Mature Twin Neonates Exhibit Oxidative Stress via Nitric Oxide Synthase Dysfunctionality: A Prognostic Stress Marker in the Red Blood Cells and Umbilical Cord Vessels
Abstract
:1. Introduction
2. Methods and Materials
2.1. Human Samples
2.2. Sample Processing for Immunostaining
2.3. Immunolabelling on Umbilical Cord and Cord Blood Samples
2.4. Statistical Analysis
3. Results
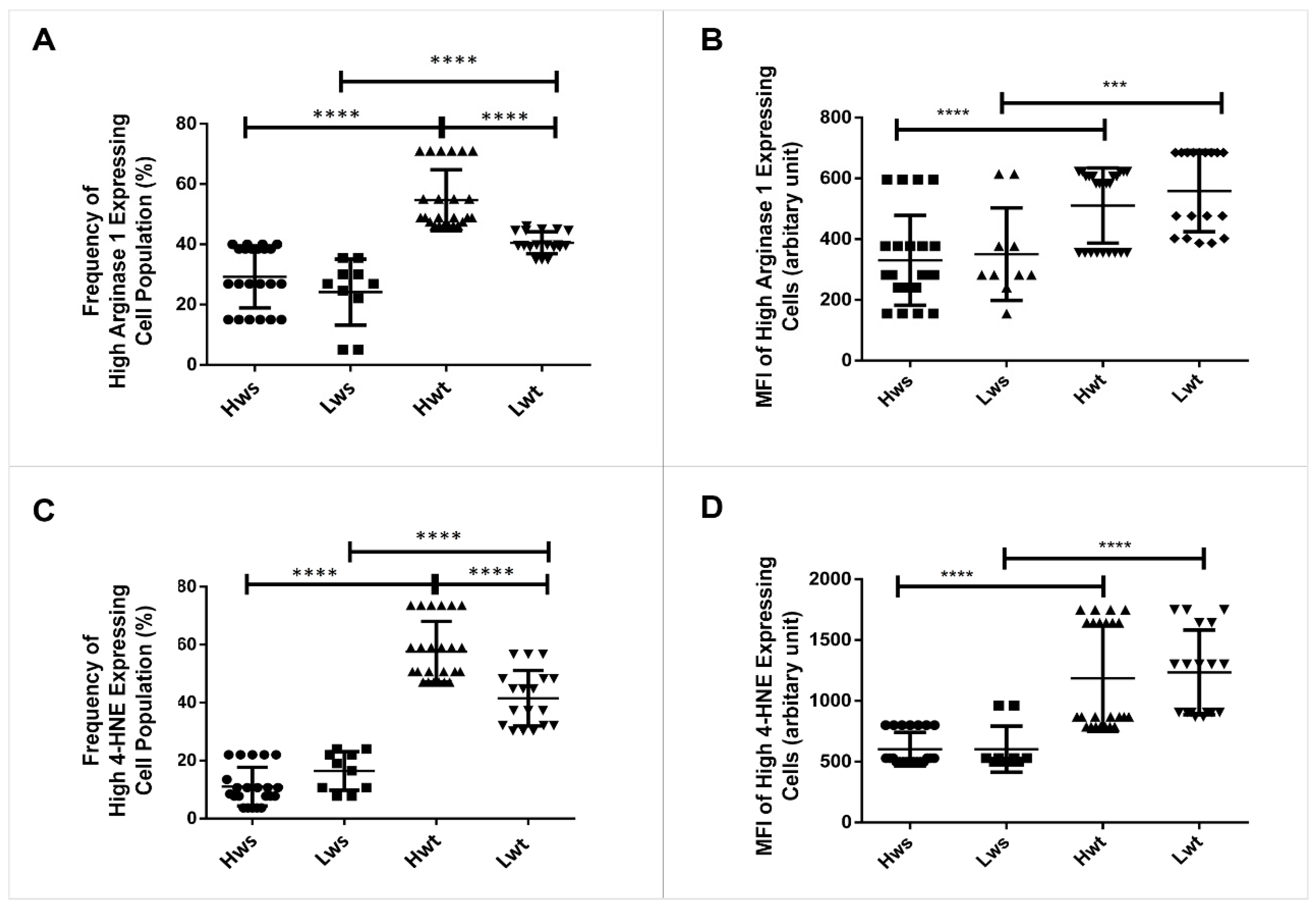
3.1. NOS3 Expression and Its Activation Level Is Altered Both in the Umbilical Cord Vessels and in the Isolated RBCs
3.2. Quantitative Measurement of Lipid Peroxidation Marked with 4-Hydroxynonenal and Annexin V Positive Levels in Twin Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feltes, B.C.; Poloni, J.F.; Notari, D.L.; Bonatto, D. Toxicological Effects of the Different Substances in Tobacco Smoke on Human Embryonic Development by a Systems Chemo-Biology Approach. PLoS ONE 2013, 8, e61743. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Roszkowski, K. Oxidative stress in pregnant women. Arch. Perinat. Med. 2013, 19, 150–155. [Google Scholar]
- Umranikar, A.; Parmar, D.; Davies, S.; Fountain, S. Multiple births following in vitro fertilization treatment: Redefining success. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Thaete, L.G.; Dewey, E.R.; Neerhof, M.G. Endothelin and the regulation of uterine and placental perfusion in hypoxia-induced fetal growth restriction. J. Soc. Gynecol. Investig. 2004, 11, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, G.; Perrone, S. Biomarkers of oxidative stress in the fetus and newborn. Hematol. Meet. Rep. 2009, 2, 103–107. [Google Scholar]
- Su, R.-N.; Zhu, W.-W.; Wei, Y.-M.; Wang, C.; Feng, H.; Lin, L.; Yang, H.-X. Maternal and neonatal outcomes in multiple pregnancy: A multicentre study in the Beijing population. Chronic Dis. Transl. Med. 2015, 1, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Hayes, E. Review of “Multiple Pregnancy: Epidemiology, Gestation & Perinatal Outcome. Second Edition”. J. Exp. Clin. Assist. Reprod. 2005, 2, 12. [Google Scholar] [CrossRef]
- Acharya, G.; Sonesson, S.E.; Flo, K.; Räsänen, J.; Odibo, A. Hemodynamic aspects of normal human feto-placental (umbilical) circulation. Acta Obstet. Gynecol. Scand. 2016, 95, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Reyes-Hernández, C.G.; López de Pablo, A.L.; Carmen González, M.; Arribas, S.M. Implication of oxidative stress in fetal programming of cardiovascular disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef] [Green Version]
- Brain, S.D.; Grant, A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004, 84, 903–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purcell, T.L.; Buhimschi, I.A.; Given, R.; Chwalisz, K.; Garfield, R.E. Inducible nitric oxide synthase is present in the rat placenta at the fetal-maternal interface and decreases prior to labour. Mol. Hum. Reprod. 1997, 3, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, B.R.; Pernomian, L.; Bendhack, L.M. Contribution of oxidative stress to endothelial dysfunction in hypertension. Front. Physiol. 2012, 3, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cau, S.B.A.; Carneiro, F.S.; Tostes, R.C. Differential modulation of nitric oxide synthases in aging: Therapeutic opportunities. Front. Physiol. 2012, 3, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özüyaman, B.; Grau, M.; Kelm, M.; Merx, M.W.; Kleinbongard, P. RBC NOS: Regulatory mechanisms and therapeutic aspects. Trends Mol. Med. 2008, 14, 314–322. [Google Scholar] [CrossRef]
- Lucas, R.; Fulton, D.; Caldwell, R.W.; Romero, M.J. Arginase in the vascular endothelium: Friend or foe? Front. Immunol. 2014, 5, 589. [Google Scholar] [CrossRef] [Green Version]
- Shu, X.; Keller, T.C.S.; Begandt, D.; Butcher, J.T.; Biwer, L.; Keller, A.S.; Columbus, L.; Isakson, B.E. Endothelial nitric oxide synthase in the microcirculation. Cell. Mol. Life Sci. 2015, 72, 4561–4575. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, A.; Tengbom, J.; Alvarsson, M.; Wernly, B.; Zhou, Z.; Pernow, J. Red Blood Cell Peroxynitrite Causes Endothelial Dysfunction in Type 2 Diabetes Mellitus via Arginase. Cells 2020, 9, 1712. [Google Scholar] [CrossRef]
- Pretini, V.; Koenen, M.H.; Kaestner, L.; Fens, M.H.A.M.; Schiffelers, R.M.; Bartels, M.; Van Wijk, R. Red blood cells: Chasing interactions. Front. Physiol. 2019, 10, 945. [Google Scholar] [CrossRef] [Green Version]
- Cortese-Krott, M.M.; Kelm, M. Endothelial nitric oxide synthase in red blood cells: Key to a new erythrocrine function? Redox Biol. 2014, 2, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinbongard, P.; Schulz, R.; Rassaf, T.; Lauer, T.; Dejam, A.; Jax, T.; Kumara, I.; Gharini, P.; Kabanova, S.; Özüyaman, B.; et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006, 107, 2943–2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernow, J.; Mahdi, A.; Yang, J.; Zhou, Z. Red blood cell dysfunction: A new player in cardiovascular disease. Cardiovasc. Res. 2019, 115, 1596–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Kövamees, O.; Zahorán, S.; Hedin, U.; Alvarsson, M.; Hermesz, E.; Tratsiakovich, Y.; Nordin, F.; Mahdi, A.; Lundberg, J.O.; et al. Erythrocytes from Patients with Type 2 Diabetes Induce Endothelial Dysfunction Via Arginase I. J. Am. Coll. Cardiol. 2018, 72, 769–780. [Google Scholar] [CrossRef]
- Dugmonits, K.N.; Ferencz, A.; Zahoran, S.; Lazar, R.; Talapka, P.; Orvos, H.; Hermesz, E. Elevated levels of macromolecular damage are correlated with increased nitric oxide synthase expression in erythrocytes isolated from twin neonates. Br. J. Haematol. 2016, 174, 932–941. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, E.; Matsumoto, T.; Isige, M.; Tsuji, T.; Mugisima, H.; Takahashi, S. Comparison of capacities to maintain hematopoietic stem cells among different types of stem cells derived from the placenta and umbilical cord. Regen. Ther. 2016, 4, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Cortese-Krott, M.M.; Rodriguez-Mateos, A.; Sansone, R.; Kuhnle, G.G.C.; Thasian-Sivarajah, S.; Krenz, T.; Horn, P.; Krisp, C.; Wolters, D.; Heiß, C.; et al. Human red blood cells at work: Identification and visualization of erythrocytic eNOS activity in health and disease. Blood 2012, 120, 4229–4237. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, P.; Dugmonits, K.N.; Végh, A.G.; Hollandi, R.; Horváth, P.; Maléth, J.; Hegyi, P.; Németh, G.; Hermesz, E. Failure in the compensatory mechanism in red blood cells due to sustained smoking during pregnancy. Chem. Biol. Interact. 2019, 313, 108821. [Google Scholar] [CrossRef] [Green Version]
- Mizrahi, O.; Ish Shalom, E.; Baniyash, M.; Klieger, Y. Quantitative Flow Cytometry: Concerns and Recommendations in Clinic and Research. Cytom. Part B Clin. Cytom. 2018, 94, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Sun, L.; Zhang, D.; Zhu, L.; Wang, S.; Wang, D. Effects of red blood cell supernatants on hypoxia/reoxygenation injury in H9C2 cells. Int. J. Clin. Exp. Med. 2018, 11, 3612–3619. [Google Scholar]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes and Mechanisms of Intrauterine Hypoxia and Its Impact on the Fetal Cardiovascular System: A Review. Int. J. Pediatr. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingdom, J.C.P.; Kaufmann, P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta 1997, 18, 613–621. [Google Scholar] [CrossRef]
- Stanek, J. Hypoxic patterns of placental injury: A review. Arch. Pathol. Lab. Med. 2013, 137, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ming, X.F. Arginase: The emerging therapeutic target for vascular oxidative stress and inflammation. Front. Immunol. 2013, 4, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Y.J.; Wu, Q.; Muszynski, M.; Hansson, G.K.; Libby, P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-γ, tumor necrosis factor-α, and interleukin-1β. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 19–27. [Google Scholar] [CrossRef]
- Liu, X.M.; Chapman, G.B.; Peyton, K.J.; Schafer, A.I.; Durante, W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc. Res. 2002, 55, 396–405. [Google Scholar] [CrossRef] [Green Version]




| Clinical Parameters | Full-Term Single Neonates | Full-Term Twin Neonates |
|---|---|---|
| Numbers of Samples (N) | Hws: N = 22 Lws: N = 10 | Hwt: N = 24 Lwt: N = 18 |
| Gestational age at delivery (weeks) | 37.52 ± 0.50 (37–38.6) | 37.35 ± 0.47 (37–38.4) |
| Birth weight (kg) | Hws: 3.30 ± 0.172 (3.0–3.5) Lws: 2.59 ± 0.171 (2.49–2.8) | Hwt: 3.19 ± 0.073 (3.0–3.3) Lwt: 2.6 ± 0.174 (2.2–2.9) |
| APGAR score at 10 min | 9.92 ± 0.27 (9–10) | 9.69 ± 0.53 (8–10) |
| Ratio of vaginal delivery/caesarean section | 28:4 | 34:8 |
| Maternal age (years) | 29.5 ± 6.11 (20–42) | 31.9 ± 7.12 (24–40) |
| Blood sample pH | 7.26 ± 0.13 (7.03–7.42) | 7.29 ± 0.12 (7.19–7.44) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, P.; Dugmonits, K.N.; Orvos, H.; Hermesz, E. Mature Twin Neonates Exhibit Oxidative Stress via Nitric Oxide Synthase Dysfunctionality: A Prognostic Stress Marker in the Red Blood Cells and Umbilical Cord Vessels. Antioxidants 2020, 9, 845. https://doi.org/10.3390/antiox9090845
Chakraborty P, Dugmonits KN, Orvos H, Hermesz E. Mature Twin Neonates Exhibit Oxidative Stress via Nitric Oxide Synthase Dysfunctionality: A Prognostic Stress Marker in the Red Blood Cells and Umbilical Cord Vessels. Antioxidants. 2020; 9(9):845. https://doi.org/10.3390/antiox9090845
Chicago/Turabian StyleChakraborty, Payal, Krisztina N. Dugmonits, Hajnalka Orvos, and Edit Hermesz. 2020. "Mature Twin Neonates Exhibit Oxidative Stress via Nitric Oxide Synthase Dysfunctionality: A Prognostic Stress Marker in the Red Blood Cells and Umbilical Cord Vessels" Antioxidants 9, no. 9: 845. https://doi.org/10.3390/antiox9090845
APA StyleChakraborty, P., Dugmonits, K. N., Orvos, H., & Hermesz, E. (2020). Mature Twin Neonates Exhibit Oxidative Stress via Nitric Oxide Synthase Dysfunctionality: A Prognostic Stress Marker in the Red Blood Cells and Umbilical Cord Vessels. Antioxidants, 9(9), 845. https://doi.org/10.3390/antiox9090845





