Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Design
2.2. Interventional Study
2.3. Retinal Tissue Processing
2.4. Western Blotting
2.5. Immunofluorescence Analysis
2.6. Statistical Analysis
3. Results
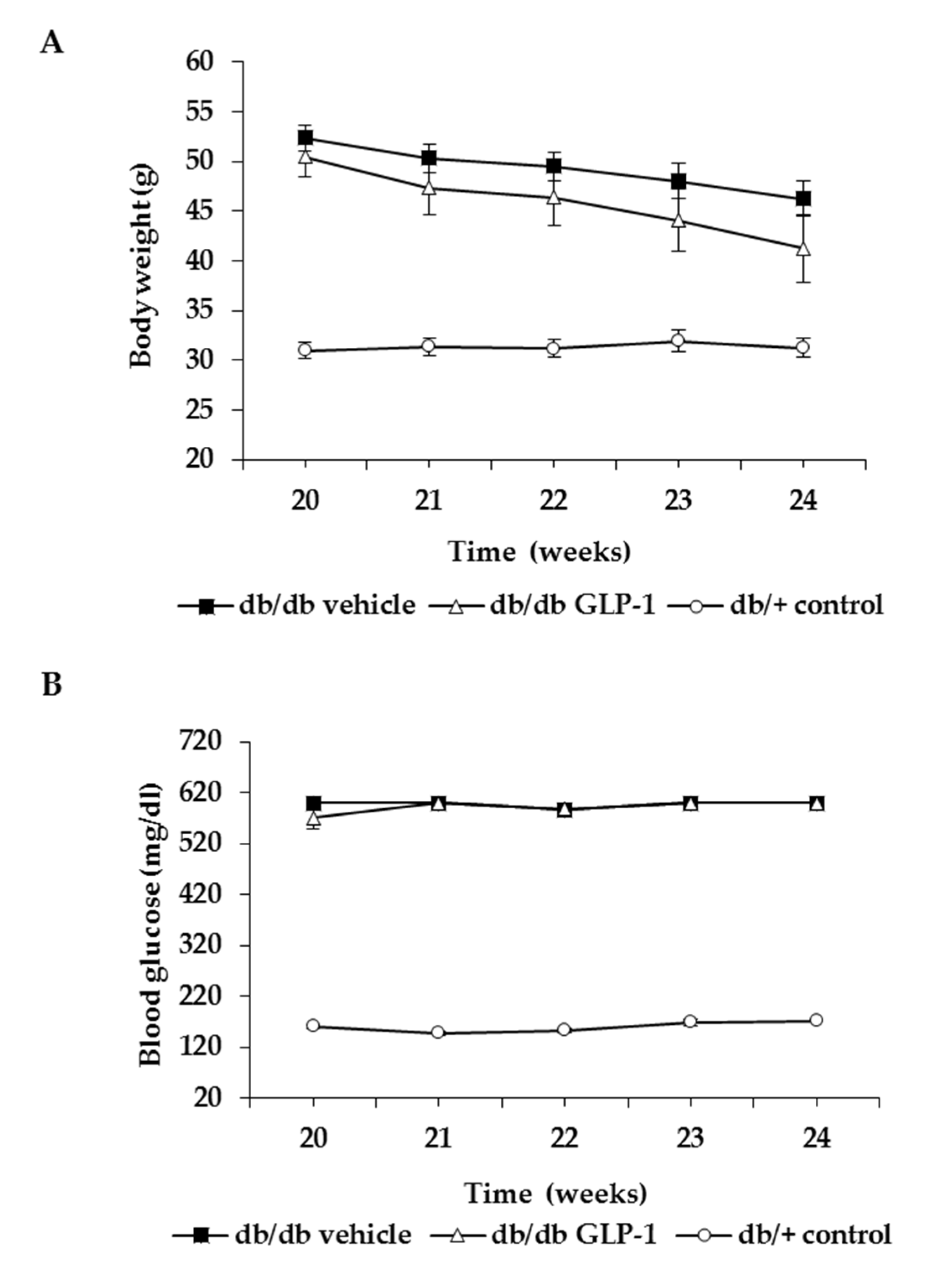
3.1. Topical Administration of GLP-1 has no Effect on Body Weight and Systemic Blood Glucose Levels
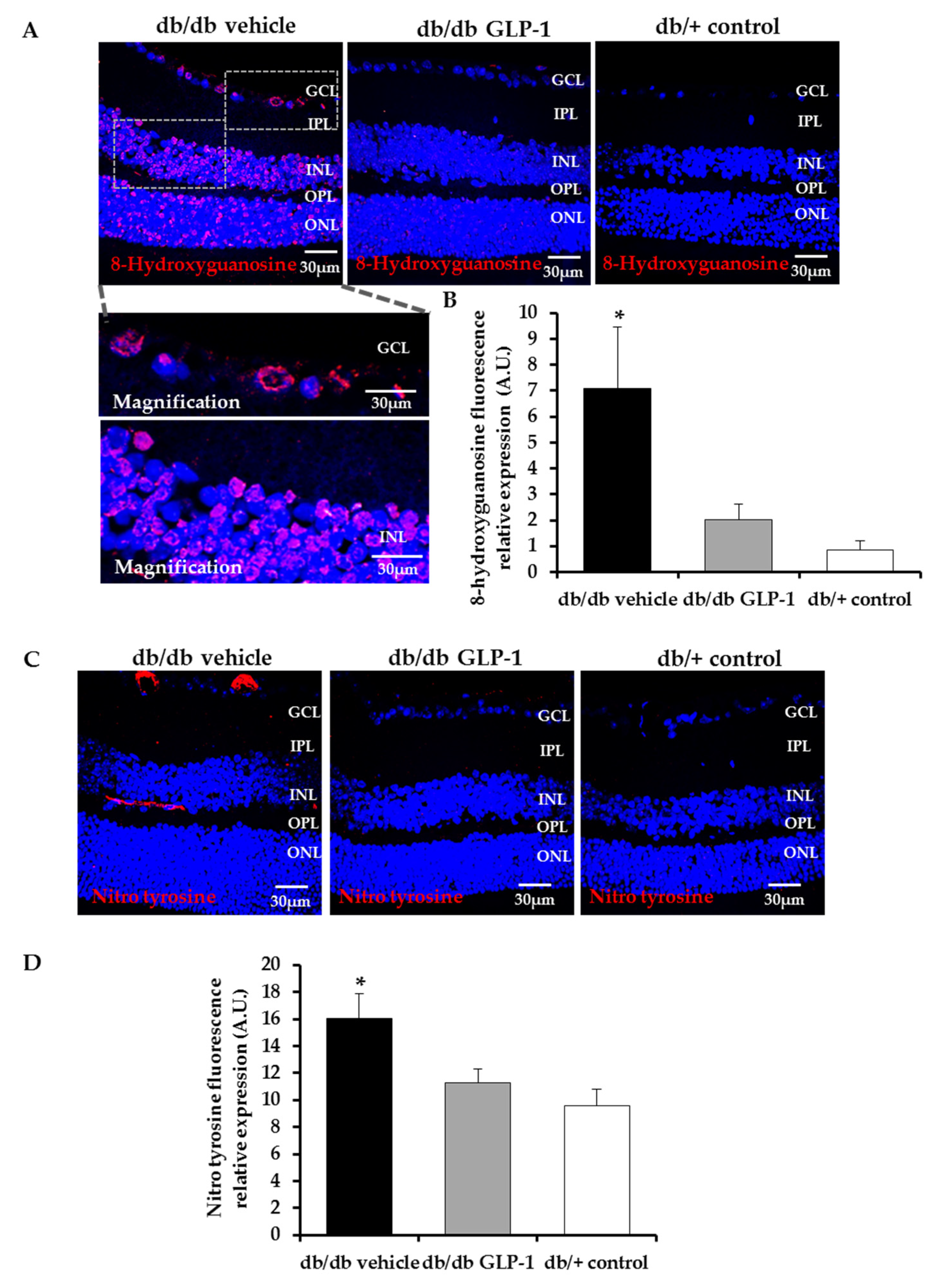
3.2. Topical Administration of GLP-1 Reduces DNA/RNA Damage through the Decrease of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) Induced by Diabetes in the Retina
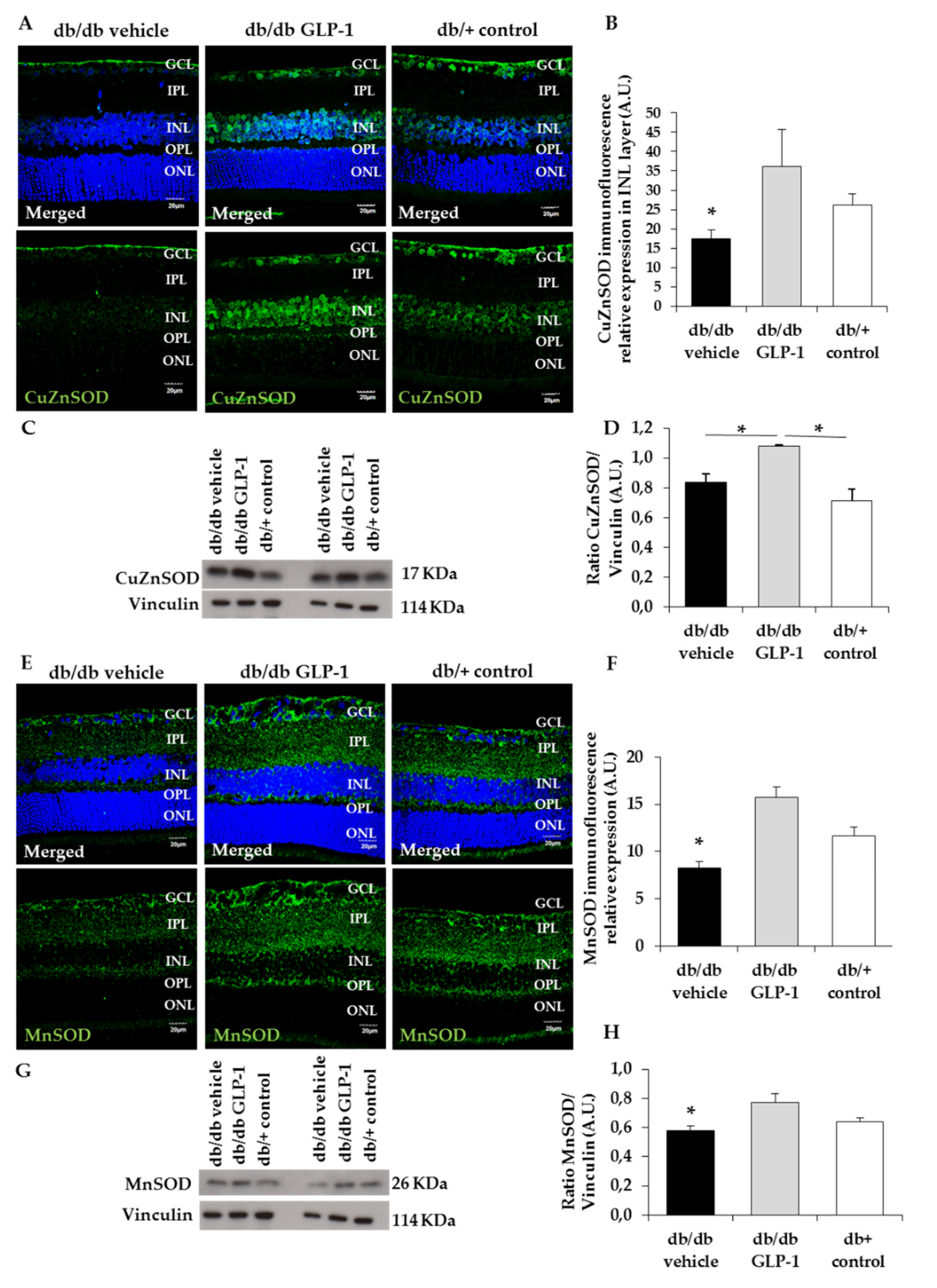
3.3. GLP-1 Eyedrops Protect from Oxidative Stress by Increasing the Protein Levels of Glutathione Reductase, Glutathione Peroxidase and Copper–Zinc and Manganese Superoxide Dismutases (CuZnSOD and MnSOD) in Diabetic Retinas
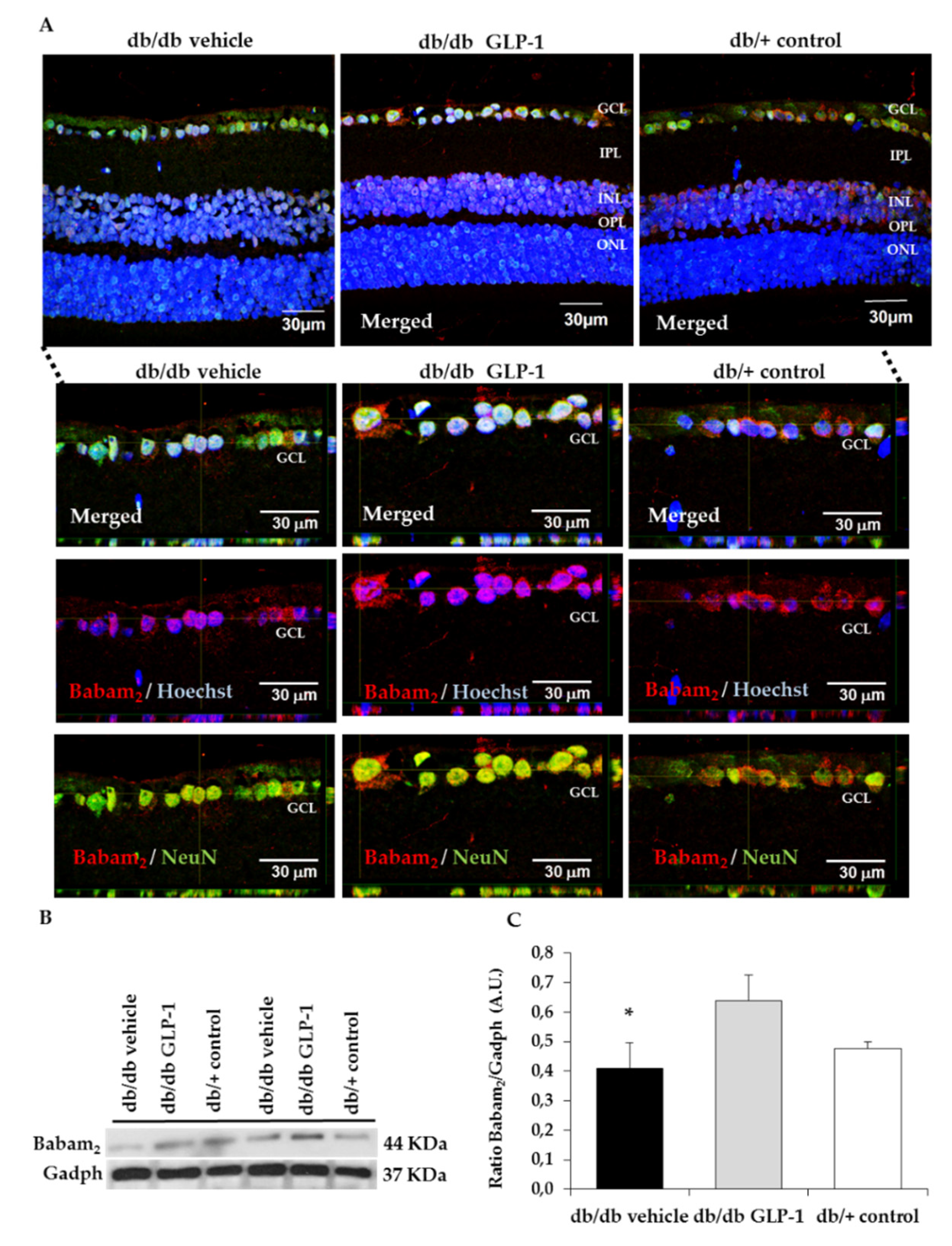
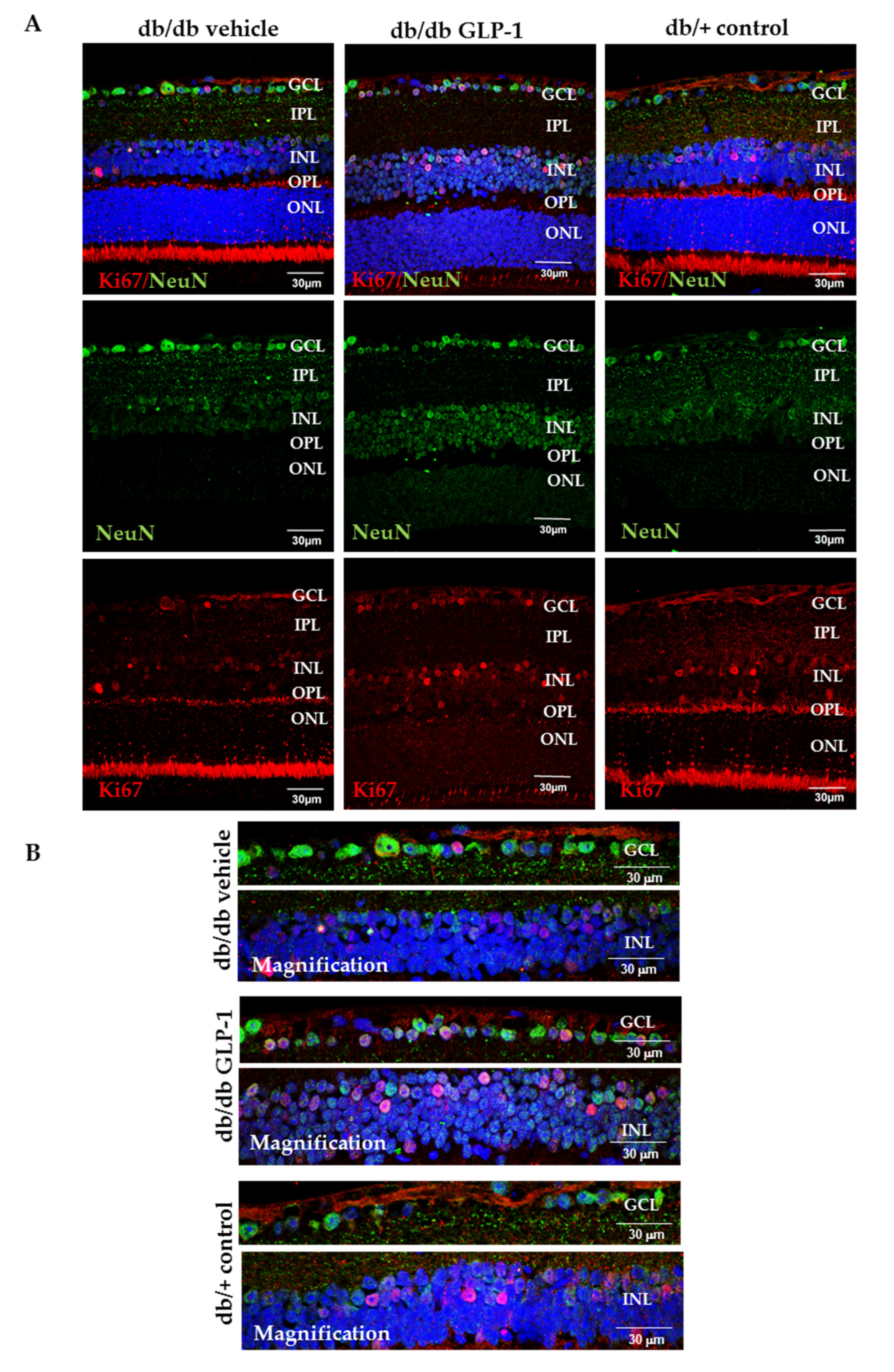
3.4. Topical Administration of GLP-1 Activates the Expression in the Retina of Proteins Involved in DNA Repair (Babam2) and Cell Proliferation (Ki67)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernández, C.; Bogdanov, P.; Corraliza, L.; García-Ramírez, M.; Solà-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simó, R. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C. GLP-1R as a Target for the Treatment of Diabetic Retinopathy: Friend or Foe? Diabetes 2017, 66, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, J.; Bogdanov, P.; Ramos, H.; Solà-Adell, C.; Turch, M.; Valeri, M.; Simó-Servat, O.; Lagunas, C.; Simó, R.; Hernández, C. New Insights into the Mechanisms of Action of Topical Administration of GLP-1 in an Experimental Model of Diabetic Retinopathy. J. Clin. Med. 2019, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhou, H.; Kuang, H. The potential benefits of glucagon-like peptide-1 receptor agonists for diabetic retinopathy. Peptides 2018, 100, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 17, 16012. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. BBA Mol. Basis Dis. 2015, 1582, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Smith, M.A.; Miller, C.M.; Kern, T.S. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J. Neurochem. 2002, 80, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Chan, P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A. Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes 2003, 52, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Atasi, L.; Ho, Y.S. Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Tang, M.K.; Yao, Y.; Tang, C.; Chui, Y.L.; Lee, K.K.H. BRE plays an essential role in preventing replicative and DNA damage-induced premature senescence. Sci. Rep. 2016, 6, 23506. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Li, L.; Miao, J.; Cai, D.Q.; Lee, K.K.; Chui, Y.L. Differential expression of a novel gene BRE (TNFRSF1A modulator/BRCC45) in response to stress and biological signals. Mol. Biol. Rep. 2010, 37, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Burcelin, R.; Nathanson, E. Neuroprotective properties of GLP-1: Theoretical and practical applications. Curr. Med. Res. Opin. 2011, 27, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.E.; Rakipovski, G.; Raun, K.; Lykkesfeldt, J. Does Glucagon-like Peptide-1 Ameliorate Oxidative Stress in Diabetes? Evidence Based on Experimental and Clinical Studies. Curr. Diabetes Rev. 2016, 12, 331–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, P.; Wang, Y.; Li, W.; Wang, C.; Sun, D.; Zhang, R.; Su, T.; Ma, X.; Zeng, C.; et al. Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes 2013, 62, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Erdogdu, Ö.; Eriksson, L.; Xu, H.; Sjöholm, Å.; Zhang, Q.; Nyström, T. Exendin-4 protects endothelial cells from lipoapoptosis by PKA, PI3K, eNOS, p38 MAPK, and JNK pathways. J. Mol. Endocrinol. 2013, 50, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Millán, E.; Martín, M.A.; Goya, L.; Lizárraga-Mollinedo, E.; Escrivá, F.; Ramos, S.; Álvarez, C. Glucagon-like peptide-1 improves beta-cell antioxidant capacity via extracellular regulated kinases pathway and Nrf2 translocation. Free Radic. Biol. Med. 2016, 95, 16–26. [Google Scholar] [CrossRef] [PubMed]

| Antibodies | Description |
|---|---|
| Babam2 | Rabbit monoclonal; 1:1000; ab177960; Abcam, Cambridge, UK |
| Cyclophilin A | Rabbit polyclonal; 1:10,000; BML-SA296; Enzo Life Sciences, Lausen, Switzerland |
| CuZnSOD | Rabbit polyclonal; 1:1000; GTX100554; GeneTex, Hsinchu, Taiwan |
| Gadph | Mouse monoclonal; 1:10,000; sc-32233; Santa Cruz, Dallas, Texas, USA |
| Glutathione peroxidase | Rabbit polyclonal; 1:1000; GTX116040; GeneTex, Hsinchu, Taiwan |
| Glutathione reductase | Rabbit polyclonal; 1:1000; GTX114199; GeneTex, Hsinchu, Taiwan |
| MnSOD | Rabbit polyclonal; 1:1000; ab13533; Abcam, Cambridge, UK |
| Vinculin | Mouse monoclonal; 1:7000; sc-73614; Santa Cruz, Dallas, Texas, USA |
| Primary Antibodies | Description |
|---|---|
| DNA/RNA damage (8-hydroxy-guanosine) | Mouse monoclonal; 1:100; ab62623; Abcam, Cambridge, UK |
| Babam2 | Rabbit monoclonal; 1:100; ab177960; Abcam, Cambridge, UK |
| CuZnSOD | Rabbit polyclonal; 1:100; GTX100554; GeneTex, Hsinchu, Taiwan |
| Ki67 | Rabbit polyclonal; 1:500; ab15580 (Abcam, Cambridge, UK) |
| MnSOD | Rabbit polyclonal; 1:100; ab13533; Abcam, Cambridge, UK |
| NeuN | Mouse monoclonal; 1:200; ab104224; Abcam, Cambridge, UK |
| Nitro tyrosine | Mouse monoclonal; 1:100; ab7048; Abcam, Cambridge, UK |
| Secondary Antibodies | Description |
| Alexa Fluor 488 Goat anti-mouse | Goat polyclonal; 1:600; #A-11032; Abcam, Cambridge, UK |
| Alexa Fluor 488 Goat anti-rabbit | Goat polyclonal; 1:600; ab150081; Abcam, Cambridge, UK |
| Alexa Fluor 594 Goat anti-mouse | Goat polyclonal; 1:600; ab150113; Life Technologies (Thermo Fisher Scientific) Waltham, MA, USA |
| Alexa Fluor 594 Goat anti-rabbit | Goat polyclonal; 1:600; A-110012; Life Technologies (Thermo Fisher Scientific) Waltham, MA, USA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, H.; Bogdanov, P.; Sampedro, J.; Huerta, J.; Simó, R.; Hernández, C. Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress. Antioxidants 2020, 9, 846. https://doi.org/10.3390/antiox9090846
Ramos H, Bogdanov P, Sampedro J, Huerta J, Simó R, Hernández C. Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress. Antioxidants. 2020; 9(9):846. https://doi.org/10.3390/antiox9090846
Chicago/Turabian StyleRamos, Hugo, Patricia Bogdanov, Joel Sampedro, Jordi Huerta, Rafael Simó, and Cristina Hernández. 2020. "Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress" Antioxidants 9, no. 9: 846. https://doi.org/10.3390/antiox9090846
APA StyleRamos, H., Bogdanov, P., Sampedro, J., Huerta, J., Simó, R., & Hernández, C. (2020). Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress. Antioxidants, 9(9), 846. https://doi.org/10.3390/antiox9090846






