interval Training–Induced Changes in Inflammatory Mediator-, Calcium-, and Mitochondria-Related Signaling in Recreationally Active Elderly Humans" Antioxidants 9, no. 9: 879. https://doi.org/10.3390/antiox9090879
APA StyleWyckelsma, V. L., Venckunas, T., Brazaitis, M., Gastaldello, S., Snieckus, A., Eimantas, N., Baranauskiene, N., Subocius, A., Skurvydas, A., Pääsuke, M., Gapeyeva, H., Kaasik, P., Pääsuke, R., Jürimäe, J., Graf, B. A., Kayser, B., Place, N., Andersson, D. C., Kamandulis, S., & Westerblad, H. (2020). Vitamin C and E Treatment Blunts Sprint Interval Training–Induced Changes in Inflammatory Mediator-, Calcium-, and Mitochondria-Related Signaling in Recreationally Active Elderly Humans. Antioxidants, 9(9), 879. https://doi.org/10.3390/antiox9090879








